Abstract
HIV is associated with an excess cancer risk, particularly of lymphoid malignancies. Modern therapeutics has changed the landscape of HIV disease and typical opportunistic complications of AIDS are now largely avoided. Although the risk of lymphoma has decreased, it still remains high. Nevertheless, treatment outcomes have improved due both to improvements in HIV medicine and in cancer therapeutics for the common lymphomas occurring in those with HIV infection. Other hematologic malignancies are rarely seen in HIV-infected patients, but the standardized risk ratio for many of these cancers is higher than in the background population. Principles of cancer care and appreciation for HIV infection as a comorbid condition can guide physicians in setting realistic goals and treatment for this patient population. In many cases, expected outcomes are very similar to the HIV-unrelated patients and therapeutic planning should be based on this understanding. Treatment tolerance can be predicted based on the status of the HIV disease and the cancer therapy being administered. For those hematologic cancers in which transplantation is part of standard care, this modality should be considered an option in those with HIV infection.
Introduction
Excess cancer and infection risk in the late 1970s and early 1980s heralded a new immunodeficiency syndrome now known as AIDS. The advent of modern HIV therapeutics has revealed a second act for HIV-related cancer and infection epidemiology. HIV cancer rates have shifted and treatment outcomes for the commonly occurring hematologic malignancies in HIV have improved. This article focuses on the role that combination antiretroviral therapy (cART) has played in this emerging epidemiology and underline what the optimal clinical approach to the various hematological malignancies should be in the context of advanced HIV therapeutics.
Burden of hematologic cancers in the HIV-infected population
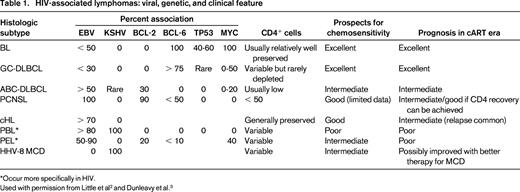
The principal hematologic malignancies occurring with increased frequency in association with HIV infection are lymphoid neoplasms, principally lymphomas (Table 1). Therefore, all patients presenting with classical Hodgkin lymphoma (cHL), Burkitt lymphoma (BL), or diffuse large B-cell lymphoma (DLBCL), including primary CNS lymphoma (PCNSL) should be tested for HIV infection. Indeed, we recommend that anyone with a diagnosis of hematologic malignancy be tested for HIV infection.
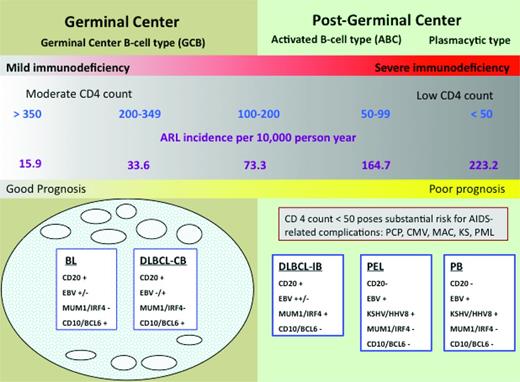
Immunologic status affects susceptibility to lymphoid neoplasms. The ability of cART to favorably modulate immunologic status appears to be the explanation for the changing epidemiology and clinical outcomes of these tumors in the setting of HIV infection. Comparing the incidence of AIDS-related lymphomas (ARLs) before and after the widespread availability of cART, Besson et al found a 50% decrease in ARL incidence.1 Strikingly, within distinct CD4 strata, there was no change in incidence across treatment periods (Figure 1). How can overall incidence decrease and yet remain unchanged within distinct CD4 strata? As CD4 cells decline, the risk of lymphoma increases. Therefore, with cART, more patients maintain relatively well-preserved CD4+ cell counts so their lymphoma risk is lower, translating into fewer incident cases overall. Of huge importance is the relationship between the degree of CD4+ cell depletion and the type of lymphoma that develops. As shown in Figure 1, the spectrum of lymphomas that occur in patients with higher CD4+ cells are characterized by more favorable biology and are more likely to be amenable to cure. Indeed, this immunologic shift consequent to widespread cART use most likely explains the survival improvement of HIV-related lymphomas in the cART era.2,3 However, these welcome factors do not belie the continued elevated lymphoma risk that persists in the cART era. The standardized incidence ratio (SIR) is > 70 (145 if CD4 is < 100; 35.8 if CD4 is ≥ 500) compared with the background population.4
Risk of NHL is inversely related to CD4 cell count. The lowest risk is between 350 and 500 CD4 cells/mm3 and does not change appreciably as the CD4 cells increase beyond that level. The risk increases substantially below 200 CD4 cells/mm3. Preserving CD4 cells with cART thus decreases the incidence of lymphoma and shifts toward the favorable subtypes to the left in the diagram. AIDS complications risk can also be ameliorated with cART. Note that within CD4+ cell strata, the ARL incidence has not changed comparing the cART and pre-cART eras.1 CB indicates centroblastic; IB, immunoblastic; PCP, Pneumocystis jiroveci pneumonia; MAC, mycobacterium avium complex; KS, Kaposi sarcoma; KSHV, Kaposi sarcoma–associated herpes virus; and PML, progressive multifocal leukoencephalopathy. Adapted with permission from Besson et al,1 Little et al,2 and Dunleavy et al.3
Risk of NHL is inversely related to CD4 cell count. The lowest risk is between 350 and 500 CD4 cells/mm3 and does not change appreciably as the CD4 cells increase beyond that level. The risk increases substantially below 200 CD4 cells/mm3. Preserving CD4 cells with cART thus decreases the incidence of lymphoma and shifts toward the favorable subtypes to the left in the diagram. AIDS complications risk can also be ameliorated with cART. Note that within CD4+ cell strata, the ARL incidence has not changed comparing the cART and pre-cART eras.1 CB indicates centroblastic; IB, immunoblastic; PCP, Pneumocystis jiroveci pneumonia; MAC, mycobacterium avium complex; KS, Kaposi sarcoma; KSHV, Kaposi sarcoma–associated herpes virus; and PML, progressive multifocal leukoencephalopathy. Adapted with permission from Besson et al,1 Little et al,2 and Dunleavy et al.3
The immunologic risk contribution to lymphoid cancer development is complex and varies according to tumor type. For example, cHL occurs with relatively high frequency during the first few months after initiation of cART as the CD4 cell counts are increasing and the HIV viral loads are decreasing, suggesting that cHL may be driven by immune recovery rather than by CD4+ cell count depletion.5 The relationship between the immune status and cancer is nevertheless complex. For example, a sudden precipitous CD4+ cell decrease may herald the onset of cHL months to a year in advance and is not necessarily a sign of cART failure.6 Interestingly, the SIR for cHL appears to be higher in cART users (SIR = 36.2) compared with nonusers (SIR = 11.4), although these estimates have overlapping 95% confidence intervals. In addition, other hematologic cancers may have elevated risk that are less clearly related to CD4+ cell count and may be partly explained by other factors such as the ability of HIV-infected individuals to live into older age with modern HIV therapy. For example, multiple myeloma (SIR = 9.8) and myeloid leukemias (SIR = 2.2) may be slightly elevated in HIV, but the absolute numbers are very small and determining the effects of immune status versus other factors that may contribute to the risk is difficult.7 It is also noteworthy that ∼ 3.2% of HIV-associated lymphomas are peripheral T-cell lymphomas; sporadic cases of B-cell and T-cell acute lymphoblastic leukemia also occur.8 The role of HIV infection and the immune environment on these tumors has not been well elucidated because of the rarity of the tumors.
Management of lymphoid and myeloid tumors in HIV-infected individuals
Optimal management requires an understanding of the patient's HIV disease as well as the curative potential of the malignancy. In other words, one must consider what needs to be done in relation to the HIV disease and then decide whether that has any impact on cancer management. To address these issues, HIV infection should be viewed as any other comorbid condition. There is a spectrum of comorbid disease that modulates therapeutic decision making in any given case and one assesses whether the impact is nearly negligible or severe and thus whether it imposes limits on cancer therapy. This is as true for HIV infection as for any other comorbid condition.
The comorbid status of HIV disease can be assessed using reasonably objective criteria. The main elements of this are the HIV viral load, sensitivity of the virus to available antiretroviral drugs, CD4+ cell counts, and prior history of AIDS-related complications. The mosaic of these data reveals a spectrum of HIV disease. On one end of the spectrum are those who are quite healthy in terms of HIV and would be expected to live a normal life span were it not for the cancer.9 The other end of the spectrum consists of end-stage AIDS patients who are highly likely to succumb to the HIV disease even if the current tumor had never developed. Fortunately, in areas where cART is widely used, most patients are closer to the healthy end of the spectrum. Figure 1 provides a graphic representation of this concept.
In general, patients with CD4+ counts > 200 cells/mm3 are at low risk of AIDS-related complications, and cancer therapeutics should be based on experience from the HIV-unrelated population. Those with lower CD4+ cells (100-200 CD4+ cells/mm3) also do well with standard therapy but may require more supportive care. In patients with < 50 CD4+ cells/mm3, there is without question a higher risk of treatment-related and opportunistic complications, as well as treatment-related mortality. However, this high-risk group is not homogenous, and prior HIV treatment status modifies the risk assessment considerably. Consultation with experts in HIV infection management may be key to understanding the prospects for successful long-term HIV management, and this can be invaluable toward planning best cancer therapy.
A first reflex may be to assign the advanced CD4+-depleted group to the end-stage AIDS end of the spectrum. Before cART, this would have been correct. However, those who are treatment naive are very different from those who have a long history of complicated AIDS and highly treatment-resistant HIV. Increasingly, patients with advanced CD4+ cell depletion are treatment naive. The AIDS epidemic is shifting demographically to communities with disadvantaged medical access or high HIV stigma that creates a barrier to HIV testing and care. Estimates suggest that up to 25% of people in these communities have unknown HIV infection.10 For patients with newly diagnosed advanced HIV disease, long-term cancer-free survival is possible in a large proportion with BL, DLBCL, PCNSL, and multicentric Castleman disease (MCD), as well as nonhematologic cancers such as germ cell tumors. In addition, nearly 40 anti-HIV drugs or combinations are Food and Drug Administration (FDA) approved, making HIV salvage therapy more feasible. If a decision is made to recommend palliative-only cancer care, in most cases, it should be based on the cancer outcome prospects rather than on HIV.
Another important concern specific to patients with HIV is what to do about cART during chemotherapy. Our strategy is to suspend cART before BL and DLBCL chemotherapy and resume after all cycles have been completed. It is important not to use a repeated stop-and-start strategy because this promotes HIV drug resistance. Our strategy avoids overlapping toxicity, pharmacokinetic interactions, and possible adherence problems associated with chemotherapy-related toxicity that could promote HIV drug resistance. We also recognize that the preponderance of CD4+ cells (> 500/mm3) are lost because of chemotherapy rather than HIV infection, which would lead to the loss of < 50 CD4 cells/mm3 during the time it takes to complete treatment.11 Therefore, concomitant cART during chemotherapy does not appear to protect against CD4 cell depletion. However, it is noteworthy that many experts in the field, including infectious disease experts, disagree with this approach. Much of their rationale is based on a study entitled SMART (Strategic Management of Antiretroviral Therapy).12 This large, randomized study demonstrated that continuous cART decreased the death hazard compared with a strategy of stopping cART until CD4+ cells decreased below 250 cells/mm3 and then restarting it until they reach 350/mm3 and continuing to cycle this way. Extrapolation of these data to cancer patients is problematic on at least 2 counts: (1) cancer patients were not eligible for the SMART study and (2) it is highly doubtful a difference would have been found in survival had the treatment interruption been restricted to one occasion (as we recommend during polychemotherapy for hematologic cancer).
Our concerns about adherence to cART during chemotherapy have not been borne out.13 Most studies show that HIV viral loads remain undetectable with concomitant cART and chemotherapy. However, if cART is administered with chemotherapy, we strongly recommend meticulous attention to toxicity. If early-cycle dose reductions are needed, one should consider the possibility of drug-drug interactions as the cause of enhanced toxicity and suspend cART. Abrogation of treatment-related toxicity by instituting chemotherapy dose reductions rather than removing the pharmacokinetic offender may adversely affect cancer cure. In some cases, preemptive cART suspension may be best. For example, the very real risk of drug-induced pancreatitis from asparaginase is likely to be compounded by effects of many antiretroviral drugs that are also implicated as causative agents in pancreatitis.
DLBCL and BL
In the cART era, the outcome for patients with aggressive lymphomas who receive optimal therapy is equivalent to that of the background population, and this should be kept in mind when selecting up-front treatment. In the pre-cART era, in an attempt to reduce toxicity, approaches such as the use of CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone/prednisolone)–like regimens for BL were widely practiced and resulted in dismal outcomes. Such approaches are now recognized as inadequate and should not be practiced. It is critical that these patients are approached in the same way as those with HIV-unrelated lymphoma and treated with curative intent.
The clinical evaluation should proceed without delay irrespective of whether the patient has previously known or newly diagnosed HIV infection, just as it would with any other patient. This evaluation should include assessment of the HIV disease parameters including CD4 cell count and HIV viral load. In addition to routine tests (including assessment of hepatitis B and C coinfection), thorough evaluation of the brain and CSF should be included. Having observed relatively high rates of isolated CNS progression and relapse in our HIV-infected patients, more than a decade ago, we implemented CNS prophylaxis for all patients with aggressive B-cell lymphomas.
The preferred regimen in our judgment for HIV-DLBCL is EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab) infusional chemotherapy, as described previously.2,3,13,14 Unfortunately, there are no randomized phase 3 data to provide the highest level of evidence one would like in support of this statement. However, there are single-institution and multicenter phase 2 data, as well as analyses combining trial data that strongly support our opinion. Interest in performing the definitive phase 3 study of R-CHOP versus EPOCH-R in this population has been low because of the disparities in the phase 2 outcomes of the 2 regimens.
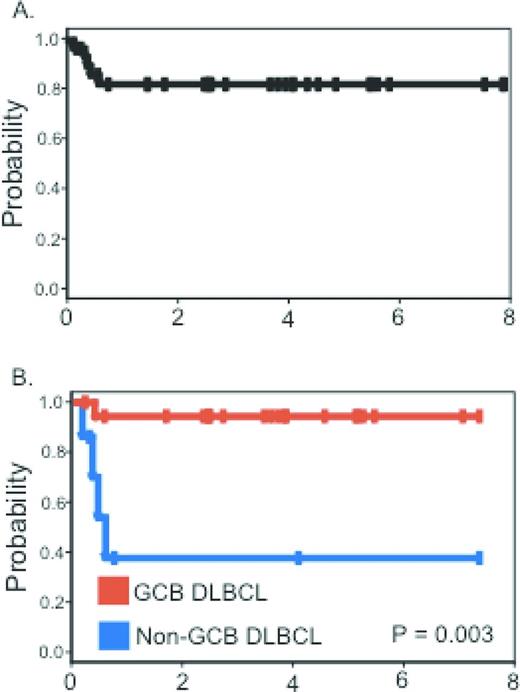
The initial National Cancer Institute (NCI) experience with 39 patients published in 2003 focused attention away from ARL as a lethal or preterminal AIDS event to one that was highly curable. After EPOCH chemotherapy and before rituximab availability, complete remission was achieved in 74% of patients and, at 53 months median follow-up, disease-free and overall survival were 92% and 60%, respectively.2 Comparison with similar HIV-unrelated cases showed equivalent outcomes. Subsequently, 33 subjects treated with the short-course EPOCH-RR (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin - double dose rituximab) regimen had a progression-free and overall survival of 84% and 68%, respectively, at 5 years median follow-up3 Tumor histogenesis was the only characteristic associated with lymphoma-specific outcome. The progression-free survival at 5 years was 95% for those with germinal center B-cell-like DLBCL and 44% for non-germinal center B-cell-like DLBCL (Figure 2). A pooled analysis of 150 patients treated on AIDS Malignancy Consortium (AMC) studies of either R-CHOP or EPOCH-R shows the hazard ratios for event-free survival and overall survival favor the EPOCH-R regimen.13
Progression-free survival. (A) Progression-free survival in all patients. (B) Progression-free survival for germinal center B-cell-like and non-germinal center B-cell-like DLBCL patients treated with short-course (SC)-EPOCH-RR. Used with permission from Dunleavy et al.3
Progression-free survival. (A) Progression-free survival in all patients. (B) Progression-free survival for germinal center B-cell-like and non-germinal center B-cell-like DLBCL patients treated with short-course (SC)-EPOCH-RR. Used with permission from Dunleavy et al.3
The findings of the short-course EPOCH-RR regimen are potentially important for treatment planning in the nonresearch setting for those patients with serious HIV-associated comorbidities or for patients who are unusually intolerant to chemotherapy. For example, if on restaging after 2 cycles, there is a complete response, administering 1 or 2 more cycles is reasonable and supported by these data.3 This is likely to be a much more successful strategy than repeated dose reductions and dose delays in an attempt to administer 6 cycles of therapy.
In HIV-unrelated DLBCL, a randomized phase 3 study comparing R-CHOP and dose-adjusted (DA)-EPOCH-R has recently completed enrollment. Why not await those study results before so strongly recommending the EPOCH-R regimen in HIV-DLBCL? First, the phase 3 results are unlikely to be available for a few years. Second, HIV-associated DLBCL is frequently characterized by high tumor proliferation, a feature that appears to confer resistance to CHOP but not to EPOCH.15 The phase 2 single-institution and multicenter experience with the EPOCH-R regimen is significantly more encouraging than with any other approaches reported. This holds for studies in which cases were restricted to favorable-risk ARL and yet 31% died of lymphoma with R-CHOP,16 unlike the case with EPOCH-R, in which the progression-free survival exceeds 80% (Figure 2A). In addition, the risk profiles of combining chemotherapy with rituximab in HIV strongly favors the EPOCH regimen, for which excess toxicity has not been reported.14,17 Salvage strategies should mirror the HIV-unrelated setting, including the use of hematopoietic transplantation as indicated, preferably on a research protocol.18
Striking preliminary evidence shows EPOCH-R to be highly active in both HIV-related and HIV-unrelated BL without the toxicity associated with more standard approaches.19 Of 30 patients with BL, 11 were HIV infected. No differences in outcome were seen between the HIV-positive patients and the overall group. At a median follow-up of 73 months, the progression-free and overall survival were 100% and 90%, respectively. Only 16% of the cycles administered were associated with fever and neutropenia. This compelling data was reviewed by the NCI Lymphoma Steering Committee, which recommended funding a national multicenter, single-arm phase 2 study aimed at providing a strong level of evidence for this approach. The study is now being conducted and is available to all AMC, Southwest Oncology Group (SWOG), Alliance for Clinical Trials in Oncology, and Eastern Cooperative Oncology Group (ECOG) members to enroll BL and cMYC+ DLBCL patients regardless of HIV status. Until the outcome of this study is known, it is highly recommended to refer patients for participation in the study. In addition, several small studies have shown that regimens such as CODOX-M (cyclophosphamide, vincristine, doxorubicin, methotrexate) IVAC (ifosfamide, etoposide and cytarabine) and hyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) are effective in HIV-BL, though considerably toxic (Table 2).20-22 Significant infectious complications and toxic deaths (17%) have been reported with these regimens.23
The important message here is that BL in the setting of HIV is highly curable. Inferior outcomes are documented using CHOP-like bolus therapy, which until recently was the standard practice in HIV-BL.24 A retrospective analysis of the Kaiser Permanente experience revealed outcome disparities for HIV-infected cases compared with HIV-unrelated cases, with increased 2-year mortality hazard risk doubled for the HIV cases.25 Examination of that study seems to reveal more about patterns of care rather than true survival prospects with optimized therapy, highlighting the importance of rapidly translating meaningful treatment advances into clinical practice.
Plasmablastic and primary effusion lymphoma
Plasmablastic lymphoma (PBL) and primary effusion lymphoma (PEL) are both oncogenic virus–driven tumors (EBV for PBL and HHV-8 for PEL) associated mainly with HIV advanced immune depletion. Both of these tumors carry a poor prognosis, although the occasional patient appears to have long-term survival and even cure. There is no established standard of care.
PBL involves multiple anatomical sites rather than mainly the oral cavity, as originally described. It represents < 10% of HIV-DLBCL with reported median survivals of 4 to 11 months.26,27 Our anecdotal experience suggests good outcome in those with limited-stage disease. We administer abbreviated cycles of EPOCH chemotherapy and then consider involved-field radiotherapy if all of the initial disease is confined to one radiation port. Because ∼ 50% of PBL cases are MYC positive, regimens such as EPOCH that can overcome high tumor proliferation are logical strategies.28 Because this tumor is EBV driven, we continue cART (modified to minimize pharmacokinetic interactions) during chemotherapy. Given the immune escape that appears central to the pathogenesis for this tumor, we recommend consideration for investigational allogeneic transplantation for relapse.
PEL is associated with HHV-8 in 100% of cases and is required to establish the diagnosis. In 70%, the tumor cells also harbor EBV. Nonlineage antigens including CD30 can be present, possibly suggesting a role for the use of brentuximab vedotin therapeutically. Outcome is generally poor, although long-term cures are occasionally seen. A strategy of draining the effusions while administering therapy is used in our clinic, but sufficient data to recommend this as generally effective or increasing the curative potential are not available. From a palliative perspective, at least it relieves effusion-related symptoms.
Primary DLBCL of the CNS
AIDS-related PCNSL (AR-PCNSL) is truly an opportunistic, EBV-driven cancer, nearly always restricted to patients with < 50 CD4+ cells/mm3. It is rarely seen in patients benefiting from cART. Cases seen in the setting of high CD4 cells are unlikely to be AR-PCNSL; especially if EBV unrelated, these cases are more likely to be more akin to PCNSL seen in the background population.
The main educational point to be emphasized is the necessity to abandon the diagnostic approach standardized in the early 1980s. That strategy calls for first an empiric trial of therapy for toxoplasmosis and, on progression shown by cranial imaging, treatment for PCNSL is then commenced. In the cART era, this is not a medically sound strategy. Because risk of toxoplasmosis is high in these patients and may occur concurrently with PCNSL, treatment for both may sometimes be required.
The presence of EBV in AR-PCNSL and its nuclear medicine imaging avidity, thallium-201–based tomography or fluorodeoxyglucose (18F) positron emission tomography (FDG-PET), offer an important diagnostic biomarker algorithm.29 If the CSF is positive for EBV by PCR and the FDG-PET (or thallium-201 scan) is also positive, the positive predictive value for AR-PCNSL approaches 100%. Although biopsy is always preferred and should be performed in all cases if feasible, if it is not possible, lymphoma therapy may be initiated in certain cases without further delay. If both tests are negative, lymphoma is ruled out with near 100% predictive value. If the results of the FDG-PET and EBV PCR are discordant, the predictive values are too low to act on and biopsy must be performed. Prompt initiation of treatment for both the HIV and the brain tumor may improve the prognosis. Our own clinical experience is that rituximab and high-dose methotrexate given with aggressive leucovorin rescue and concomitant cART is very active, with some long-term cures.30 The main feature limiting favorable outcome has been very sick patients referred after lengthy diagnostic delays that contributed to irreversible profound neurologic deficits.
HL
Mixed-cellularity cHL and lymphocyte-depleted cHL are the most common types of HL observed in the setting of HIV, and 80% of cases are EBV associated. Therapeutically, HIV-cHL should be approached in the same way as HIV-unrelated HL. Regimens such as ABVD should be standard. Recent data show that HIV status does not influence outcome in patients with cHL treated with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) chemotherapy in the cART era.31 Optimal curative potential rests on adherence to the dose and schedule of ABVD. Neurotoxicity and undue neutropenia should be first attributed to cART and the first maneuver should be to discontinue it to maintain the chemotherapy dose and schedule. This is potently highlighted by experience with excess neurotoxicity and subsequent dose reductions of vinblastine when given with ritonavir-based cART.32 This raises concerns for similar, though perhaps less obvious, effects on other vinca alkaloids, including vincristine.
Myeloid and lymphoid leukemias
There are not sufficient data to inform the approach to HIV patients with leukemia. Based on the principles of achieving tumor control while maintaining immune integrity, one can draw useful guidance for developing a rational approach in treatment planning for patients with these diseases. The chronic leukemias, especially with newer and developing therapeutics, are treated over the long-term with tyrosine kinase inhibitors and other targeted agents. Therefore, it is necessary to coadminister cART carefully planned to minimize drug-drug interactions. For acute leukemias, suspension of cART may be the best option. If there is to be a prolonged consolidation and maintenance phase, selection of antiretroviral agents should be handled in consultation with knowledge of pharmacokinetics of the various drugs. These are rare situations, and seeking out prominent experts for assistance is encouraged. Hematopoietic stem cell transplantation as part of standard care should be incorporated into therapeutic planning for HIV-infected individuals. Referral to investigational studies for this purpose should be prioritized.
Kaposi sarcoma–associated herpes virus–associated MCD
MCD is a neoplastic inflammatory condition with no standard therapy yet informed by adequate data. Patients with this condition are clearly best served by referral to research studies. Rituximab can be helpful, as well as novel therapies directed against human or viral IL-6. Because the Kaposi sarcoma–associated herpes virus encodes for kinase that phosphorylate nucleoside analogs such as zidvoudine and ganciclovir, these have been used as part of rationally designed interventions with some success.33
Conclusion
For the most part, hematologic cancer therapeutic prospects are equivalent in the HIV-related and HIV-unrelated settings. Appropriate assessment of HIV as a comorbid condition is essential to optimizing therapeutic strategies. Modern HIV medicine has influenced the epidemiology of hematologic cancers in HIV, and those tumors that do occur tend to have biological characteristics making them more amenable to successful therapy. Management of cART during cancer therapy must be individualized according to the type of malignancy and the specific therapy being administered.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: azidothymidine and ganciclovir for treatment of Kaposi sarcoma-associated herpes virus–associated MCD.
Correspondence
Richard Little, National Cancer Institute, National Institutes of Health, 31 Center Drive, MSC 2062, Bldg 31, Rm B1-W30, Bethesda, MD 20892; Phone: 240-276-6560; Fax: 240-276-7892; e-mail: littler@mail.nih.gov.