Abstract
What is the correct use of established clotting factors, prothrombin complex concentrates (PCCs), and activated factor VII in bleeding complications of trauma, surgery, and old and new oral anticoagulants? How will new clotting factors, specifically the long-acting factors, change the hemostatic management of coagulation deficiency disorders? From bench to bedside, comparative coagulation studies and clinical trials of modified clotting factors are providing insights to help guide hemostatic management of congenital and acquired bleeding disorders. Comparative thrombin-generation studies and preclinical and clinical trials suggest that PCCs and fresh-frozen plasma are effective in reversing the anticoagulant effects of warfarin, yet there are few data to guide reversal of the new oral anticoagulants dabigatran and rivaroxaban. Although coagulation studies support the use of PCCs to reverse new oral anticoagulants, correlation with clinical response is variable and clinical trials in bleeding patients are needed. For congenital bleeding disorders, exciting new technologies are emerging from the bench. Data from clinical trials of molecularly modified coagulation factors with extended half-lives suggest the possibility of fewer infusions, reduced bleeds, and better quality of life in persons with hemophilia. Preclinical studies of other novel prohemostatic approaches for hemophilia and other congenital coagulation disorders include RNA interference silencing of antithrombin, monoclonal anti-tissue factor pathway inhibitor (anti-antibody, anti-tissue factor pathway inhibitor) aptamer, bispecific anti-IXa/X antibody, and fucoidans. Understanding the comparative coagulation studies of established prohemostatic agents, the pharmacokinetics of new long-acting clotting factors, and their correlation with bleeding outcomes will provide opportunities to optimize the hemostatic management of both congenital and acquired hemostatic disorders.
Hemostasis in surgery, trauma, and oral anticoagulant reversal
Background
Hemostatic management of patients with excessive bleeding in trauma, surgery, and with oral anticoagulant therapy continues to be a major challenge. Increasing evidence from the bench and bedside indicates that prohemostatic agents such as prothrombin complex concentrates (PCCs), factor eight inhibitor bypass activity (FEIBA), and activated factor VII (rFVIIa) improve quality and quantity of clot formation and reverse excessive bleeding. Current recommendations for surgical/trauma hemostasis and warfarin reversal are based on data from comparative coagulation studies and preclinical and clinical trials, yet few data exist regarding new oral thrombin and Xa inhibitors and clinical trials on these are not available. The purpose of this update is to review current laboratory and clinical studies of prohemostatic agents to guide hemostatic management of excess bleeding in trauma, surgery, and anticoagulant reversal.
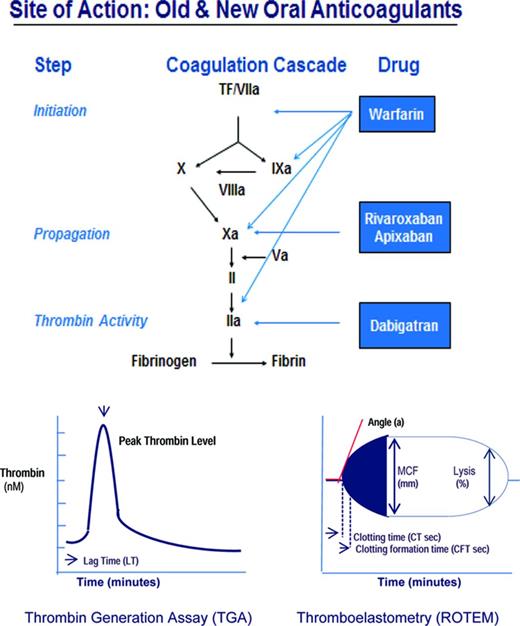
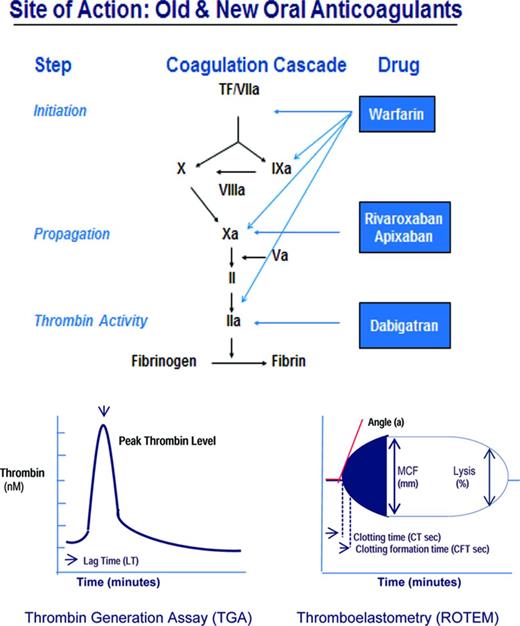
Whether induced by trauma, surgery, or excess anticoagulation, bleeding occurs through vessel injury. Vessel injury initiates coagulation through its interaction with coagulation factors, the vessel wall, and platelets that ultimately ensures the formation of a lifesaving fibrin clot (Figure 1). The damaged tissue from injured vessels (tissue factor, TF) activates factor VII (fVIIa), triggers a complex series of reactions that successively activate the tenase complex (the conversion of X by IXa with cofactor VIIIa) and the prothrombinase complex (the conversion of II by Xa with factor Va), generating thrombin (IIa; Figure 1). Thrombin activates fibrin clot formation, promotes clot stabilization and cross-linking through activation of fXIII, and provides clot protection through activation of thrombin-activatable fibrinolysis inhibitor. This process can be monitored by qualitative and quantitative laboratory measures of clot formation, specifically the thrombin generation assay (TGA) and thromboelastometry (ROTEM), which provide more comprehensive clot assessment than the prothrombin time (PT) and activated partial thromboplastin time (aPTT), which measure one-dimensional time to clot formation.1 The TGA assay measures the capacity of plasma to form thrombin, including endogenous thrombin potential (ETP; area under the curve) and lag time (LT; the time to initiation of thrombin generation); and ROTEM quantifies the effect of plasma and its cellular components on clot formation, including clotting time, maximum clot firmness, and clot lysis.2 The TGA and ROTEM assays can be used to assess the relative prohemostatic effects of PCCs, FEIBA, and rFVIIa in the management of hemorrhage with surgery, trauma, and excess anticoagulation. The purpose of this section is to review TGA and ROTEM findings and clinical bleeding outcomes after PCCs and rFVIIa in excessive bleeding with surgery, procedures, or anticoagulant reversal (Table 1) and current guidance for their use.
PCCs
PCCs are factor concentrates that contain factors II, VII, IX, and X, as well as protein C and S, originally developed to control bleeding in hemophilia patients with inhibitors to factor VIII or IX, to “bypass” factor VIII and factor IX in clot formation; and in hemophilia B patients. The so-called 4-factor PCCs such as Beriplex and Octaplex, which are manufactured outside of the United States, and Kcentra, which was recently licensed in the United States, contain factors II, VII, IX, and X, whereas the 3-factor PCCs such as Bebulin and Profiline contain factors II, IX, and X, but low levels of VII. Activated PCCs such as FEIBA were subsequently developed to increase the content of activated clotting factors in PCCs to enhance hemostasis, specifically the content of VIIa, IIa, and Xa. Although the use of PCCs for other reasons than the control of bleeding in hemophilia, for example, for blood loss or anticoagulation reversal, is considered off-label in the United States, it is a licensed indication in Europe. PCCs have a rapid onset of action (within minutes) and viral inactivation is by filtration, nanofiltration, and either pasteurization or solvent detergent treatment. Potential complications of PCC use include thrombosis and thromboembolic events.
rFVIIa
rFVIIa is a recombinant protein originally licensed to treat or prevent bleeding in patients with hemophilia A or B with inhibitors (antibodies) to factor VIII or IX, in patients with congenital factor VII deficiency, and, in Europe, for use in Glanzann thrombasthenia. The mechanism by which rFVIIa corrects hemophilia A or B inhibitors is as a “bypass” therapy, meaning bypassing VIII or IX, which are unavailable for clot formation (due to the inhibitor), and instead activating coagulation through VIIa. rFVIIa is also widely used off-label for life-threatening bleeding after major surgery or trauma or with oral anticoagulants. The mechanism of rFVIIa action is thought to be by boosting clot formation via the production of a “thrombin burst” through activation of IX, X, and II on the surface of activated platelets. In clinical settings, however, where no congenital factor deficiency exists and thrombin formation is adequate, rFVIIa may actually promote excess thrombin, that is, above-normal thrombin generation, resulting in thromboembolic complications3,4 that may be fatal in up to 27% of patients.5 Therefore, rFVIIa in off-label settings, which constitutes 97% of its use,5 may be dangerous, remains unsupported, and is specifically advised against in Europe. The drug is expensive, its risk-benefit profile is not well understood,4 and there is no validated test to monitor its use.
PCCs and rFVIIa in surgery and trauma
Comparative coagulation and preclinical studies
In animal coagulopathy models, clot stability is improved and blood loss is reduced after PCCs and, to a lesser extent, rFVIIa.6 In a porcine model of dilutional coagulopathy, PCCs provided better hemostatic control than rFVIIa. After liver laceration, pigs receiving 4-factor PCCs (35 U/kg) or rFVIIa (6 μg/kg) each demonstrated improved ROTEM clot strength, but there was more durable thrombin generation and less blood loss with PCC-treated animals.7 Similarly, in a porcine trauma model induced by standardized spleen injury, pigs receiving 4-factor PCC (35 U/kg) showed stronger, more rapid thrombin generation and clinical hemostasis than pigs receiving rFVIIa (180 μg/kg).8 In ROTEM studies, PCCs were also superior to rFVIIa in protecting against fibrinolysis in V-, VIII-, IX-, or X-deficient plasmas.2,9 ROTEM studies in plasmas with low heparin concentration, typical of cardiac bypass surgery, further revealed that adequate clot stability after rFVIIa required the addition of fibrinogen and aprotinin.2 These preclinical data provide in vitro and in vivo evidence that PCCs are more effective than rFVIIa in restoring thrombin generation and reducing blood loss in surgery or anticoagulation reversal.10
Clinical studies
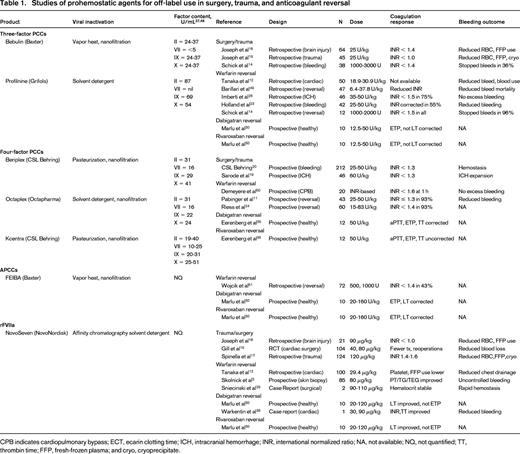
Clinical studies of coagulopathic patients undergoing surgery or procedures receiving PCCs or rFVIIa indicate that these patients have reduced blood loss and improved hemostasis (Table 1). In a prospective randomized, controlled trial of 43 patients with international normalized ratio (INR) > 2 requiring anticoagulant reversal for procedures or acute hemorrhage, 4-factor PCCs given at 25, 35, or 50 U/kg for INR < 4, INR 4-6, or INR > 6, respectively, corrected the INR to < 1.3 within 30 minutes in 93% of patients and effectively controlled bleeding.11 In patients undergoing cardiopulmonary bypass, 3-factor PCCs (18.9-30.9 U/kg) reduced transfusion requirement—fresh-frozen plasma (FFP) by 22% and platelet transfusions by 12%—to a greater degree than rFVIIa (90-120 μg/kg).12,13 Among surgical patients with severe perioperative bleeding, 4-factor PCCs (2000-3000 U) corrected INR from 1.7 to 1.4, improved hemoglobin and arterial blood pressure, and reduced surgical bleeding.14
Several studies indicate that rFVIIa provides rapid INR correction and shortens ROTEM clot formation and TGA lag time, although clinical response may be variable and thrombosis more common. In coagulopathic cardiac bypass patients undergoing aortic repair, treatment with rFVIIa alone (90-120 μg/kg) reduced chest tube drainage and transfusion requirement.13 In contrast, in a randomized controlled trial of 24 warfarin-treated subjects undergoing punch skin biopsy, pretreatment with rFVIIa (80 μg/kg) improved clot formation in TGA and ROTEM assays but failed to reduce blood loss or bleeding duration.3 In a postoperative cardiac surgery study, whereas rFVIIa shortened ROTEM clot formation and thrombin generation, fibrinogen was required to achieve clot stability.2 In a cardiac surgery study in which rFVIIa (40 or 80 μg/kg) reduced blood loss and transfusion requirement, there was an increase in thromboembolic events compared with placebo.15 In fact, a systematic review of 16 randomized clinical trials and 48 observational studies of off-label rFVIIa demonstrated the highest thrombotic complication rate occurred in cardiac surgery and intracranial hemorrhage (ICH) patients.4
In coagulopathic trauma patients, half of whom were receiving warfarin, 3-factor PCCs (25 U/kg) rapidly corrected the INR and reduced the RBC requirement, but, as with most studies of rFVIIa in trauma, showed no survival benefit.16 A recent retrospective review of 124 severely injured trauma patients requiring massive transfusion, however, found that early use of rFVIIa (120 μg/kg) within 2 hours of injury not only reduced hemorrhage and coagulopathy but also decreased 24-hour and 30-day mortality.17 Despite this finding, controversy remains regarding rFVIIa use given its high cost and lack of dosing guidelines.
A comparison study of 3-factor PCCs (25 U/kg) with rFVIIa (90 μg/kg) in 85 traumatic brain injury patients revealed significantly greater reduction in RBC and FFP requirement and lower mortality rate in the PCC group.18 The combination of rFVIIa (1.0 mg) and 3-factor PCCs to treat 46 patients with intracerebral hemorrhage led to rapid INR reversal that was maintained during hospitalization, with hemorrhagic expansion in only 4 patients.19 In a randomized trial in patients with acute major hemorrhage with surgical procedures, all of whom also received vitamin K, the 4-factor PCC Kcentra at 25 U/kg for INR 2.0-3.9, 35 U/kg for INR 4-6, and 50 U/kg for INR > 6, was significantly superior to FFP, shortening the INR to 1.3 in the first 24 hours, with correction beginning by 30 minutes.20
Finally, it is noteworthy that the use of ROTEM testing to guide PCC therapy in coagulopathic surgical patients has reduced transfusion requirement, blood loss, ICU admission, and deaths compared with routine monitoring without ROTEM.21
PCCs and rFVIIa to reverse warfarin
Comparative coagulation and preclinical studies
The prohemostatic effects of PCCs and FFP have been assessed by in vitro thrombin generation in warfarin-treated whole blood. PCCs were shown to be more effective than FFP in restoring PT/INR and thrombin generation (peak and lag time) and, among the PCCs, 4-factor PCCs were more effective than 3-factor PCCs.22 The degree of PT/INR correction is related to the starting INR: whereas 3- and 4-factor PCCs restore PT/INR in warfarin-treated plasma samples with INR < 3, only 4-factor PCCs correct PT/INR and thrombin generation in factor VII-deficient plasma when the INR is > 10.22 The latter is attributed to the low factor VII content of 3-factor PCCs and is consistent with the improved thrombin generation when FFP is combined with 3-factor PCCs in warfarin-treated plasma with INR > 4.23
Clinical studies
During warfarin reversal in 60 coagulopathic patients, some requiring emergency surgery, 4-factor PCCs (15-83 U/kg) corrected the INR to 1.4 within 1 hour in 92% of patients (Table 1).24 In 3 randomized trials of warfarin reversal for ICH, 3-factor PCCs (25-50 U/kg) provided significantly faster INR correction than FFP.25 The addition of FFP to 3-factor PCCs improved warfarin reversal response rate: in 40 patients with INR > 5; 50% achieved INR correction with either FFP or 3-factor PCCs (25-50 U/kg) alone, whereas 89% achieved correction with the combination of FFP and 3-factor PCCs.23
In a study of preoperative warfarin reversal a single dose of 4-factor PCCs (1000-2000 U) provided INR correction from 2.8 to 1.5 with no postoperative bleeding.14 INR correction was more rapid with 3-factor PCCs (4.8 vs 7.3 hours) and also when 4-factor PCCs were combined with FFP (3 vs 8.9 hours) than with FFP alone.12 INR correction with 3-factor PCCs, however, was limited by the initial INR. Although 4-factor PCCs corrected INR despite the starting INR, 3-factor PCCs corrected INR in only 33% of those with INR > 4 and in none of those with a starting INR ≥ 6.11,26,27 In 11 retrospective studies of acute warfarin reversal for acute bleeding, including ICH, rFVIIa (10-90 μg/kg) rapidly corrected the INR but, unlike with PCCs, the reduction in bleeding was equivocal.28 Similar to the greater degree of clinical bleeding response with PCCs compared with rFVIIa, ROTEM clot stability and clot lysis time assays in warfarin-treated patients improved to a greater degree with PCCs than with rFVIIa.29
PCCs and rFVIIa to reverse new oral anticoagulants
Introduction
With efficacy and safety data from clinical trials of new oral anticoagulants (NOACs), the thrombin inhibitor dabigatran and the Xa inhibitors rivaroxaban and apixaban have been increasingly used in the clinical setting. Despite short half-life, short duration of action, no requirement for monitoring, few drug interactions, and lower bleeding rate, reversal of these agents for life-threatening bleeding is challenging because there are no specific antidotes. However, despite laboratory improvement with hemostatic agents in thrombin generation (TGA), LT, and time to peak thrombin, other assays may not correct30 and clinical bleeding may continue unchecked. Therefore, management of bleeding with NOACs is challenging and, in the absence of clinical trials, clinical guidance is based on data from animal models, healthy volunteers, and case reports.
Comparative coagulation and preclinical studies
In a dabigatran-induced murine coagulopathy model after tail transection injury,26 FEIBA improved bleeding time significantly better than PCCs, rFVIIa, or the combination of PCCs and rFVIIa, but none of these interventions reduced blood loss.31 In the dabigatran-induced rabbit coagulopathy model after standardized kidney incision, PCCs in increasing doses of 20, 35, and 50 U/kg incrementally increased peak thrombin generation and speed of thrombus formation and reduced PT, but blood loss was completely normalized only at the highest dose.32 In a third model, the dabigatran-induced murine ICH model induced by striatal collagenase injection, ICH could be prevented by 4-factor PCCs pretreatment (25-100 U/kg), but not rFVIIa (8 mg/kg) or FFP, and reduction in clinical bleeding as measured by 24-hour ICH expansion was only observed with PCCs and, to a lesser extent, with FFP, but not with rFVIIa.33 In rat and baboon continuous bleeding models induced by continuous infusion rivaroxaban, the administration of PCCs (25 or 50 U/kg), aPCC (50 or 100 U/kg), or rVIIa (400 μg/kg) each reversed the anticoagulant effects of rivaroxaban, as measured by PT and thrombin time (TT), but rFVIIa did not correct thrombin antithrombin complexes by TGA and, moreover, none completely corrected coagulation values to baseline.34 Further, there was poor correlation with bleeding response, measured by mesenteric bleeding time, which failed to completely correct, and this was attributed to the slow generation of Xa to neutralize rivaroxaban and completely stop bleeding.34
Clinical trials
Although there are no clinical trials of thrombin or Xa inhibitor reversal, several randomized trials in healthy volunteers have investigated laboratory reversal of these agents. In volunteers anticoagulated with rivaroxaban, PCCs (50 U/kg) completely reversed rivaroxaban-induced PT prolongation and TT prolongation and improved ETP.30 In contrast, PCCs at the same dose failed to reverse the anticoagulant effect of dabigatran in these volunteers, as measured by aPTT, TT, and ETP.35 In a second study of healthy volunteers anticoagulated with dabigatran or rivaroxaban, thrombin generation correction by FEIBA (20-120 U/kg) was greater than that by 4-factor PCCs (12.5-50 U/kg) or rFVIIa (20-120 μg/kg).30 Although LT and ETP corrected after FEIBA, only LT corrected after PCCs and only LT corrected after rFVIIa.30 Few data exist in bleeding patients about how improvement in clot formation in TGA relates to clinical bleeding response. In a single available case report of dabigatran-associated bleeding in a patient undergoing aortic valve and coronary artery bypass surgery, high-dose rFVIIa (21.6 mg in 5 doses of 2.4 mg or 7.2 mg each) followed by hemodialysis successfully reduced chest and pericardial blood loss.36
Treatment guidelines
Bleeding in surgery and trauma
PCCs are more effective than rFVIIa or FFP in restoring thrombin generation and/or INR reversal and reducing blood loss in surgery and trauma regardless of whether there has been previous warfarin use.10,11,16,20 In randomized trials, 3-factor PCCs (25-50 U/kg) have demonstrated more rapid INR correction than FFP25 and when FFP is administered with 3-factor PCCs, response rates improve.23 Although the combination of 3-factor PCCs with low-dose rFVIIa is effective in reversing INR19 and rFVIIa may provide earlier correction of coagulopathy,2,3,17 safety issues remain. In addition, given the thrombotic risk associated with each agent alone, recommendations regarding the combination must await randomized trials comparing safety and efficacy in bleeding patients. The 4-factor PCCs provide rapid, sustained correction of INR in coagulopathic surgical and trauma patients with better efficacy than FFP and without the associated fluid overload,11,16,20 but should be avoided in patients with recent thromboembolism (ie, within the previous 3 months).20 Further, the use of PCCs and rFVIIa should be judicious, with INR-based dosing for PCCs and careful monitoring for thrombosis.20
Warfarin reversal
Few clinical trials have addressed the management of anticoagulation-related bleeding complications. The current 2012 Chest guidelines, which are based on small observational studies, case reports, and expert opinion, suggest that patients with major vitamin K antagonist–associated bleeding should be rapidly reversed with PCCs, FFP, or rFVIIa, with 4-factor PCCs preferred over plasma, supplemented by IV vitamin K (5-10 mg).10 The latter provides rapid onset of action, within 6-8 hours, and thus helps to sustain the response of other products with shorter half-lives.10 Dosing recommendations for 3-factor PCCs, as for the recently Food and Drug Administration (FDA)–approved 4-factor PCCs, for warfarin reversal include 25 U/kg for INR 2.0-3.9, 35 U/kg for INR 4.0-6.0, and 50 U/kg for INR > 6.0.11,37 Whereas rFVIIa is considered an alternative to PCCs, both agents are associated with thrombosis risk, especially in those with previous thrombosis,20 and the lowest effective dose of rFVIIa is not established.
Reversal of NOACs
Whether PCCs or rFVIIa can reverse the anticoagulant effects of thrombin or direct Xa inhibitors is not established. In the absence of clinical trials, recommendations for NOAC reversal in bleeding patients are based on preclinical studies, case reports, and expert opinion. The short half-life and lack of a specific antidote suggest that for urgent reversal, NOACs and antiplatelets agents should be held and transfusion support and surgical hemostasis provided if indicated.38 Dialysis with activated charcoal, if initiated within 2 to 4 hours of dabigatran ingestion, will remove it from the circulation,38-41 but not rivaroxaban because it is highly protein bound. Although FFP, rFVIIa, and PCCs may be considered, studies of these agents in humans to reverse NOACs are lacking, there is little correlation between coagulation assay correction and reversal of bleeding, and dosing and monitoring are not established.30,32,34-36,38-44
Experimental agents
Several novel antidotes to reverse NOACs are in development and some are in early clinical trials development for reversal of anticoagulants. These include an Xa congener that neutralizes Xa coagulation inhibitor function, but has no in vivo effects.45 Also being studied is a thrombin double mutant, W215A/E217A, which shortens thrombin inhibitor-associated aPTT prolongation thrombin generation in vitro.46 Another novel drug in development is the dabigatran-specific antidote aDabi-Fab. This antidote mimics thrombin structure but not thrombin function in vitro, with 350-fold greater affinity for dabigatran than for thrombin. Studies have demonstrated that aDabi-Fab induces a rapid and prolonged reversal of dabigatran-prolonged TT and aPTT.47 Development of such antidotes, if shown to be safe and effective in early phase studies, are anticipated to be evaluated in later-phase clinical trials. These agents may provide additional options for emergency reversal of NOACs.
Long-lasting clotting factors for hemophilia B
Background of long-acting FIX proteins
Long-lasting products are generating a great deal of excitement within the field. The new long-lasting protein technologies represent a potential major advance in hemophilia management. There are 13 ongoing long-acting factor VIII and IX clinical trials listed at www.clinicaltrials.gov involving 12 to 93 sites on 6 continents and 12 to 172 patients. Differences in factor VIII and IX half-life translate into shorter duration products for factor VIII derivatives (for more information, please see the chapter by Shapiro in this publication regarding long-acting factor VIII proteins). Early-phase long-acting FIX studies52-54 and one phase 3 study55 have been completed, and the latter is under FDA review. Given the progress in hemophilia treatment, with replacement clotting factors enabling a lifespan comparable to that in unaffected men, why the interest in long-acting factor proteins? Despite revolutionary advances in safety and purity of factors, the standard of care for prevention of hemophilic joint disease is prophylaxis or 2 or 3 times weekly IV factor infusion, which is invasive, burdensome, and, in young children, requires venous access devices that may be complicated by thrombosis and infection. Therefore, there is a theoretical advantage for clotting factors with longer half-lives because they could result in potentially fewer infusions, less frequent bleeding, fewer venous access devices, and better quality of life.
Technologies of long-acting factor IX proteins
Recombinant factor IX products with longer plasma half-lives than currently commercially available products have been in development over the last decade. The so-called “long-acting” factor IX proteins are composed of standard recombinant proteins either conjugated to hydrophilic polymers such as polyethylene glycol (PEG)52 or linked or fused to albumin56 or the Fc fragment of IgG1.57 PEG prolongs half-life by causing steric hindrance of the factor IX protein, reducing its elimination efficiency and protecting clotting proteins from proteolytic degradation.52 Alternatively, fusion technology prolongs half-life through fusion of the recombinant factor IX to albumin56 or to the Fc fragment of IgG1.57 In the case of Fc fusion protein, the Fc fragment binds to the neonatal Fc receptor, is taken up by endothelial cells or macrophages by pinocytosis and recycled within lysosomes to the plasma membrane, protecting it from lysosomal degradation, and improving its circulation half-life.58 When these long-acting factor IX proteins are physiologically activated, the PEG, albumin, and linker are cleaved,56 leaving the recombinant factor IX, with similar function to standard factor IX in chromogenic and activity assays and, for everything but the albumin protein, similar in immunogenicity assays.
Preclinical and clinical studies
Animal studies demonstrate prolonged half-life in the circulation of these long-acting factor IX proteins,52,56,57 compared with standard recombinant factor IX. Improved survival has been demonstrated for the glycopegylated and Fc fusion factor IX proteins after tail vein transection when the procedure is preceded by long-acting factor IX and longer-lived hemostasis compared with recombinant factor IX.59 Early-phase safety and pharmacokinetic studies in patients with severe factor IX deficiency have demonstrated safety and prolonged activity with half-lives ranging from 82 to 93 hours after single-dose pharmacokinetic assessment of the glycopegylated factor IX, N9-GP,52 the factor IX Fc fusion protein rFIXFc,53 and the albumin fusion factor IX, rIX-FP54 compared with recombinant factor IX (Table 2). In one phase 3 study in patients with severe factor IX deficiency, the long-acting factor IX appears to have comparable efficacy to recombinant factor IX in the treatment and prevention of bleeds, with a prolonged half-life and a factor IX level above 1%, the level below which spontaneous bleeds may occur, up to at least 14 days in 50% of patients.55 Preliminary data show no evidence of inhibitor antibodies, anaphylaxis, or allergy and no drug-related serious adverse events.
Conclusions
For the first time, long-lasting clotting factor therapy provides the possibility of a simpler, more durable treatment regimen for persons with hemophilia. If safety, efficacy, enhanced recovery, and half-life are confirmed in large clinical studies, it is anticipated that long-acting factors may provide a new paradigm in hemophilia management, with prolonged protection against spontaneous bleeds, decreased infusion frequency, and improved quality of life. An unanswered question is whether individual pharmacokinetics will be necessary to personalize or optimize dosing of long-acting clotting factors or if empiric dosing, as is commonly practiced today, will be sufficient to provide maximal bleed protection. Although cost and patient choice may also shape discussions regarding implementation of these products, the new pharmacokinetic characteristics of long-acting proteins offer the potential to reduce disease burden and improve quality with fewer treatments, constituting a major improvement in hemophilia management.
Future hemostatic approaches
It is important to recognize that, whereas long-acting factor proteins have significant potential to reduce burden of disease, considerable efforts continue to be spent on the development of alternative modalities for the treatment of hemophilia and other congenital disorders. Although the current strategy of regular factor infusions to prevent bleeds (ie, prophylaxis) reduces morbidity and improves quality of life, the most difficult treatment complication, inhibitor formation, remains in up to 30% of patients. Therefore, better approaches are still needed. The newest approach, in contrast to infusing factor proteins with prolonged factor half-life, is to block clot inhibitors, thereby enhancing thrombin generation and hemostasis. Some of these novel prohemostatic approaches are in early-phase studies and are beginning to be developed for phase 3 trials in individuals with hemophilia and other congenital bleeding disorders. These include inhibitors of RNA interference silencing of antithrombin,60 monoclonal anti-TFPI antibody,61 anti-TFPI aptamer,62 bispecific anti-IXa/X antibody,63 and fucoidans.64 If proven safe and effective in phase 3 trials, these novel agents may provide for long-lasting, inhibitor-free therapy for hemophilia because they avoid exposure of hemophilia patients to standard factor VIII or factor IX to which inhibitor formation is directed, a potentially game-changing innovation.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Biogen Idec and National Hemophilia Foundation Medical and Scientific Advisory Committee; has received research funding from Baxter, Bayer, Biogen Idec, CSL Behring, and Novo Nordisk; and has consulted for Baxter and Biogen Idec. Off-label drug use: PCCs and rFVIIa for reversal of NOACs.
Correspondence
Margaret V. Ragni, MD, MPH, Professor of Medicine, Clinical and Translational Science, Department of Medicine, Division Hematology/Oncology, University of Pittsburgh, Director, Hemophilia Center of Western Pennsylvania, 3636 Boulevard of the Allies, Pittsburgh, PA 15213-4306; Phone: 412-209-7288; Fax: 412-209-7281; e-mail: ragni@dom.pitt.edu.