Abstract
Hemophilia is a genetic disease caused by a deficiency of one of the coagulation proteins. The term usually refers to either hemophilia A, factor VIII (FVIII), with an incidence of ∼1 in 5000 male births, or hemophilia B, factor IX (FIX), with an incidence of ∼1 in 30 000 male births. When severe, the disease leads to spontaneous life-threatening bleeding episodes. Current therapy requires frequent intravenous infusions of therapeutic factor concentrates. Most patients administer the infusions at home every few days and must limit their physical activities to avoid bleeding when the factor activity levels are below normal. In March 2014, a new therapeutic FIX preparation was approved for clinical use in Canada and the United States and, in June 2014, a new FVIII preparation was approved for clinical use in the United States. Over the next couple of years, other new factor products for FIX, FVIIa, and FVIII, which are currently in late stages of clinical trials, will likely also be approved. These new factors have been engineered to extend their half-life in circulation, thus providing major therapeutic advances for patients with hemophilia primarily by allowing treatment with fewer infusions per month. In the clinical trials so far, >500 patients have successfully used these extended half-life products regularly for >1 year to prevent spontaneous bleeding, to treat successfully any bleeding episodes, and to provide effective coagulation for major surgery. Essentially all infusions were well tolerated and effective. These promising new therapies should allow patients to use fewer infusions to maintain appropriate clotting factor activity levels in all clinical settings.
Learning Objective
To be able to identify the new extended t1/2 products for hemophilia A, B, and inhibitors and to understand the differences found to date between these products in clinical and preclinical studies
Introduction
Hemophilia is a genetic disease caused by mutation of one of the genes for coagulation proteins. The term usually refers to either hemophilia A, factor VIII (FVIII), or hemophilia B, FIX deficiency. The incidence of hemophilia A is the same in all geographic regions, populations, and ethnic groups studied: ∼1 in 5000 male births.1 When severe, defined as clotting activity <1%, patients are at risk for spontaneous, life threatening bleeding episodes. Individuals with moderate hemophilia, between 1% and 5% clotting activity, or with mild hemophilia will usually suffer abnormal bleeding only after minor trauma or surgery.
When untreated, patients with severe hemophilia have a short life expectancy of ∼20 years but, over the past several decades, the clinical management for hemophilia has improved dramatically.2,3 Factor replacement therapy has reduced the morbidity and mortality of hemophilia through reduction in the frequency of bleeding episodes and improvement in the quality of life.4,5 Regular intravenous infusions of hemophilia factor concentrates 2-3 times each week, termed prophylactic therapy, reduce the development of hemophilic arthropathy and are now the standard of care for children and, increasingly, for adults as well.6,7 Despite this progress, both children and adults still experience bleeding episodes when the factor activity level is low (Figure 1). As few as only 1 or 2 bleeding episodes in a single joint can initiate the process of inflammation, leading to synovitis and chronic joint damage or hemophilic arthropathy.8,9 In addition, although the life expectancy of persons with severe hemophilia has improved significantly, death rates due to bleeding remain higher than in the general population.10 Twenty years ago, the development of recombinant factors provided safe and reproducible sources of the factors and increased the supply, but these therapies are expensive; annual costs have risen to ∼$150 000 per patient in the United States and these rigorous regimens are difficult so adherence remains a problem.11 In addition, convenient access to peripheral veins is a problem and many children require the use of central venous access devices, with the concomitant risks of infection and thrombosis.12,13 Despite recent promising success in gene therapy for hemophilia B, a cure for hemophilia is not yet available.14 Therefore, improved factor preparations are needed.
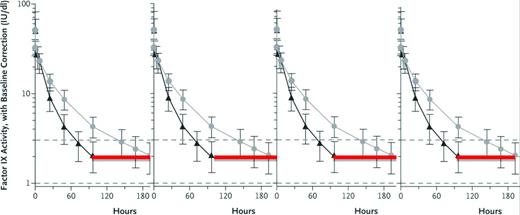
Expected FIX activity levels after rFIXFc or rFIX. Shown are expected FIX activity after doses (50 IU/kg body weight) of rFIXFc or rFIX administered intravenously at time 0 hours and followed for the specified intervals. The immediate postdose recovery giving a FIX activity level of 50% is as expected for both the short-acting rFIX and the extended t1/2 rFIXFc. The red line indicates the hours when the FIX activity level is <2% in this individual after taking the short-acting rFIX, and thus when he would be at risk of bleeding. Although the lines represent 1 individual's experience with the 2 different FIX preparations, the vertical lines represent the range of FIX activity levels for most persons with hemophilia. (Figure modified with permission from Powell et al.28 )
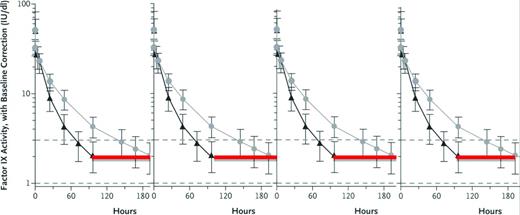
Expected FIX activity levels after rFIXFc or rFIX. Shown are expected FIX activity after doses (50 IU/kg body weight) of rFIXFc or rFIX administered intravenously at time 0 hours and followed for the specified intervals. The immediate postdose recovery giving a FIX activity level of 50% is as expected for both the short-acting rFIX and the extended t1/2 rFIXFc. The red line indicates the hours when the FIX activity level is <2% in this individual after taking the short-acting rFIX, and thus when he would be at risk of bleeding. Although the lines represent 1 individual's experience with the 2 different FIX preparations, the vertical lines represent the range of FIX activity levels for most persons with hemophilia. (Figure modified with permission from Powell et al.28 )
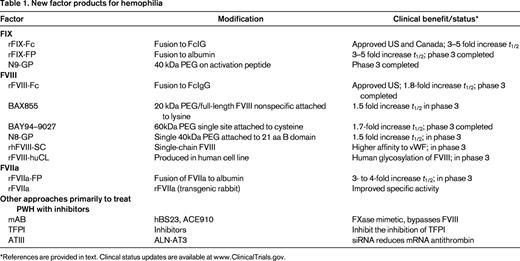
Various methods are in development to improve the treatment of hemophilia.15-20 The different approaches, including the use of bioengineered coagulation factors, can be summarized in 4 groups, efforts to extend the half-life (t1/2) of FVIII, FIX, or FVIIa and other alterations in the coagulation cascade to compensate for the missing clotting activity in hemophilia (Table 1). Each group will be discussed separately. As the first extended t1/2 factor product approved for clinical use, eftrenonacog alfa (FIXFc) will be discussed in greater detail than the others still in development, but comparative clinical experience eventually will be necessary to determine the relative clinical advantages of the various new products.
Fusion proteins
In March 2014, the first factor concentrate with an extended t1/2, eftrenonacog alfa (FIXFc), was approved in Canada and the United States for clinical use in children and adults with hemophilia B. The protein is composed of a single molecule of recombinant FIX covalently fused to the dimeric Fc (fragment crystallizable) domain of IgG1, with no intervening linker sequences.19 This approach combines 2 molecular structures, each with a long history of safety and efficacy in clinical use.19 Recombinant FIX has been in clinical use for nearly 20 years. The IgG constant region (Fc) has been molecularly engineered to create fusion proteins that prolong the circulating t1/2 of Fc fusion–based drugs used clinically (eg, etanercept, romiplostim) and others in development.21,22 After nearly a decade of experience in clinical use, these protein fusions support the safety of the Fc fusion approach.
With Fc fusion proteins, the neonatal Fc receptor interacting with the endogenous IgG recycling pathway delays lysosomal degradation of IgG and the fusion proteins, recycling them back into circulation and thus prolonging the plasma t1/2.23,24 The Fc domain has a t1/2 of 3 weeks.23 Fc-fusion proteins that are internalized by endothelial cells bind to the Fc receptor present in the acidified endosome in a pH-dependent manner and are subsequently recycled back to the surface and released at neutral pH, thereby escaping degradation by the lysosome. Although Fc fusions are typically expressed as homodimers formed through a disulfide bond, this dimeric structure was not effective for large clotting factors. However, fusion of the monomeric form of the IgG1 Fc to human FIX, FVIIa, or B-domain-deleted FVIII, with secretion as the dimeric Fc molecule with one molecule of clotting protein, was effective and demonstrated increases in plasma t1/2.24 In addition, the larger-sized molecules are expected to experience slower renal clearance.25 An additional advantage during manufacture is that the Fc domain can improve the solubility and stability of the partner molecule both in vitro and in vivo, and allows for easy cost-effective purification by protein-G/A affinity chromatography.19 A human cell line, HEK-293H, is used for expression and cotransfected with the expression cassette for PC5 (proprotein convertase 5),26 a processing enzyme, to ensure full cleavage of the FIX propeptide. Cell lines are grown in serum-free suspension medium in the presence of vitamin K. Purification of rFIXFc monomer is by column chromatography with the use of a protein A capture step and 2 anion exchange steps, Fractogel DEAE and Q Sepharose. The last ion exchange step involves pseudoaffinity elution19 from a Q Sepharose resin with low ionic strength CaCl2 to obtain rFIXFc with highest specific activity.
Preclinical studies of eftrenonacog alfa
Eftrenonacog alfa (FIXFc) demonstrated safety and prolonged efficacy in bleeding models compared with rFIX in mice, rats, hemophilic dogs, and cynomolgus monkeys.19 Biochemical characterization confirmed proper propeptide processing and appropriate extensive posttranslational modification after expression in HEK293H cells.27 rFIXFc possessed a greater degree of β-hydroxylation of Asp 64 than did rFIX or pdFIX. Peters et al19 showed that rFIXFc has a 3- to 4-fold longer terminal t1/2 in mice expressing human neonatal Fc receptor (FcRn) and beta 2 microglobulin (β2m) compared with rFIX, whereas both proteins have similar short terminal half-lives in mice lacking FcRn (FcRn/β2m KO). These studies confirm that FcRn mediates the longer t1/2 of rFIXFc, thus using the natural pathway responsible for protecting IgG antibodies from degradation. Levels of activated FIX are extremely low in rFIXFc, <10% of the levels reported for currently marketed rFIX and 5% of the levels reported for pdFIX products.19 Whether the lower levels of activated FIX seen with rFIXFc are clinically significant remains to be determined with more extensive clinical experience.
Clinical studies for FIXFc
Shapiro et al reported the first in human phase 1/2a clinical trial of rFIXFc.17 This open-label, dose-escalation trial (www.ClinicalTrials.gov identifier #NCT00716716) in previously treated adult subjects with hemophilia B examined the safety and pharmacokinetics of rFIXFc. Subjects with a variety of hemophilia B genotypes, such as stop codon/nonsense and missense mutations, were included. Several subjects had near absence of FIX antigen that correlated with markedly reduced FIX activity, whereas others with missense genotypes had more antigen than activity, indicating a dysfunctional circulating protein. A total of 16 adverse events were reported by 7 subjects, distributed evenly across treatment groups, and most were mild or moderate. No allergic reactions, FIX inhibitors, or anti-rFIXFc antibodies were detected. There were no reports of thrombosis during the study.
After rFIXFc infusion, there was a dose-proportional increase in FIX activity followed by a biexponential decay characterized by a short distribution (α) phase and a log-linear elimination (β) phase. The mean elimination t1/2 was dose independent over the therapeutic dose range tested, that is, 56.5 ± 14.1 hours at 100 IU/kg, compared with the mean elimination t1/2 reported for rFIX (BeneFIX) of 19.3 ± 4.97 hours (range, 11.1-36.4 hours). With baseline subtraction, mean FIX activity terminal t1/2 and mean residence time for rFIXFc were 56.7 and 71.8 hours, respectively. These results are ∼3-fold longer than those reported for current rFIX products. The incremental recovery of rFIXFc was 0.93 IU/dL per IU/kg, similar to plasma-derived FIX.
The phase 3 nonrandomized, open-label, multicenter study28 was designed to compare the pharmacokinetics of rFIXFc with those of recombinant FIX and to assess the safety and efficacy of repeated administration of rFIXFc for the prevention and treatment of bleeding in adolescents and adults with severe hemophilia B. (www.ClinicalTrials.gov identifier #NCT01027364.) The participants were representative of the general adult population with severe hemophilia B (endogenous FIX level of ≤2 IU/dL or ≤2% of normal levels) and were 12 years of age or older. Reflecting different clinical regimens, the study included 4 treatment groups. Group 1 received weekly dose-adjusted prophylaxis (50 IU of rFIXFc/kg of body weight to start) with the dose adjusted as needed to maintain trough FIX activity levels at 1%–3% or higher if needed to prevent clinical bleeding. Group 2 received interval-adjusted prophylaxis (100 IU/kg every 10 days to start) with the interval adjusted as needed as for group 1. Group 3 received treatment as needed (episodic or on-demand treatment) for bleeding episodes (20-100 IU/kg) with the dose adjusted according to bleeding severity. Group 4 received treatment as needed for surgical procedures. In a subgroup of Group 1 participants, comparative sequential pharmacokinetic assessments of rFIX (BeneFIX; Pfizer) and rFIXFc were performed after infusion of a dose of 50 IU/kg and repeated at week 26. The primary efficacy end point was the annualized bleeding rate and safety end points included the development of inhibitors and adverse events. The study enrolled 123 patients, demographically diverse and with genotype profile as expected for this population. A total of 5243 rFIXFc administrations occurred during the study and, overall, 96.6% of participants in the prophylaxis groups were adherent to their treatment regimen, with most self-administering rFIXFc at home. The pharmacokinetics subgroup included 22 subjects. The terminal t1/2 of rFIXFc was significantly longer than that of rFIX (geometric mean, 82.1 vs 33.8 hours; P < .001). The incremental recovery levels for rFIXFc and rFIX were similar. The time to reach a FIX level of 1 IU/dL (1%) was 11.2 days with rFIXFc and 5.1 days with rFIX. The pharmacokinetic findings at week 26 were similar. In the group that received prophylaxis dosing based on individualized intervals to maintain trough FIX levels at 1%–3% (Group 2), >1/2 of the participants had dosing intervals of 14 or more days during the last 3 months on the study. In the weekly prophylaxis group, the median dose was 45 IU/kg. As expected, the reduction in the annualized bleeding rate with prophylaxis compared with episodic treatment was consistent across all demographic and disease-based subgroups. Participants in Groups 1 and 2 with the highest bleeding frequency before study entry had median annualized bleeding rates of 2.05 and 2.76, respectively, while enrolled in the study. Among the participants receiving prophylaxis, 23.0% in Group 1 and 42.3% in Group 2 had no bleeding episodes during the study. Adverse events observed in this study were consistent with those expected in the population of persons with hemophilia.29
In the phase 3 study, rFIXFc also demonstrated safe and effective perioperative hemostasis during the perioperative period in subjects requiring major, mostly orthopedic, surgery. In 14 major surgeries performed in 12 participants, including 5 knee replacements, the hemostatic response during the perioperative period was rated by investigators or surgeons as excellent (for 13 surgeries) or good (for 1). Blood loss was consistent with similar surgeries in subjects without hemophilia. Dosing was determined locally by the investigator based on individual rFIXFc pharmacokinetic profiles, type of surgery, and clinical status, and followed using local laboratory assay results. The strong correlation (R2 = 0.9586, P < .001) between observed and predicted FIX activity suggests that surgery did not affect rFIXFc pharmacokinetic properties. No unique safety concerns were identified during surgical rehabilitation. FIXFc was not associated with inhibitor formation in any subject, all of whom had prior exposure to FIX products. In general, for previously treated patients after 150 exposure days, the risk of developing an inhibitor after switching to another FIX product is very low. Therefore, it is not surprising that no inhibitors were detected for subjects switching to the fusion protein, composed of 2 natural proteins without any new or foreign epitopes. There was no evidence of allergic reactions or thrombogenicity as reported previously for rFIX.30
Implications from the clinical studies: how I treat hemophilia B
Previous studies have reported clear medical benefits with early individualized prophylactic therapy in hemophilia.5,31 Not all individuals with severe hemophilia B can adhere to the strict requirements of frequent infusions with previous short-acting therapies. To achieve wider use, prophylaxis therapy needs to be effective, convenient, simple, and safe. Introduction of rFIXFc replacement therapy with an extended t1/2 may represent an important step toward achieving these goals.32 These advantages were recognized by the subjects who participated in the phase 3 clinical study. When given the choice of continuing on with treatment with FIXFc on an extension study after the phase 3 clinical trial or returning to treatment with their previous shorter t1/2 FIX product, none of the subjects chose to return to treatment with their previous FIX product and all chose to continue with the extended t1/2 product. Moreover, a FIX product with an extended t1/2 may have an additional advantage in the treatment of episodic bleeds through a potential reduction in the number of follow-up treatments needed to support complete healing.33
Because rFIXFc is the first extended t1/2 product, it is expected to replace current FIX products for children and adults with hemophilia B. It is important also to evaluate dosing strategies to understand how to efficiently initiate prophylaxis with FIXFc. It is reassuring that none of the subjects experienced any clinical difficulties with switching to the new product on the clinical trials. Because there is significant individual variation in risk of bleeding, selecting one dose and interval regimen is suboptimal because it may expose patients to avoidable bleeding events or overtreatment. The use of patient information (eg, previous therapy) to guide empiric selection of dosing regimen with a new FIX therapy may prove to be a more systematic and efficient approach.
The phase 3 clinical study results provide insight into the pharmacodynamics of rFIXFc in patients with hemophilia B. The maximum concentration (Cmax) was reached immediately after infusion, suggesting a rapid onset of action similar to rFIX. This rapid onset was reflected clinically because patients reported relief of pain from joint bleeding that was as rapid with rFIXFc as they experienced with rFIX. The in vivo recovery observed for rFIXFc may represent an improvement compared with that reported for rFIX and may be attributable to the more human posttranslational modification(s) from the human cell line used to produce rFIXFc or to the Fc moiety. Data from the pharmacokinetic analyses of the clinical trials may provide a means of optimizing individualized prophylactic treatment to achieve target trough levels and to reduce peak/trough variation.34-36 The results suggest that once weekly dosing of rFIXFc at 20 IU/kg, every 10 days at 40 IU/kg, or every 2 weeks at 100 IU/kg is sufficient to maintain a mean trough of 1% above baseline in many adult patients with hemophilia B. In addition, one must consider the heterogeneity of reported clinical breakthrough bleeding events relative to trough level of plasma FIX activity,37 as well as the heterogeneity in pharmacokinetic parameters of individual subjects. Therefore, prophylaxis dosing requires individual adjustment, taking into account the many other variables that may contribute to risk of bleeding, such as more intense activity levels. Indeed, with an extended t1/2 product, there may be opportunities to infuse more often or with higher doses to achieve higher trough levels for times of increased activity.
A recombinant FIX product with a longer t1/2 than currently available FIX products would be expected to require fewer injections to maintain target FIX levels, thus reducing the need for repeated venous access and potentially improving the acceptance of prophylactic regimens by patients with hemophilia B. Reduced frequency of dosing would be expected to reduce the number of pediatric patients in whom central venous access devices are required, thus reducing the significant medical complications that accompany use of these devices: infections, sepsis, and thrombosis. These advantages should also increase adherence to prophylactic regimens in the pediatric population. Further, the increased t1/2 should decrease repeated dosing in the treatment of episodic bleeding or in surgical settings.
The initial pricing of FIXFc has been announced and its use for routine prophylaxis appears to be about the same annual cost as for current rFIX products. Certainly, actual clinical use will determine the final pricing structure and affect how rapidly the hemophilia community adopts the use of this new FIX product. In addition, experience in other clinical settings will be needed. Use in patients with mild and moderate hemophilia B has not been examined yet; but no safety issues would be expected. Hemophilia B patients who have a history of developing an inhibitor or an allergic reaction to any FIX product were excluded from the clinical trials, so use in this population has not been examined. Female carriers of hemophilia B with low FIX activity levels, including pregnant women, were also excluded from the clinical trials. Finally, there are no data on use in patients older than 65 years of age or in previously untreated patients with severe hemophilia B. No special problems in any of these populations would be expected, but clinical experience has not been reported.
It is likely that the increased t1/2 of FIXFc will significantly improve the quality of life of persons with hemophilia B, with improved joint health and higher trough levels of FIX that should allow more normal activities in patients with hemophilia B. Clinical studies are needed to demonstrate these advantages. There are other extended t1/2 FIX products currently in clinical trials that are expected to come to market in the next year or 2 and clinical experience will determine the long-term relative benefits between these products. The clinical trials for gene therapy of FIX deficiency continue slowly to make progress and it is expected that, within a couple of years, patients will have the options of using one of the new FIX products with increased t1/2 or participating in gene therapy trials. Additional clinical experience is needed to guide their choice.
Currently, close to 75% of the world's population of hemophilia B patients lack access to any FIX product for routine treatment of bleeding episodes. A major advantage of the introduction of the new FIX products will be to encourage the manufacturers of current FIX products to create new markets for these products in the underserved populations.
Other new products in development for hemophilia B
Two other extended t1/2 FIX products have completed phase 3 clinical trials and are expected to be submitted for regulatory approval in 2015. N9-GP is a recombinant FIX molecule with a 40-kDa PEG molecule attached to the activation peptide of FIX.15 During activation, the PEG moiety is removed, leaving the native FIXa. The toxicology program did not identify any PEG-related safety issues. The phase 3 trial demonstrated overall safety and efficacy. In the phase 1 trial, there was one serious adverse event reported as probably being related to N9-GP, a hypersensitivity reaction (nausea, vomiting, paresthesias, facial swelling, and diaphoresis, but no changes in blood pressure or pulse) that occurred during administration of N9-GP in a 25-year-old male patient who had no history of inhibitors nor allergic reactions to his previous plasma-derived FIX product. After the event, the patient continued with his previous plasma-derived FIX product without any complications.15
Another approach to extending the t1/2 of FIX uses fusion of the coagulation protein to albumin, which has a t1/2 of ∼20 days. The increase in t1/2 mediated by albumin fusion proteins also results from their interaction with the salvage neonatal Fc-receptor present on many cell types, in addition to the slower renal clearance for larger-sized molecules.25 The albumin fusion approach uses recombinant technology to covalently attach the clotting factor to albumin through the FIX activation peptide. Therefore, during the activation of the coagulation cascade at the site of clotting, the FIX activation peptide is cleaved, releasing the albumin part of the fusion so that only the native FIX remains at the site of clotting. Animal studies, toxicology, and the phase 1 clinical trials have demonstrated safety and efficacy with extended t1/2.20,38-40 The phase 3 trial has finished enrollment.
FVIII extended t1/2 products
In June 2014, the first extended t1/2 FVIII product to receive approval for clinical use, FVIII-Fc (efraloctocog alfa) was approved for clinical use in the United States for adults and children with hemophilia A. Similar to the FIXFc product discussed above, FVIII-Fc is the fusion product of FVIII to the Fc fragment of IgG.41,42
As for FIX, recent efforts to extend the t1/2 of FVIII have pursued conjugating the factor protein at one or a few specific sites with polyethylene glycol (PEG), a hydrophilic polymer, or use recombinant technology to fuse the factor protein with either albumin or the Fc fragment of IgG. Fusion of FVIII to albumin failed so far to preserve effective coagulation activity. Fusion of FVIII to Fc, similar to the efforts for FIX described above, was more successful. (Table 1) Although any of these approaches has substantially increased the plasma t1/2 of factors FIX and FVIIa, only limited t1/2 extension has been observed for FVIII. This is likely due to different mechanisms of clearance between FIX/FVIIa and FVIII. Previous studies demonstrated that FIX clearance is likely mediated through interaction of the aminoterminal Gla domain residues 3-11 with endothelial/collagen IV sites, although the specific mechanism remains unclear.43 In contrast, clearance of FVIII is very rapid in the absence of VWF, possibly due to binding to phospholipid surfaces of cells. In the presence of VWF, FVIII is stabilized in the plasma with a t1/2 of ∼12 hours. The clearance of FVIII from the plasma is likely dependent on the dominant role that VWF plays in regulating the clearance of FVIII, so, conversely, VWF interaction may limit the effectiveness of the different methods to extend the t1/2 of FVIII.
Approaches that use PEGylation
PEGylation involves the covalent attachment of PEG to a protein. In the late 1970s, random PEG addition was shown to reduce the immunogenicity of proteins.44 The most common method of PEG addition is through covalent attachment to lysine residues or N-terminal amines, but this often reduces activity of the protein and the extent of PEG addition is variable, producing a heterogeneous product and complicating reliable synthesis for consistent effectiveness. A new approach involving targeted, site-specific PEGylation has significant advantages.45 One such approach is attachment of PEG-maleimide to cysteine residues. For FVIII, the PEGylation was highly specific and the site of PEGylation on the FVIII molecule was critical to PEGylation efficiency, preservation of the coagulation activity, and improvement in pharmacokinetic parameters.46-48
Nearly a dozen PEGylated protein therapeutics have been approved for use, including anti-TNFα mAb Fab fragment,49 VEGF-aptamer, epoietin β, and IFN-α 2α.50 Although no long-term safety concerns due to the PEGylation have arisen with any of the approved therapeutics, appropriate clinical observations will be needed as the clinical trials for the coagulation proteins progress. There are several PEGylated coagulation factors that are in clinical development. In hemophilia animal models of bleeding, all of these newer PEGylated FVIII molecules have full coagulation activity and, in early clinical trial experience, there are no indications of adverse events, toxicity, and/or immunogenicity in previously treated persons with hemophilia. Extensive safety data for PEGylated proteins containing high-molecular-weight PEG do not indicate any safety concerns after chronic use in animal models or patients. However, there are differences in how the PEGylation is achieved and subtle clinical effects may become apparent through additional clinical experience.
Small PEG molecules are more rapidly cleared than large ones. Larger PEG molecules do not penetrate into tissues as well as smaller ones. With >10-kDa PEG, there is increased pinocytotic uptake into macrophages and Kupffer cells; with >30-kDa PEG, renal clearance decreases; and with >50-kDa PEG, liver clearance increases.51,52 All of the PEGylated FVIII products in clinical trials currently use PEG adducts >15 kDa; specific toxicology experiments for each will need to be evaluated. For example, there was no toxicity associated with acute high-dose administration of BAY 94-9027, a site-specific PEG-conjugated FVIII molecule, or upon repeated dosing with up to 11 mg/kg every other day for 4 weeks in rats. The PEG amount in BAY 94-9027 is ∼4 μg/kg in a dose of 60 IU/kg rFVIII. Over a 1-year period, a 50-kg patient would receive ∼11 mg of PEG. Most experts consider PEG to be inert in the human body.
Several studies have found that PEGylated proteins in general show reduced immunogenicity compared with their unmodified parent molecules.50,51,53 Interestingly, the PEG modification at L491C in the A2-domain of FVIII reduced inhibitory activity for a monoclonal antibody that reacts to this highly immunogenic region of FVIII, suggesting that such modifications may limit the activity of inhibitory antibodies.48
Recently, a summary was reported for toxicology and preclinical results for BAX 855, a full-length rFVIII that 2 20 kDa branched pegylation molecules added nonspecifically by chemical means at lysine residues. Assessment of animal toxicity was based on mortality, clinical observations, clinical pathology, male fertility in rats, organ weights, and pathology evaluations. No PEG-related effects were observed.53 This FVIII product is in a phase 3 clinical trial with results expected to be reported in 2015.
A B-domain-deleted FVIII product has been PEGylated at a site-specific mutation to cysteine with a 40 kDa pegylation molecule. Again, no safety signals have been seen in extensive toxicology studies. This extended t1/2 FVIII, BAY 94-9027, is in a phase 3 clinical trial with results expected to be submitted for regulatory approval in 2015.
In a phase 1 clinical trial, Novo Nordisk demonstrated a glycoPEGylated FVIII that increased plasma t1/2 by 1.6-fold. A single dose of up to 75 U/kg N8-GP was well tolerated in patients with hemophilia A, with no safety concerns.54 Recently, the top level results for the phase 3 clinical trial have been reported to the press and, in 184 previously treated hemophilia A subjects, one neutralizing inhibitor developed. Further details will be needed to assess the significance of this finding.
Two other new FVIII products in clinical trial need to be included in this discussion. One is rhFVIII-hCL, a recombinant FVIII manufactured in a human cell line and currently in phase 3 in adults and children and in clinical trial for previously untreated patients with hemophilia A.55,56 The potential advantages are improved t1/2 and possibly a lower rate of inhibitor development in previously untreated patients due to human glycosylation patterns. Another new FVIII product was modified by recombinant technology so that the FVIII is a single chain molecule, scFVIII. It has a higher affinity to VWF, which may translate into a longer t1/2, and it is currently in a phase 3 clinical trial.18,39,57,58
Use of extended t1/2 products for surgery in hemophilia
All of the clinical trial data for each of the extended t1/2 products indicate that coagulation is as effective for the extended product as for either plasma-derived or recombinant factor product, but with improved t1/2 and pharmacokinetics. The 2 recently approved products were also approved for use in surgery. The phase 3 clinical trials for each product include an arm for use in surgery, but all of the results have not yet been reported. Nonetheless, it is reasonable to conclude that the extended t1/2 products can be used for surgery effectively and safely. Dosing considerations should target the same peak and trough factor activity levels as for currently used factor products.
Clinical laboratory assays of factor activity for the extended t1/2 products
As with any new coagulation product entering clinical use, appropriate assays to monitor factor activity after infusion will need to be determined. The US Food and Drug Administration (FDA)-approved guidelines for the recently approved FIXFc product state that appropriate laboratory assays include clotting assays that use ellagic acid and, for the recently approved FVIIIFc product, appropriate assays include chromogenic assays and clotting assays. Several publications have addressed assay differences for other products currently in phase 3 clinical trials.59-61 Further studies are planned or under way to provide more detailed guidance for assays to monitor the new products as they receive approval for clinical use.
FVIIa products in clinical trials
FVIIa has been used commonly for nearly 20 years for the treatment of patients with inhibitory antibodies and now there are other FVIIa products in development. One of these, rFVIIa-FP, uses the same fusion technology described above for FIX. It may be a promising extended t1/2 product for patients with FVII deficiency; however, whether the extended t1/2 also provides extended coagulation protection in hemophilia patients with high responding inhibitors remains to be tested in planned clinical trials62
Other novel approaches for hemophilia therapies
There are also several novel approaches being pursued in preclinical work or early phase 1 trials. These novel approaches are intriguing, but much remains to be determined as they enter clinical development.
FXase complex mimetics
FXase complex mimetics represent an interesting but complex opportunity to ameliorate the coagulation defect of hemophilia. FVIIIa serves as a cofactor for FIXa to increase the Vmax and decrease the Km for FIXa-mediated cleavage of FX in the presence of negatively charged phospholipids provided by the surface of activated or damaged cells. It is possible that small molecules might replace the FVIII function by promoting the assembly of FIXa and FX in a manner that stimulates the rate of FXa generation. Such a small molecule could be delivered subcutaneously or even orally. In this direction, a humanized bispecific mAb to FIXa and FX was derived (hBS23) that displayed a 2-week t1/2 in a cynomolgus monkey model of acquired hemophilia.63,64 A phase 1 study in 64 Japanese and Caucasian healthy adults indicated that ACE910 (hBS23 with additional minor molecular engineering) at doses up to 1 mg/kg had medically acceptable safety and tolerability profiles and a new phase 1/2 study has recently been initiated to assess prophylactic efficacy as well as safety and pharmacokinetics in patients with/without inhibitors.65
Inhibition of tissue factor pathway inhibitor
Another novel approach in development involves manipulation of the antithrombotic pathways that control coagulation. The tissue factor/FVIIa/FXa complex forms small amounts of thrombin to initiate coagulation. The tissue factor pathway inhibitor (TFPI) inhibits this complex through its 2 Kunitz domains: Kunitz domain 1 interacts with FVIIa and Kunitz domain 2 interacts with FXa. A monoclonal antibody to the second Kunitz domain neutralizes the inhibitory effect of TFPI on extrinsic pathway activation. Subcutaneous administration of this antibody (mAb 2021) 24 hours before injury prevented bleeding in hemophilic rabbits (Table 1).66,67
Recently, several therapeutic agents that inhibit TFPI have been described as potential hemophilia treatments. One is a nucleic acid aptamer that binds tightly and specifically to TFPI and inhibits its function in vitro and in vivo.68 A nonanticoagulant sulfated polysaccharide (NASP) BAX 513 demonstrated efficacy in hemophilia dogs.69 TFPI-antagonist peptides have been designed and have demonstrated hemostatic efficacy in vitro and in vivo in mouse models.68,70-72 Each type of inhibitor of TFPI provides unique advantages and interactions with clotting factors. There is some concern that inhibition of TFPI by certain inhibitor types may worsen bleeding tendencies. Further studies in animals and humans will be needed to sort out the therapeutic opportunities of these potentially exciting approaches.
Targeting antithrombin
Another novel approach is represented by the development of ALN-AT3, a synthetic, GalNAc-conjugated RNAi therapeutic designed to suppress liver production of antithrombin (AT) mRNA after subcutaneous injection. Reducing AT levels has the potential to reduce the stoichiometric inhibition of thrombin and thus improve hemostasis for patients with hemophilia. Subcutaneous administration of ALN-AT3 resulted in dose-dependent and reversible reduction of circulating AT, with a single-dose ED50 of ∼1 mg/kg in multiple species.73,74 Reduction in AT was associated with significant increases in thrombin generation and enhanced hemostasis in hemophilia A and B mouse models. In a microvessel laser injury in hemophilia A and B mice, functional improvements in hemostasis were observed after treatment with ALN-AT3. Animals administered a single dose of 1 or 30 mg/kg ALN-AT3 10 days before injury showed dose-dependent accumulation of platelets and fibrin at the site of injury, as quantified by intravital microscopy. Stable hemostatic plug formation was observed at all sites of injury in treated animals. Extensive toxicology studies of ALN-AT3 have been conducted in several species and, as expected, the toxicity observed in wild-type animals was due to the increased procoagulant pharmacology of ALN-AT3. Greater than 90% reduction in AT in wild-type animals led to thrombotic events. In contrast, reduction by ALN-AT3 to 5% AT levels was well tolerated in hemophilia A and B mice, with no evidence of thrombosis.75 Further studies are required in models that have functional FVIII and/or FIX to demonstrate long-term safety, and a phase 1 clinical trial is planned.
Future concerns of novel molecules
A major concern in hemophilia A is the development of neutralizing antibodies (inhibitors) that prevent further use of therapeutic FVIII. Current options for treatment of these patients with inhibitors are very expensive. Therefore, there are significant potential implications as the clinical trials with the new therapies evolve. It may be demonstrated in the planned clinical trials with previously untreated patients, that the extended t1/2 products will have lower rates of inhibitor development. Some other PEGylated proteins have had reduced immunogenicity compared with the parent protein; PEGylation may block potential inhibitory antibody binding sites and the increased exposure to FVIII antigen may promote peripheral tolerance. However, as with any new product, there is the risk of higher rates of developing inhibitors. These issues will only be resolved by planned clinical trials in previously untreated patients.
Recombinant FVIIa has not produced neutralizing antibodies in hemophilia patients, probably because they have native FVIIa and do not recognize the infused FVIIa as a foreign protein.76 Several pharmaceutical companies have development programs to generate molecularly modified FVIIa proteins with either longer t1/2 or improved efficacy. The 2 programs with the most clinical trial data to date were based on molecular constructs that introduced conservative amino acid substitutions into native FVIIa. After no problems were seen in extensive animal testing and phase 1 clinical trials, both programs were terminated in phase 3 trials due to the detection of neutralizing inhibitors. These results will need further investigation, but serve as a potential warning for programs based on molecular alterations of clotting proteins.
Conclusion
The past quarter-century has witnessed a tremendous expansion in our understanding of mechanisms that regulate hemostasis in vivo and the development of extended t1/2 factor products with promise to improve treatment of hemophilia. FIXFc is the first fruit from these advances. Its marked increase in t1/2 for FIX is expected to allow less frequent infusions of factor, to allow higher factor trough levels so there may be increased potential for preventing spontaneous bleeding, and, perhaps most importantly, to allow individuals with hemophilia B to have a more normal quality of life. In the near future, there will be other factor products with extended t1/2 for both hemophilia A and B, and there may be alternative approaches for preventing bleeding in hemophilia that may offer even greater advantages. Future concerns include: (1) how will the costs of these new factor products influence their clinical use?; (2) will there be any issues with inhibitor formation with the new factor products in previously untreated patients?; and and (3) will there be any reason for concern in using these new factor products in an increasingly elderly hemophilia population? As was the case over the past 25 years, progress in hemophilia will depend on enthusiastic participation by physicians and patients in appropriate clinical trials to obtain clear answers to guide future hemophilia treatment.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Bayer, Biogen, Octapharma, CSL Behring, NovoNordisk, Baxter, and REV-Bio. Off-label drug use: None disclosed.
Correspondence
Jerry S. Powell, Division of Hematology and Oncology, Suite 3016, University of California Davis Medical Center, 4501 X Street, Sacramento, CA 95817; e-mail: jspowell@ucdavis.edu.