Abstract
Pregnancy-associated venous thromboembolism (VTE) is a leading cause of maternal mortality, but is relatively uncommon. It is clear that the antepartum and postpartum periods have different magnitudes of risk and distinct risk factors for VTE and therefore must be considered separately. Absolute daily risks of VTE must be understood and explored when deciding to prescribe antepartum or postpartum thromboprophylaxis and must also be balanced against the downsides of prophylaxis. When the risks for VTE and bleeding are both low, other burdens of thromboprophylaxis must be weighed in and a decision made after an individualized patient values- and patient preferences–based discussion. Risk stratification is essential to ensure that the practicing clinician strikes the right balance.
Learning Objective
To prescribe and recommend thromboprophylaxis rationally in pregnancy and the postpartum period
Introduction
Symptomatic pregnancy associated venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is estimated to occur antepartum (from conception to delivery, ie, ∼40 weeks) in 5-12 per 10 000 pregnancies and to occur postpartum (6 weeks) in 3-7 per 10 000 deliveries.1 VTE remains a leading cause of direct maternal death in the developed world, causing 0.8-4.7 VTE deaths per 100 000 maternities.2,3 In the period between 2006 and 2009, there were 644 maternal deaths due to PE in the United States (http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/PMSS.html#n8).
We recently completed the Thrombophilia in Pregnancy Prophylaxis Study (“TIPPS”), which offered some new insights on preventing VTE in pregnancy.4 In this chapter, we review an evidence-based and absolute risk–based approach to preventing VTE in pregnancy.
Pathophysiology of VTE
The pathophysiology of VTE in the antepartum period includes the following. First, venous stasis caused by progesterone-induced venodilation, pelvic venous compression by the gravid uterus, and pulsatile compression of the left iliac vein by the right iliac artery,5 leading to the marked propensity for left leg DVT in pregnancy (>80%).6 Second is hypercoagulability developing as the hemostatic system is progressively activated to prepare the pregnant women for the hemostatic challenges of delivery (including reduced anticoagulant activity of protein S, increased activated protein C resistance,7 and increased procoagulant activity through higher levels of fibrinogen and factor V, VIII, IX, and X), leading to increased thrombin production7 as measured by increased thrombin antithrombin complexes, increased soluble fibrin, and F 1.2 levels.8 Finally, there is reduced fibrinolysis due to increased plasminogen activator inhibitor type 1 and 2 (PAI-1 and 2) activity and decreased tissue plasminogen activator (t-PA) activity.8 The pathophysiology of VTE in the postpartum period includes vascular damage to the pelvic vessels that can occur after normal vaginal, assisted vaginal, or cesarean section deliveries and postpartum immobilization. The hypercoagulability of pregnancy, although maximally present in the early postpartum period, gradually returns to the nonpregnant state, as evidenced by progressive normalization of markers of coagulation activation to prepregnancy levels.9,10
Striking the right balance: aiming to prevent enough VTE to warrant the downsides of prophylaxis
Rational prevention requires optimal balancing of an increased bleeding risk from pharmacologic thromboprophylaxis and reducing VTE, which, although serious and at times tragic, is relatively uncommon. Therefore, we must focus on known high-risk groups with the understanding that recommendations for prophylaxis, even in high-risk groups, are based on limited data. A recently updated Cochrane Review addressed the effectiveness and safety of prophylaxis for VTE in pregnancy and the early postpartum period. The reviewers conclude that: “There is insufficient evidence available from the randomized controlled trials included in this review to guide clinical decision making. In the absence of clear randomized controlled trial evidence practitioners must rely on consensus-derived clinical practice guidelines or recommendations.”11
The absolute risk of VTE during the time interval of interest (eg, antepartum or postpartum) is a key fact that should underpin decisions about choice, cost, intensity, and duration of VTE prevention. At the extremes, the risk of postpartum VTE in women with prior unprovoked VTE and thrombophilia without thromboprophylaxis exceeds 5% during the short postpartum period,12 whereas the risk of antepartum VTE in unselected pregnant women is <0.1% over 40 weeks of pregnancy.1 With these absolute event rates, even assuming an optimistic 95% relative risk reduction with thromboprophylaxis, we can calculate a number needed to treat (NNT) of ∼20 and ∼1000 for each scenario, respectively; that is, we would need to treat 20 women with prior unprovoked VTE and thrombophilia for the short postpartum interval to prevent one postpartum VTE. Conversely, we would need to treat >1000 average-risk women for the entire antepartum period to prevent one VTE. Clearly, patients, clinicians, and policy makers would find an NNT of 20 for the short postpartum period reasonable, but none would consider an NNT of 1000 reasonable for the 40 week antenatal period. Therefore, the goal is to identify the group of women in whom the intervention is clinically effective, cost-effective, and a reasonable NNT is achieved.
First let us consider nonpharmacologic approaches. Nonpharmacologic tools to prevent VTE have the attraction of not causing major bleeding. It would be tempting to recommend their universal adoption on this basis alone. Certainly, early ambulation postpartum is without risk, likely effective, and should be universally adopted. However, compression stockings, previously assumed to similarly be beneficial and benign, have become controversial. Recent large randomized controlled trials (RCTs) in stroke patients suggest that knee-high compression stockings cause DVT and thigh high stockings provide no benefit.13,14 Intermittent pneumatic compression devices have not been studied in pregnancy, but are effective in other populations; however, they are costly and cumbersome for patients.15
In terms of pharmacologic approaches, low-molecular-weight heparins (LMWHs) are the preferred agent. There are no clinical data with the new oral anticoagulants in pregnant or lactating women,16,17 and preliminary data from animal studies suggest that these agents cross the placenta and be secreted into breast milk; therefore, they contraindicated in pregnant and lactating women.17 Warfarin is teratogenic with antepartum use and, although safe to use in breastfeeding mothers, requires frequent laboratory monitoring, which is a challenge in mothers with newborn children. This inconvenience makes warfarin use impractical as thromboprophylaxis in short-term indications.18 Unfractionated heparin must be administered subcutaneously 2-3 times per day and is associated with a 10-fold higher risk of heparin-induced thrombocytopenia than LMWH.19 Furthermore, unfractionated heparin increases the risk of osteoporotic fracture with long-term use, with absolute event rates as high as 2.2%,20 compared with no change in bone mineral density with prophylactic-dose long-term LMWH.21 For these reasons, this discussion on pharmacoprophylaxis will focus on LMWH. Prophylactic doses of LMWH include enoxaparin 40 mg, dalteparin 5000 units, or tinzaparin 4500 units, all given subcutaneously daily. Note that it remains controversial whether LMWH should be weight adjusted or doubled after 20 weeks, when increases in plasma volume and glomerular filtration rate may lead to a reduced anti-Xa effect. Whether these lower anti-Xa levels lead to a reduced efficacy is unknown.
To be clinically effective, the NNT should be low and clearly exceed the number needed to harm (NNH). The major harm associated with LMWH use is major bleeding. In Table 1, I have summarized and provide a pooled proportion of major bleeds in LMWH versus no LMWH RCTs in pregnancy. In the antepartum period, the risk of major bleeding with prophylactic LMWH is very low [0%; 95% confidence interval (95% CI) 0%–0.6%; Table 1]. In other words, in a worst-case scenario, the NNH for major bleeding is 167. However, we must interpret this information with caution because some reports do not include placental abruption or bleeding associated with miscarriage as being LMWH related, but in reality, they may be exacerbated by LMWH.22,23 Not surprisingly, the risk of major bleeding is likely higher in the postpartum period: 0.3% (95% CI: 0%–1%; Table 1). Therefore, the NNH in the postpartum period is likely 333 and, in a worst-case scenario, 100. In addition, we must consider the other downsides of LMWH. This is especially important when the NNH is low and the NNT is high. NNH is low and NNT is high in many antepartum prophylaxis scenarios. Among the other downsides of LMWH is the increased risk of minor bleeding,24,24-26 which, although not life threatening, is at the very least a nuisance and can be anxiety provoking. LMWH use also increases liver enzymes (of unknown clinical significance),24,24 causes heparin-induced thrombocytopenia (albeit rare),27 wound complications after cesarean delivery, allergic reactions (skin reactions 1.8%; anaphylaxis is rare), and antepartum LMWH use until term complicates obstetric analgesic options at delivery. Finally, the cost and inconvenience of LMWH use, especially through the prolonged antepartum period (up to 400 injections per pregnancy at >$15 000 US), should not be minimized.
To estimate the net clinical benefit, we must also take into consideration the different case fatality rates (CFRs) of VTE and major bleeding in addition to NNT and NNH. These CFRs are unknown in pregnancy due to the rarity of fatal events and the lack of pregnancy-specific VTE and LMWH studies. However, in other populations, the CFR of major VTEs, the proportion of major VTEs that are fatal, has been determined to be 1.4% (95% CI: 0.9%–2.2%).28 In contrast, the CFR of major bleeding associated with prophylactic anticoagulants is estimated to be 3.6% (95% CI: 3.2%–3.9%).28 This suggests that any incremental major bleeding risk estimate must be inflated 2- to 3-fold to counterbalance a reduction in major VTE if a pharmacologic prophylaxis strategy is to be expected to achieve a mortality benefit. Taking the CFRs into account, patients would require a postpartum VTE risk of >1% for LMWH prophylaxis to likely provide a net clinical benefit in the postpartum period (recall point estimate of major bleeding with postpartum LMWH use of 0.3% and above) and >3% to almost certainly provide benefit (recall upper bound of 95% CI for postpartum major bleed of 1%). Similarly, in the antepartum period, in a worst-case scenario, we would require an absolute antenatal VTE risk of >1.8% to likely provide a net clinical benefit (recall upper bound of 95% CI of 0.6% for antenatal LMWH use) and almost certainly provide a net clinical benefit if the antenatal VTE risk were >3%. Taking into consideration all of the other downsides of prolonged antenatal LMWH use, most clinicians and patients would not likely consider antenatal LMWH if the antenatal risk of VTE were <1%. Each scenario requires an individual patient discussion, taking into account patient preferences and values, to come to a common decision on prophylaxis. For example, some patients with needle phobia may be more comfortable with a higher VTE risk thresholds before instituting prophylaxis and some patients with a family history of life-threatening PE in a first-degree relative may be more comfortable with a lower VTE risk threshold to accept prophylaxis.
Preventing antenatal VTE: who is at high enough risk to warrant LMWH?
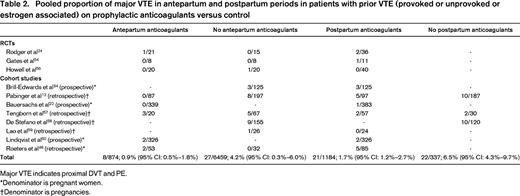
As outlined above, most clinicians/patients would seek an absolute risk of antenatal VTE that exceeds 1% before even considering antepartum LMWH prophylaxis. In Tables 2 and 3, I have summarized the studies exploring the risk of pregnancy-associated VTE in women with prior VTE and provide pooled proportions of risk of recurrent VTE. Given an absolute risk of antenatal VTE of 0.1% in the general pregnant population, we need to identify risk factors that increase the risk of antenatal VTE by >10-fold before even considering antepartum LMWH prophylaxis. The risk factors for antepartum VTE that appear to increase the risk of antenatal VTE >10-fold or are associated with >1% absolute risk of VTE include: prior VTE if unprovoked or if associated with a prior estrogen hormone exposure (exogenous estrogen or pregnancy; Tables 2 and 3); immobilization (strict bed rest for a week or more in the antepartum period and BMI ≥25 kg/m2; odds ratio = 62.3; 95% CI: 11.5-337)29 ; and women with potent thrombophilias such as homozygous factor V Leiden (FVL)30,31 or homozygous prothrombin gene variant (PGV),31 antithrombin deficiency,32 and those who are double heterozygotes for thrombophilia.31 . It should be noted that published data on VTE risk in otherwise asymptomatic pregnant women with antithrombin deficiency are limited to small case series, but most of the literature supports a high enough risk to warrant thromboprophylaxis.33
It is noteworthy that the risk of antenatal VTE in women with prior provoked VTE (surgery, trauma, or immobilization) without thrombophilia and without an estrogen hormone trigger for their VTE (exogenous estrogen or pregnancy) is low, so it is likely that this subgroup can have prophylaxis withheld in the antenatal period.34 It is also likely that this recommendation can be extended to all women with prior provoked VTE that are not estrogen associated (Table 3) regardless of thrombophilia, but uncertainty remains because the high upper bound of the 95% confidence interval around the pooled estimate is higher than 3% (Table 3).
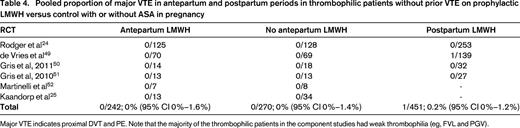
Women without prior VTE with weak thrombophilias (eg, heterozygous FVL or heterozygous PGV) have a low antepartum risk of VTE and should not receive anticoagulant prophylaxis. Large prospective cohort studies of patients with FVL and PGV35-38 and RCTs (Table 4) demonstrate a low antenatal VTE risk with these common thrombophilias without antenatal prophylaxis.
Postpartum period is associated with higher risk and has distinct risk factors for VTE
Compared with age-matched, nonpregnant controls, the daily risk of VTE is increased 7- to 10-fold for antepartum VTE, but is 15- to 35-fold for postpartum VTE.39,40 The heightened clinical risk of VTE after delivery rapidly diminishes during the postpartum period,39 returning to the antenatal level of risk by 3 weeks postpartum and then to nonpregnant levels after the 6th to 12th postpartum week.29,41,42
We seek an absolute risk of postnatal VTE that exceeds 1% to consider postpartum LMWH prophylaxis and to definitely suggest postpartum LMWH prophylaxis if the risk exceeds 3%. Therefore, given an absolute risk of postpartum VTE of 0.05% in unselected populations, we need to identify risk factors that increase the risk of postnatal VTE by >20-fold before considering postpartum LMWH prophylaxis and a >60-fold increased risk would definitely suggest LMWH prophylaxis is indicated. The risk factors for postpartum VTE that increase risk >20-fold or are associated with a >1% absolute risk of VTE are: immobilization (strict bed rest for a week or more in the antepartum period and BMI ≥25 kg/m2; odds ratio = 40.1; 95% CI: 8.0-201.5),29 homozygous FVL,43 and homozygous PGV.43 The risk factors that increase risk of VTE >60-fold or are associated with an absolute risk of VTE >3% are antithrombin deficiency,44 combined thrombophilias, and prior VTE (all prior VTEs regardless of whether unprovoked, provoked, or estrogen associated; Table 2). Finally, although not well explored, perhaps other combinations of independent risk factors would exceed these thresholds. These other risk factors might include family history of VTE,45 prior superficial phlebitis,46 weaker thrombophilias (heterozygous FVL or heterozygous PGV or protein C deficiency or protein S deficiency), emergency C-section, postpartum infection, postpartum hemorrhage, smoking, BMI >25 kg/m2, intrauterine growth restriction, preeclampsia, stillbirth, varicose veins, inflammatory bowel disease, preterm birth, and age >35 years.29,47 Further research is required to identify which of these latter combinations of risk factor subgroups is high enough risk to warrant postpartum thromboprophylaxis.
It is notable that the risk of postpartum VTE in women with heterozygous FVL or heterozygous PGV without a personal history of VTE or a family history of VTE is likely lower than 1%, so it is very debatable if these women should receive postpartum prophylaxis in the absence of other risk factors.18
At the other extreme, the high risk of postpartum VTE in patients with prior VTE despite prophylaxis is striking in some studies (>5%12,48 ) and the pooled estimate of 1.7% (Table 2) suggests a need for research to explore alternative strategies that, again, will need to be balanced against a higher bleeding risk. Indeed, the Highlow RCT (www.ClinicalTrials.gov identifier #NCT01828697) is currently under way exploring and is whether higher doses of LMWH prophylaxis (50%–75% of full treatment dose) are superior to the usual fixed, low-dose prophylaxis. Consensus guidelines also suggest adding mechanical methods to pharmacoprophylaxis in high-risk patients in the postpartum period18 and certainly patients with prior VTE would warrant this added measure.
Patient counseling
In addition to the individualized discussion regarding anticoagulant prophylaxis suggested, all women at high risk of pregnancy-associated VTE should also be counseled about the signs and symptoms of DVT and PE and an action plan developed should these symptoms arise. It is important to explain to patients that VTE-mimicking symptoms are common in pregnancy. They should not be alarmed by the gradual development of bilateral leg edema or the gradual onset of dyspnea in late pregnancy.
Women with a prior VTE who remain on vitamin K antagonists should be counseled to discontinue them as soon as they become pregnant (missed menses and/or positive urine pregnancy test). In women on oral direct factor Xa inhibitors or direct thrombin inhibitors, reliable contraceptive methods are advised until a planned pregnancy. Before a planned pregnancy, these drugs should be discontinued and replaced by vitamin K antagonists or LMWH. In women who become pregnant and have had a recent VTE, the urgency and aggressiveness of ongoing treatment should be dictated by the age of the recent VTE. In the absence of pregnancy-specific research to guide us, my approach is to start full-dose therapeutic LMWH immediately if the VTE occurred in the last month (eg, enoxaparin 1 mg/kg q 12 h or dalteparin 200 units/kg q 24 h, or tinzaparin 175 units/kg q 24 h). I offer aggressive prophylaxis in the form of intermediate-dose (eg, 3/4 of full treatment dose) LMWH initiated in the next 24 hours if the VTE occurred in the last 12 months and I consider prophylactic-dose LMWH if the VTE occurred >12 months previously (eg, enoxaparin 40 mg, dalteparin 5000 units, or tinzaparin 4500 all given sc daily).
In summary, as our knowledge of the absolute risks of pharmacoprophylaxis in pregnancy and VTE risk stratification in pregnancy has evolved, we have developed a clearer picture of who should and who should not receive pharmacoprophylaxis to prevent pregnancy-associated VTE (Table 5). Clinicians should arm themselves with this knowledge to strike the right balance in preventing pregnancy-associated VTE.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: LMWH to prevent VTE in pregnancy.
Correspondence
Marc Rodger, Chief, Division of Hematology, Senior Scientist, Ottawa Hospital Research Institute, Ottawa Hospital, General Campus, 1812-E Box 201, 501 Smyth Road, Ottawa, ON, Canada K1H 8L6; Phone: (613)737-8899, ext. 74641; Fax: (613)739-6102; e-mail: mrodger@ohri.ca.