Abstract
Liver disease results in complex alterations of all 3 phases of hemostasis. It is now recognized that hemostasis is rebalanced in chronic liver disease. The fall in clotting factor levels is accompanied by a parallel fall in anticoagulant proteins. High von Willebrand factor levels counteract defects in primary hemostasis. Conventional coagulation tests do not fully reflect the derangement in hemostasis and do not accurately predict the risk of bleeding. Global coagulation assays (thrombin generation, thromboelastography) reflect the interaction between procoagulant factors, anticoagulant factors, platelets, and the fibrinolytic system and show promise for assessing bleeding risk and guiding therapy. These assays are not yet commercially approved or validated. Prevention of bleeding should not be aimed at correcting conventional coagulation tests. Thrombopoietin receptor agonists were shown to increase the platelet count in cirrhotic patients undergoing invasive procedures but may increase the risk of thrombosis. Rebalanced hemostasis in liver disease is precarious and may be tipped toward hemorrhage or thrombosis depending on coexisting circumstantial risk factors. Bacterial infection may impair hemostasis in cirrhosis by triggering the release of endogenous heparinoids. There are no evidence-based guidelines for hemostatic therapy of acute hemorrhage in liver disease. There is currently inadequate evidence to support the use of recombinant FVIIa, prothrombin complex concentrates, or tranexamic acid in acute variceal or other hemorrhage.
Learning Objectives
The reader will understand how alterations in hemostasis in liver disease result in a rebalance between procoagulant, anticoagulant, and fibrinolytic systems
The reader will understand the limitations of currently available laboratory tests for evaluating the coagulopathy of liver disease
The reader will be able to identify triggers of acute bleeding in liver disease
The liver has a pivotal role in hemostasis by synthesizing all clotting factors except von Willebrand factor (VWF) and natural anticoagulants, as well as several fibrinolytic proteins. Liver disease results in complex alterations of all 3 phases of hemostasis: primary hemostasis, coagulation, and fibrinolysis. This overview summarizes the changes in hemostasis that occur in chronic liver disease, limitations of laboratory tests used to assess bleeding risk, and clinical implications for prevention and management of bleeding.
Alterations in hemostasis
Primary hemostasis
Platelet number and platelet function may both be adversely affected by liver disease. Mild to moderate thrombocytopenia occurs in up to 76% of patients with cirrhotic liver disease although the platelet count is rarely < 30 000-40 000. The etiology of thrombocytopenia in liver disease is multifactorial. Platelet sequestration due to congestive splenomegaly reflects portal hypertension and is characterized by redistribution of circulating platelets to the splenic pool. Platelet counts correlate inversely with spleen size in some but not all studies. Bone marrow suppression by antiviral therapy, alcohol, or folate deficiency impairs platelet production. Impaired hepatic synthesis of thrombopoietin (TPO), the primary physiologic regulator of platelet production, may contribute to thrombocytopenia. TPO mRNA levels are significantly reduced in cirrhotic liver tissue, and serum TPO levels are lower in patients with cirrhosis.1 Accelerated platelet turnover may contribute to thrombocytopenia in chronic liver disease.1 “Hypersplenism” reflects an exaggeration of the normal function of splenic macrophages to remove senescent cells. Immune-mediated platelet destruction occurs in patients with hepatitis C and primary biliary cirrhosis. Platelet consumption may also occur secondary to cirrhosis related hypercoagulability resulting in platelet activation. The widely variable extent of splenic sequestration, TPO levels, and platelet survival reported suggests that different mechanisms predominate in different patients.
There are conflicting data on platelet function in chronic liver disease. A variety of platelet function defects have been identified although their clinical significance is debated. Under normal conditions, platelets have a dual function. They adhere to the site of vascular injury by binding to the multimeric adhesive protein VWF. Platelets also support thrombin generation by assembling activated clotting factors on their surface. Platelet procoagulant activity assessed by thrombin generation assays using platelet rich plasma is preserved in patients with cirrhosis.2 Adhesion of platelets from patients with cirrhosis was similar to that of control platelets as long as platelet count and hematocrit were adjusted to normal levels.3 A substantial proportion of patients with cirrhosis have impaired platelet aggregation in vitro, although the pattern of abnormalities is not consistent. Elevated levels of markers of platelet activation were found in plasma from cirrhotic patients suggesting platelet hyperfunction possibly triggered by oxidant stress.4
Coagulation
Liver parenchymal cells produce all of the coagulation factors involved in the generation of a fibrin clot except for FVIII, which is primarily synthesized by the hepatic endothelium and extrahepatic endothelial cells.5 Chronic liver disease is characterized by reduced synthesis of procoagulant proteins (FII, FV, FVII, FIX, FX, and FXI). Clotting factor levels usually fall in parallel with the progression of liver disease, although levels vary considerably.6 Fibrinogen levels are normal or increased in most patients with stable cirrhosis. An acquired dysfibrinogenemia develops in 50%-78% of patients with chronic liver disease. Regenerating hepatocytes synthesize an abnormal fibrinogen with increased sialic acid residues, which impair polymerization of fibrin monomers. FVIII levels are often increased several fold in patients with stable cirrhosis. High FVIII levels may result from increased synthesis in response to cytokine release from necrotic tissue, as well as reduced clearance due to impaired hepatic expression of low density lipoprotein receptor and stabilization by high levels of VWF characteristic of liver disease.7,8
Natural anticoagulant protein levels fall progressively with increasing severity of liver disease.6 Antithrombin, protein C and protein S levels range from 10% to 65% of normal, similar to the range of values found in patients with inherited deficiencies.6,9 Tissue factor pathway inhibitor (TFPI) is synthesized by endothelial cells. TFPI levels are normal or elevated in patients with chronic liver disease. However, the TPFI anticoagulant pathway is functionally impaired by low levels of protein S which functions as a cofactor for TFPI-mediated downregulation of thrombin generation.10
Fibrinolysis
All of the profibrinolytic and antifibrinolytic proteins are synthesized by the liver except tissue plasminogen activator (tPA) and PAI-1 which are synthesized by endothelial cells. PAI-1 is produced by a variety of sources including adipose tissue. Levels of plasminogen, α2antiplasmin, thrombin-activatable fibrinolysis inhibitor (TAFI), and FXIII levels are often reduced in chronic liver disease.11 In contrast, plasma levels of tPA are usually elevated due to release by activated endothelial cells and reduced hepatic clearance. PAI-1 levels are variable and may be normal or increased.11 Fibrinolytic activity varies considerably between individuals. Ascitic fluid in cirrhosis has fibrinolytic activity. Reabsorption of large volumes of ascitic fluid into the systemic circulation may contribute to accelerated fibrinolysis in some cases.12
Rebalanced hemostasis
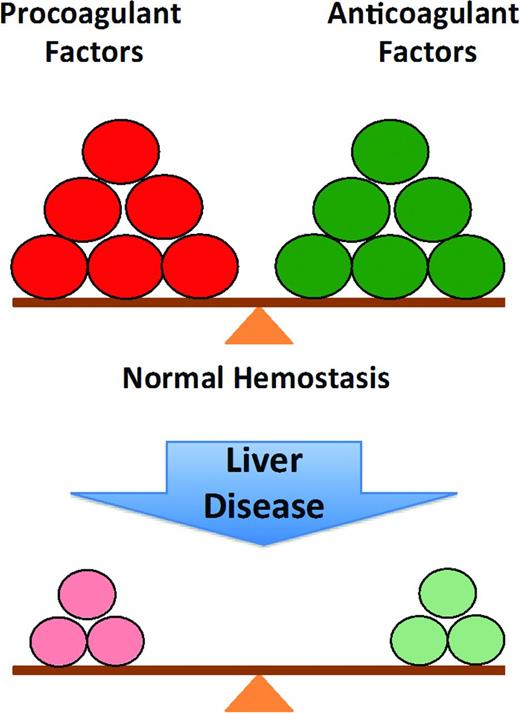
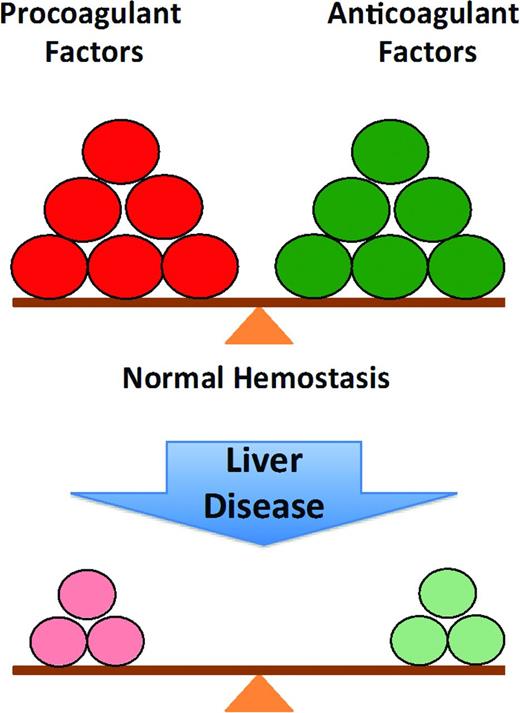
Historically, the changes in hemostasis that occur in liver disease were assumed to reflect an acquired bleeding disorder. This classical interpretation of the coagulopathy of liver disease has been replaced by the concept of “rebalanced hemostasis” (Figure 1). According to this model, the hemostatic alterations that occur result in a new balance within and between procoagulant, anticoagulant and fibrinolytic systems (Figure 2).7 Because of the relative deficiency of procoagulant and anticoagulant factors, the hemostatic balance is more precarious and may tip toward bleeding or thrombosis depending on provoking circumstantial risk factors.
The normal balance of hemostasis and rebalanced hemostasis in liver disease: because of the relative deficiency of both procoagulant and anticoagulant factors in chronic liver disease, the balance is more fragile and more easily tipped toward bleeding or thrombosis.
The normal balance of hemostasis and rebalanced hemostasis in liver disease: because of the relative deficiency of both procoagulant and anticoagulant factors in chronic liver disease, the balance is more fragile and more easily tipped toward bleeding or thrombosis.
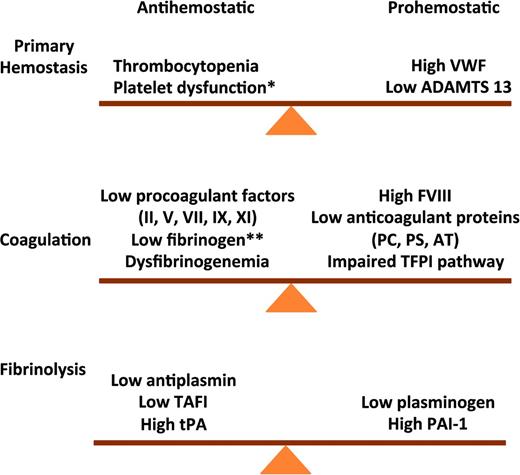
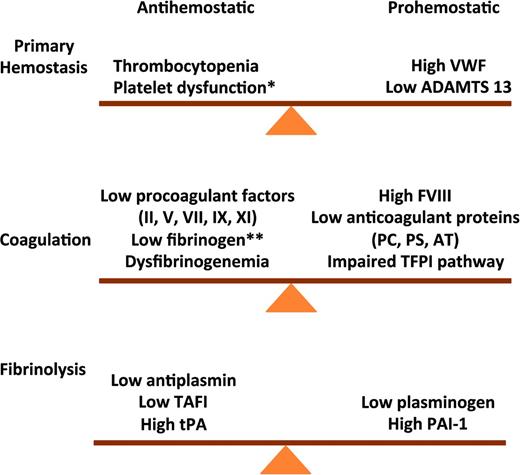
Rebalanced hemostasis in chronic liver disease. Primary hemostasis: high VWF levels and low ADAMTS 13 levels counteract defects in primary hemostasis. Coagulation: reduced levels of procoagulant factors are balanced by a parallel decline in anticoagulant factors. Fibrinolysis: fibrinolysis is rebalanced by parallel changes in profibrinolytic and antifibrinolytis proteins. VWF indicates von Willibrand factor; ADAMTS 13, a disintegrin & metalloproteinase with thrombospondin type 1 motif 13; PC, protein C; PS, protein S; AT, antithrombin; * does not occur consistently in chronic liver disease; and **end-stage liver disease.
Rebalanced hemostasis in chronic liver disease. Primary hemostasis: high VWF levels and low ADAMTS 13 levels counteract defects in primary hemostasis. Coagulation: reduced levels of procoagulant factors are balanced by a parallel decline in anticoagulant factors. Fibrinolysis: fibrinolysis is rebalanced by parallel changes in profibrinolytic and antifibrinolytis proteins. VWF indicates von Willibrand factor; ADAMTS 13, a disintegrin & metalloproteinase with thrombospondin type 1 motif 13; PC, protein C; PS, protein S; AT, antithrombin; * does not occur consistently in chronic liver disease; and **end-stage liver disease.
This rebalance of hemostasis was demonstrated in plasma from patients with cirrhosis which generates as much thrombin as plasma from healthy individuals using in vitro assays that reflect the activity of both procoagulants and anticoagulants.13 VWF levels are markedly elevated in patients with cirrhosis and correlate with the severity of liver disease.3 High VWF levels counteract defects in primary hemostasis due to thrombocytopenia and platelet dysfunction. In vitro experiments suggest very high VWF levels may restore platelet adhesion to the subendothelium at sites of vascular injury.3 ADAMTS 13, a plasma metalloproteinase that cleaves multimeric VWF into smaller less adhesive multimers is produced in hepatic cells. A decline in ADAMTS13 levels with increasing severity of liver disease also promotes normal platelet function.14
Fibrinolysis is also rebalanced in chronic liver disease due to parallel changes in profibrinolytic and antifibrinolytic proteins. Laboratory evidence of hyperfibrinolysis was demonstrated in some11 but not all15 studies. The conflicting results reported may reflect differences in disease severity and limitations of testing. Most observations were based on measurement of individual components of the system rather than overall fibrinolytic activity resulting from both profibrinolytic and antifibrinolytic factors. Hyperfibrinolysis may be triggered by additional insults such as surgery or sepsis, which shift the balance between profibrinolytic and antifibrinolytic factors.
Laboratory tests
PT/INR
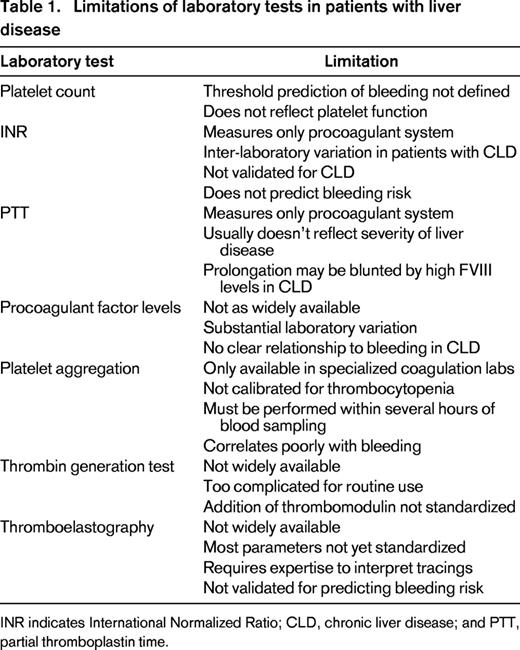
Conventional coagulation tests [prothrombin time (PT) and activated partial thromboplastin time (aPTT)] do not fully reflect the derangement in hemostasis in liver disease and do not reliably predict the risk of bleeding (Table 1). The international normalized ratio (INR) was developed to standardize PT reporting for patients on stable anticoagulation with vitamin K antagonists (VKA) and is not validated for patients with liver disease. The INR is calculated from the PT ratio (patient PT/control PT) adjusted for the international sensitivity index (ISI). The ISI reflects the sensitivity of a particular thromboplastin to a reduction in vitamin K-dependent clotting factors, and is derived from a cohort of patients on stable VKA. Patients with chronic liver disease have a more complex and less predictable coagulation profile than patients on warfarin with similar INR values. There is substantial interlaboratory variability of the INR in patients with cirrhosis, especially at high INR values.16 This interlaboratory variability is largely due to differences in the reagents and instruments used.
An alternative INR system has been proposed for patients with liver disease to standardize reporting. Calibration of the ISI using plasma samples from patients with liver disease (ISI liver) rather than VKA samples was shown to normalize PT results across thromboplastins. However, implementation of the INR liver presents logistical challenges that prevent its routine use, for example a requirement for the manufacturer to provide 2 ISI values (ISIVKA and ISI liver) for each thromboplastin. Another strategy is the use of thromboplastins with similar ISI values calibrated with plasma samples from patients on VKA and with liver disease.16
Prediction of bleeding
Conventional coagulation tests do not accurately predict the risk of bleeding in patients with chronic liver disease. There is no evidence that a prolonged PT/INR predicts bleeding at the time of invasive diagnostic procedures.17,18 A systematic review found no significant difference in bleeding rates between patients with and those without a prolonged PT prior to liver biopsy.19 The American Association for the Study of Liver Disease (ASSLD) Practice Guidelines for liver biopsy acknowledge that there is no specific PT/INR cutoff at or above which bleeding complications can be reliably predicted.20 There is evidence that the platelet count is a better predictor of bleeding than the INR. In several studies, a platelet count <60 000-75 000 was associated with a significantly increased rate of post-procedure bleeding.21 However, a threshold platelet count below which the bleeding risk clearly increases has not been defined.
Global tests of hemostasis
Conventional coagulation tests such as the PT/INR and PTT measure procoagulant factors but do not reflect the reduction in anticoagulant factors or the complex interactions between cells and coagulation factors in whole blood. The PT/INR and PTT also do not measure clot strength and stability because the endpoint of these tests is the initiation of fibrin polymerization, which occurs at very low levels of thrombin generation. In contrast, global coagulation assays reflect the interaction between procoagulants, natural anticoagulants, platelets, and the fibrinolytic system. Thrombin generation tests (TGT) dynamically measure the total amount of thrombin generated during in vitro coagulation.22 In the presence of thrombomodulin, the primary physiologic activator of protein C, the TGT measures the balance between procoagulant and anticoagulant factors in liver disease.13 The TGT was used to demonstrate that plasma from patients with stable cirrhosis generates thrombin at a normal or even increased rate despite prolonged PT/INR and PTT values.13,23 The TGT is still primarily a research tool and not validated for predicting bleeding risk in patients with liver disease.
Thromboelastography (TEG, Hemonetics Corporation, Braintree, MA) and thrombelastometry (ROTEM, TEM International GmbH, Munich, Germany) are 2 commercially available global tests of hemostasis, which reflect the interaction of plasma, platelets and blood cells. Using whole blood, these tests analyze all components of hemostasis including the dynamics of clot formation (balance of procoagulant and anticoagulant factors), clot strength (platelets and fibrinogen), and clot stability (fibrinolysis and FXIII). Both devices measure the force exerted on a small metal rod suspended in whole blood during clot formation while either the cuvette (TEG) or the rod (ROTEM) is rotated.22,24
Early studies showed that TEG guided factor replacement reduced red cell transfusions and the volume of plasma infused during liver transplantation. TEG is now widely used during liver transplantation to guide transfusion, coagulation factor replacement, and antifibrinolytic therapy. Limited data suggest TEG more accurately reflects the balance of hemostasis in patients with chronic liver disease outside of surgery. TEG was used to demonstrate that patients with compensated cirrhosis often maintain normal global hemostasis.25 However, increased severity of cirrhosis was associated with ROTEM evidence of decreased velocity of clot formation, delayed clot formation time, and reduced maximum clot strength.26
TEG may have clinical utility for evaluating the severity of coagulopathy and risk of bleeding in chronic liver disease. Cirrhotic patients with active variceal bleeding who experienced early re-bleeding had significantly more hypocoagulable TEG parameters than those who did not.27 However, there are no prospective studies validating the accuracy of TEG or ROTEM for predicting procedural bleeding risk in patents with liver disease.
Prevention of bleeding
Vitamin K
Patients with cirrhosis and abnormal coagulation screening tests often receive vitamin K despite a lack of evidence supporting benefit. Supplemental vitamin K may correct abnormal coagulation tests in patients at high risk for deficiency due to biliary disease or gut sterilization from broad-spectrum antibiotics. However, in cirrhotic patients with reduced synthetic function, the benefit of vitamin K is uncertain. A single 10 mg subcutaneous dose of vitamin K resulted in minimal improvement in PT and PTT in patients with cirrhosis with no effect on the levels of several vitamin K dependent proteins. Cirrhotic patients had normal PIVKA (Protein Induced in Vitamin K Absence) levels suggesting vitamin K deficiency does not play a major role in the coagulopathy of liver disease.28
Fresh frozen plasma
Fresh frozen plasma (FFP) is often used for prophylaxis prior to invasive procedures in patients with cirrhosis despite the absence of clinical trials demonstrating benefit. Multiple studies have demonstrated that transfusion of FFP has minimal effect on a mildly prolonged INR. Thrombin generation was normal in patients with cirrhosis despite prolonged routine coagulation tests suggesting that prophylactic infusion of FFP prior to invasive procedures is unlikely to have clinical benefit.29 A small randomized trial in patients with cirrhosis undergoing dental extractions found that intranasal desmopressin was as effective as infusion of FFP for prevention of bleeding.30
The routine use of FFP for primary prophylaxis prior to invasive procedures is not recommended. Prevention of bleeding should not be aimed at correcting abnormal routine coagulation tests (INR, PTT). The large volumes of FFP required to significantly increase clotting factor levels may result in volume overload and exacerbation of portal hypertension paradoxically increasing the risk of bleeding. Prophylactic infusion of FFP may also delay procedures and expose patients to unnecessary risks. It is still unclear whether global assays of hemostasis such as TEG may be effective for guiding prophylactic therapy. The results of a recently completed study of the use of TEG to guide blood product transfusion in cirrhotic patients undergoing invasive procedures are not yet available (NCT02362178).
Platelets
Thrombocytopenia may be an obstacle to the timely performance of invasive procedures. There is no consensus on the threshold platelet count for prophylactic transfusion in patients with liver disease. Cirrhotic plasma with a platelet count adjusted to at least 56 000 showed a level of thrombin generation corresponding to the lower limit of the normal reference range.2 These in vitro results suggest that severe thrombocytopenia may limit thrombin generation in patients with cirrhosis and support the common platelet count threshold of 50 000 for high-risk procedures. AASLD guidelines for liver biopsy recommend consideration of platelet transfusion prior to liver biopsy for a platelet count <50 000-60 000 (Class I level C).20 However, there are no prospective studies demonstrating that prophylactic platelet transfusions reduce the risk of bleeding associated with liver biopsy or other invasive procedures. Prophylactic platelet transfusions do not ensure adequate hemostasis. In a small study of patients with cirrhosis and thrombocytopenia undergoing endoscopic variceal ligation, transfusion of a single unit of platelets resulted in only a slight increase in platelet count without normalization of thrombin generation or TEG parameters.31
Thrombopoietin receptor agonists
The demonstration of reduced TPO production and activity in chronic liver disease provides a rationale for use of thrombopoietin receptor (TPO R) agonists to increase platelet production. In a recent phase II study, patients with chronic hepatitis C, cirrhosis, and thrombocytopenia (<50 0000) received romiplostim for up to 4 weeks prior to elective surgery. A fixed 2 mcg weekly dose increased the platelet count above the target threshold value of 70 000 required for surgery in 94% of patients and there were no bleeding or thrombotic complications.32 A randomized trial in patients with chronic liver disease and thrombocytopenia (<50 000) demonstrated that administration of the oral TPO R agonist, eltrombopag for 14 days prior to elective invasive procedures reduced the need for platelet transfusion (28% patients) compared with placebo (81% patients). The study was terminated early because of an increased incidence of portal vein thrombosis in patients receiving eltrombopag (4%) versus placebo (1%).33 A 7 day pre-procedural course of the second-generation TPO R agonist, avatrombopag, achieved a significant increase in platelet count within 3–7 days in ∼50% of cirrhotic patients with thrombocytopenia. The lower incidence of portal vein thrombosis (1.1%) in this study may reflect dose selection and the shorter duration of therapy resulting in a less exuberant platelet response.34
Thrombotic complications may reflect a disruption of rebalanced hemostasis by a rapid and sustained increase in activated platelets. These initial studies of TPO R agonists demonstrate how small perturbations can overcome compensatory mechanisms and rapidly shift the balance toward thrombosis in an individual patient. Additional trials are necessary before TPO R agonists can be routinely recommended for patients with liver disease undergoing elective invasive procedures or surgery.
Management of active bleeding
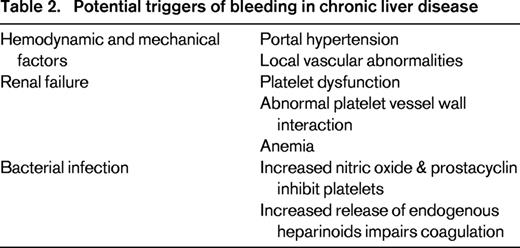
Bleeding complications in chronic liver disease are infrequently related to abnormal hemostasis. The majority of clinically significant bleeding episodes are due to increased portal pressure rather than deranged hemostasis. Risk factors for variceal bleeding are primarily related to hemodynamic and mechanical factors (portal pressure, varix size).35 Few data suggest that the coagulopathy of liver disease is directly related to variceal bleeding, although it may affect the severity of bleeding in some cases.
Rebalanced hemostasis in patients with liver disease is potentially unstable and may be tipped toward hemorrhage by several triggers including progression of portal hypertension, renal failure, and infection (Table 2). Renal failure develops in a substantial number of patients with end stage liver disease and may result in a bleeding tendency due to acquired platelet dysfunction and abnormal endothelial function. Bacterial infections may act as a trigger for variceal bleeding and are associated with failure to control bleeding.27 Bacterial endotoxin induced nitric oxide and prostacyclin may impair platelet aggregation. Endotoxin may also release endogenous heparinoids, the glycosaminoglycans that maintain the physiologic antithrombotic surface of the endothelium. There is evidence that bacterial infections may impair hemostasis in cirrhosis by triggering the release of these heparin-like substances. Several studies found that bacterial infections induced a heparin-like effect detected by TEG, which reversed after antibiotic treatment and resolution of the infection.36 Endogenous heparinoids were also detected with an anti-Xa assay in 60% of infected cirrhotic patients compared with 6.7% of those without evidence of infection.37 Anti-Xa activity was detected in 23% of patients with acute variceal bleeding compared to none of the patients with stable cirrhosis without bleeding. The presence of infection was associated with anti-Xa activity suggesting high levels of endogenous heparinoids may reflect a response to bacterial infection.38 Increased release of endogenous heparinoids may tip the balance of hemostasis toward hemorrhage and thereby explain the pathogenetic link between bacterial infection and portal hypertensive bleeding.
Management of active bleeding
Standard treatment for acute variceal hemorrhage includes a combination of a vasoconstrictor and endoscopic therapy. Recent United Kingdom consensus guidelines recommend a combination of pharmacologic (vasoconstrictor) and endoscopic therapy for initial management of active variceal bleeding.35
Transfusion
Resuscitation with red cell transfusion should aim for a target hemoglobin of 7-8 g/dL, a policy supported by recent randomized trial evidence that a restrictive transfusion protocol improves control of variceal bleeding.35,39 Over transfusion of RBC or large volumes of FFP carries a risk of increased portal pressure and re-bleeding. There are no evidence-based guidelines for treatment of abnormal hemostasis during acute bleeding. Platelet transfusion is recommended for patients with thrombocytopenia and active bleeding. There is a general consensus that the platelet count should be maintained >50 000 during acute bleeding, the level shown to ensure adequate thrombin generation in vitro.2 The increment in platelet count is often poor in patients with hypersplenism, active bleeding and/or coexistent infection. Transfusion of cryoprecipitate to maintain a fibrinogen level >100 is usually recommended although a threshold hemostatic fibrinogen level is also not well-defined. There is no evidence that prophylactic plasma or platelet transfusions reduce the risk of re-bleeding in patients with varices.
Recombinant factor VIIa
Although rfVIIa normalizes a prolonged PT/INR, there is no evidence it reduces bleeding. Several randomized studies found the use of rFVIIa in addition to standard pharmacologic and endoscopic therapy no beneficial effect on clinically relevant outcomes in patients with cirrhosis and active variceal bleeding.40 A meta-analysis found that patients who received rFVIIa had a significantly lower re-bleeding rate during the first 5 days but there was no reduction in re-bleeding rate or mortality at 6 weeks.41 A Cochrane systematic analysis also found that rFVIIa did not reduce mortality in patients with liver disease and upper GI bleeding and concluded there is insufficient evidence to support the use of rFVIIa in this setting.42 Recombinant FVIIa is not approved for use in liver disease and off-label use is associated with an increased risk of arterial thromboembolism.41 Recombinant FVIIa is rarely used as a salvage therapy and bridge to more definitive treatment in select cases of uncontrolled bleeding requiring urgent control. This decision requires careful consideration of potential thrombotic risks, as well as likely benefit.
Prothrombin complex concentrates
Prothrombin complex concentrates (PCCs) are plasma-derived products that contain vitamin K-dependent coagulation factors (FVII, FIX, FX, prothrombin) and anticoagulant proteins (protein C and protein S). The composition of different PCC products varies considerably. Three-factor PCC contain very low concentrations of FVII and little/no protein C or protein S. Four-factor PCC contain clinically adequate amounts of all the vitamin K-dependent factors including protein C and protein S. PCC have several advantages over FFP including delivery of a smaller volume with a 25-fold higher concentration of coagulation factors and more rapid correction of hemostatic parameters. However there is no evidence that administration of PCC as adjunctive or rescue therapy in cirrhotic patients with active bleeding improves outcome. PCC may increase thrombotic risk in patients with liver disease. A multicenter randomized trial is currently investigating the efficacy of preoperative administration of PCC in patients undergoing liver transplantation. In the absence of evidence confirming benefit, the routine use of PCC for bleeding complications of chronic liver disease is not recommended.
Desmopressin
There is no evidence that desmopressin improves control of bleeding or clinical outcome in patients with variceal bleeding. Several studies found that desmopressin did not reduce blood loss in patients with acute variceal hemorrhage or undergoing hepatectomy or liver transplant. DDAVP may have minimal hemostatic benefit in cirrhotic patients with elevated baseline VWF and FVIII levels.43
Antifibrinolytic agents
Although antifibrinolytic agents such as tranexamic acid were shown to reduce blood loss during liver transplantation, there is inadequate evidence of benefit outside of the transplant setting. A Cochrane systematic analysis of the use of tranexamic acid for upper GI bleeding suggested reduced mortality although the poor quality of the trials included precluded confirmation of benefit (Cochrane Database Systematic Review). The ongoing randomized controlled HALT-IT (hemorrhagic alleviation with tranexamic acid-intestinal system) trial is investigating the effectiveness and safety of tranexamic acid in patients with GI bleeding (NCT01658124). There is currently insufficient evidence to support the routine use of tranexamic acid in the setting of acute variceal hemorrhage or other bleeding.
Conclusions
It is now recognized that hemostasis is rebalanced in chronic liver disease. The balance is fragile and may be perturbed by superimposed insults such as infection or renal failure. Although clinical decisions are still often based on conventional coagulation tests such as the PT/INR, these tests are not designed to reflect the complex interaction between cells and coagulation proteins. Global assays of hemostasis show promise for assessing bleeding risk and guiding therapy but high quality data validating their use is still lacking. Bleeding in chronic liver disease is more often the result of hemodynamic and mechanical factors than deranged hemostasis. Additional studies are needed to define evidence-based guidelines for the prevention and treatment of bleeding in liver disease.
Correspondence
Jody L. Kujovich, Oregon Health & Science University, Pediatric Hematology/Oncology, Oregon Hemophilia Center, Mail Code CDRC, 707 SW Gaines St, Portland, OR 97239-3098; Phone: 503-494-8716; Fax: 503-494-2721; e-mail: kujovich@ohsu.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: I discuss the clinical trials of recombinant factor VIIa in variceal bleeding due to liver disease. I also discuss the use of prothrombin complex concentrates and the lack of evidence of benefit and the ongoing trial in liver transplantation.