Treatment of newly diagnosed and relapsed myeloma has been a rapidly moving field because of an improved understanding of disease biology and access to new drugs. Decades ago we learned that “more” was not necessarily “better”, especially when the “more” involved combinations of alkylators with steroids, which was associated with greater toxicity and more complications.1 When the “novel” agents [immunomodulatory agents (IMiDs) and proteasome inhibitors (PIs)] were brought to the field a decade ago, they subsequently demonstrated greater efficacy and were deemed safer in combinations, leading to a push to combine agents for newly diagnosed patients. This much-needed change is now extending to the relapse setting as well. Recent phase 3 trials have suggested that combinations of these new agents with each other, or with alkylator-based therapy resulted in deeper responses and prolonged duration of remission and overall survival.2-5 Given the relative wealth of new agents in development for myeloma, identifying ideal effective combination therapies with minimal overlapping toxicities at various phases of myeloma treatment is an important goal for our current treatment approaches with the intent of curing a larger fraction of newly diagnosed and relapsed myeloma patients.
Goals of therapy
The goal of therapy in both the transplant-eligible or transplant-ineligible myeloma patients in the upfront setting is to achieve the maximal possible response rapidly, with minimal toxicity and to improve performance status. In the transplant eligible patients, an additional goal is to utilize therapy that does not limit the peripheral blood stem cell mobilization. The goals of therapy in the relapsed setting are slightly different. With prior regimen and disease related toxicities, the ultimate aim of treatment here is to achieve a good balance between the efficacy and toxicity of the regimen. Several disease-related (biology of disease, staging, chromosomal abnormalities), treatment-related (prior drug therapy, regimen related toxicities, prior depth, and duration of response) and patient-related factors (comorbidities, performance status of the patient, renal and hepatic impairment, susceptibility to infections) influence the choice of the treatment regimen in relapsed patients.
Evidence demonstrating benefit with a deeper response
Multiple studies have confirmed that attaining a deeper response is the key to improving progression-free survival (PFS) and ultimately overall survival (OS).6-8 The novel agents are known to be associated with higher response rates, including deeper responses, compared with cytotoxic agents, even among some patients with high-risk disease.3,9,10 The incremental survival improvements from the last decade to the current are in part related to the use of novel agents resulting in improved depth of response. The goal of adding novel agents to backbone regimens is to increase the number of patients achieving deeper responses, provided the added toxicity is not excessive. One question, in an induction setting is how to manage the patient who does not achieve the desired maximal response after a defined number of cycles of therapy. Is there benefit to treat for a few more cycles to achieve a deeper response prior to autologous transplant? Palumbo et al suggested that maximal response with induction therapy is achieved at the completion of 4-5 cycles and perhaps the benefit to toxicity ratio starts tilting more toward the toxicity side with further therapy.11 Additional retrospective data from the Center for International Blood and Marrow Transplant Research (CIBMTR) further supports this concept.12 These observations support using a multi-agent combination therapy with minimal toxicity to achieve the maximal response rapidly, and thereby improve long-term outcomes.
As treatment improves, how to define the depth of response also has to improve. Kapoor et al evaluated the impact of achieving stringent complete response (sCR) on the outcomes of time to progression (TTP) and OS among myeloma patients post-transplant.6 Patients that have achieved a sustained sCR had 5 year OS of 91% compared with the 5 year OS of 53% among the nonsustained sCR patients, indicating that not only attaining a high-quality response, but sustaining the same response is of utmost importance to achieve better long-term outcomes. Another important marker of a deeper response is the imaging based CR. Fluorodeoxyglucose positron emission tomography integrated with computed tomography (FDG-PET/CT) demonstrates the presence of active or inactive myeloma bone disease and can be explored to monitor response and to predict the outcomes in myeloma. The 4 year PFS and OS were significantly superior for PET-negative (SUV <4.2) versus PET-positive (SUV ≥4.2) patients suggesting complete FDG suppression after HDT was an independent favorable prognostic factor on multivariate analysis for durable disease control and prolonged OS, even among patients achieving CR.13 Most important of all, minimal residual disease (MRD) negative status, as shown by the GEM (Grupo Español de MM)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) group resulted in prolonged PFS and OS. MRD negativity was the most important independent prognostic factor for PFS.14 Similarly, from the Medical Research Council (MRC) Myeloma IX study, MRD had identical prognostic significance as the International Staging System for OS, with the only other factor having a greater prognostic significance being cytogenetics.15 Ultimately, the depth of response directly influences OS and a major push should be attempted to attain this endpoint, though MRD by flow cytometry or next-generation sequencing (NGS) remains an important question for future.
Novel combination therapies in the induction setting
The role of combination therapy in the induction setting was cemented by the phase 3 trial from Cavo et al2 in younger patients. This trial randomized newly diagnosed myeloma patients to induction therapy with bortezomib, thalidomide, and dexamethasone (VTD) versus thalidomide and dexamethasone (TD), and demonstrated the importance of achieving the depth of response with a 3 drug induction followed by auto-transplant and consolidation in first response. After the first auto-transplant, complete response (CR) rates were 38% versus 23% (p = 0.0004) and ≥very good partial response (VGPR) rates were 79% versus 58% (p < 0.0001) favoring the combination arm. The PFS was significantly longer for patients on VTD arm than in those on TD (HR 0.63, 95%; 0.45-0.88; p = 0·0061). More importantly, receiving VTD induction and consolidation therapy overcame the adverse effect of t(4;14) on 3 year PFS: 69% [with t(4;14)] versus 74% [without t(4;14)] (p = 0·66), where TD arm failed to achieve similar benefit for the t(4;14) patients.2 Similar impressive results were seen with carfilzomib, thalidomide and dexamethasone (CTD) among the newly diagnosed patients with 25% of patients achieving CR, 68% achieving at least a VGPR, and 90% at least a partial response (PR) after induction therapy. ≥VGPR rates increased to 76% after auto-transplant and to 89% as best response after four cycles of consolidation therapy signifying that a PI, IMiD, and steroid combination is efficacious.16 Lenalidomide, a more potent immunomodulatory agent in combination with bortezomib and dexamethasone (RVD) exerted 100% ≥PR rates and ≥VGPR rates of 67%.17 Deeper responses were seen with less peripheral neuropathy, with the combination of carfilzomib, lenalidomide and dexamethasone (CRD) demonstrating a near complete response (nCR) rate among 62% of the patients in the trial.18 An argument has been made with regard to the use of a proteasome inhibitor in combination with cyclophosphamide and dexamethasone as a “cheaper” alternative option. The EVOLUTION study is a phase 2 study that examined combination of RVD and bortezomib in combination with cyclophosphamide and dexamethasone (VCD).19 The response rates and the 1 year PFS seem to be similar between both arms, but the study is limited by small numbers of patients enrolled and shorter duration of follow-up. In a recent retrospective study presented by Cavo et al,20 VCD was compared to VTD in 2 large phase 3 trials, the overall response rate and complete remission rate for both standard and high-risk patients was higher with the IMID/PI combination of VTD than with VCD. Randomized trials are currently ongoing to formally compare the two approaches, but for now it appears that the IMID/PI combination is superior to that of PI/cyclophosphomide, favoring IMID/PI use in newly diagnosed myeloma.20 With the availability of currently available novel agents, deeper responses are within reach for many different subsets of myeloma patients including those with high-risk disease. Table 1 illustrates the response rates achieved with novel agent 3 drug combination therapy.
Strategies to deepen the response in the current era of active agents
With the results of the 2 recently published randomized control trails supporting the benefit of oral lenalidomide therapy in the maintenance setting,21,22 the Intergroupe Francophone du Myélome (IFM) group evaluated the outcome benefit of the transplantation-based approach with the most active 3-drug combination regimen bortezomib, lenalidomide, and dexamethasone (RVD)17 as induction and consolidation followed by lenalidomide maintenance. Among the 31 patients, with median follow-up of 39 months, the estimated 3 year PFS was 77% (95% CI: 57%-88%). Among the 21 patients who have achieved MRD negativity by MFC, no one has progressed but among the 10 patients that have not achieved MRD negativity, 70% progressed, with an estimated 3 year PFS of 23% (95% CI: 4%-53%). Survival outcomes among the patients with high-risk cytogenetics were similar to the group as a whole, with an estimated 3 year PFS of 86% (95% CI: 33%-98%) suggesting that this active RVD combination therapy followed by auto-transplant, RVD consolidation and lenalidomide maintenance therapy would deliver deeper molecular responses and a lengthier PFS.23 Similar results were observed with upfront combination therapy with CRD followed by lenalidomide maintenance inducing high-quality responses of MRD negativity among newly diagnosed myeloma patients. Among the 41 patients enrolled, 14 CR/sCR and 3 nCR patients who underwent MRD assessment were negative for MRD suggesting the benefit of such combination followed by consolidation and maintenance therapy.
Can we achieve deeper responses by combining more agents?
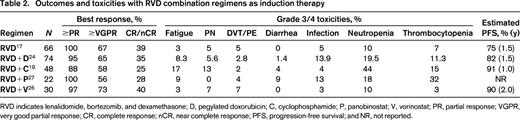
Prior studies combining more cytotoxic agents to the triplet regimens did not yield superior results. The role of quadruplet therapies remains unclear in both the transplant-eligible and -ineligible populations. The results from the EVOLUTION study that evaluated adding cyclophosphamide to RVD (VCRD) versus RVD suggested that efficacy of VCRD was similar to RVD although, the VCRD arm was associated with an increased incidence of hematologic toxicities.19 Similar results were seen with the RVDD24 regimen, which demonstrated similar efficacy to the 3-drug RVD regimen but increased toxicity. At this point, results from available studies suggest that quadruplet combinations combining cytotoxic agents are associated with greater toxicity without gaining any additional anti-myeloma effect. Taking the example of the FIRST trial, it is not the number of agents, but it is the quality of the newer agents that demonstrate superiority. Lenalidomide and dexamethasone (Rd) was superior to the alkylator-based melphalan, prednisone, and thalidomide (MPT) regimen among newly diagnosed elderly myeloma patients.25 Rather than using these historically ineffective alkylator-based therapies in combination regimens, adding an investigational biologic agent might hold promise. Induction therapy with RVD in combination with histone deacetylase (HDAC) inhibitors, such as vorinostat26 and panobinostat,27 have deepened the responses as illustrated in Table 2 with acceptable toxicity, but significant dose reductions were needed. More importantly, preliminary results with combination of daratumumab in the front-line setting gives a safety signal and able to exert good responses with various backbone therapies as depicted in Table 2. Future trials of induction therapy may incorporate the 2 active monoclonal antibodies daratumumab (anti-CD38) and elotuzumab (anti-SLAMF7) to other active regimens such as RVD or CRD with goal of attaining MRD negativity and better long-term outcomes. Thus, addition of a conventional cytotoxic agent to an IMiD/PI combination does not appear to confer benefit and future studies that include the use of monoclonal antibodies as the fourth agent, and will likely be associated with improved outcomes compared with even the best 3-drug novel agent regimen.
Novel combination therapies in relapsed setting
Recognizing the significance of attaining a deep response and with the availability of the newer anti-myeloma agents with novel mechanisms of action, a question arises as to why we do not take the same aggressive 3-drug approach in the context of early relapse (1-3 prior lines of therapy). To date the use of 2 drug salvage with bortezomib or lenalidomide has demonstrated benefit in overall survival compared with dexamethasone alone.28,29 However, additional new drugs are being studied as part of 3-drug regimens with either bortezomib- or lenalidomide-based salvage as the control arm, and are demonstrating significant benefit.
IMIDS
Lenalidomide combinations
Lenalidomide is a second-generation IMiD with greater potency and less toxicity than thalidomide. Rd is an effective regimen delivering superior responses compared to dexamethasone alone in 2 randomized phase III trials MM-00930 and MM-010.31 Lenalidomide is highly active in combination with bortezomib from the phase 2 trial by Richardson et al demonstrating high response rates of ≥PR rates of 64%, ≥VGPR rates of 28%, and a PFS of 9.5 months.32 In a different phase I combination trial with the other PI, carfilzomib, CRD resulted in >VGPR rates of 22%, and an impressive PFS of 15.4 months.33 It also exhibits synergy when combined with antibodies elotuzumab34 and daratumumab35 as shown in Table 3 and will be discussed in the antibody section.
Pomalidomide combinations
Pomalidomide is the third-generation IMiD that shares a number of the beneficial pharmacologic properties lenalidomide. The response rates are accentuated when combined with dexamethasone and further synergizes with PIs bortezomib and carfilzomib. Pomalidomide, bortezomib, and dexamethasone (PVD) in the relapsed setting resulted in PR rates of 70% and VGPR rates of 43%.36 Pomolidomide combinations with carfilzomib,37 daratumumab,38 HDAC inhibitors are ongoing and showing promising anti-myeloma effect, especially among patients with high-risk genetics features.
PIs
Bortezomib combinations
The first in class PI bortezomib not only had single-agent activity, but also combined well with many other agents. Similar to the front-line setting of myeloma treatment, VTD was shown to be superior to TD among relapsed myeloma patients. The phase 3 trial led by Gardaret et al cemented the superiority of the combination regimens by signifying the role of VTD exerting deeper responses as evident by attaining >VGPR in 56% (vs 35% for TD) of the patients and subsequently improving the median PFS of 18.3 months (vs 13.6 months for TD) and 2 year OS of 71% (vs 65% for TD; P = NS).39 Though the OS difference is not evident because of shorter follow-up or better salvage options, the authors felt that OS for VTD may prove to be greater than that for TD if the follow-up period is prolonged. Additionally, bortezomib is synergistic with lenalidomide (>PR: 64%, >VGPR 28%)32 and pomalidomide (>PR: 70%, >VGPR 43%)36 especially among heavily pretreated patients. The combination regimen of PVD demonstrated deep responses among high-risk patients suggesting that this is an active combination for high-risk myeloma. The combination with the histone deacetylase inhibitors (vorinostat40 and panobinostat41 ) also demonstrated synergy with higher response rates, as shown in Table 3. Several combination regimens with other novel agents in the pipeline (eg, ARRY-520) are in development. Though there are concerns about neurotoxicity, the use of subcutaneous and/or weekly dosing is not only associated with a lower rate of neuropathy, it is also more convenient for patients as part of a combination regimen.42 Bortezomib combined well with elotuzumab in a phase I study with ≥PR rates seen in 48% of patients, even in bortezomib-refractory patients.43 Further daratumumab-based combinations may be potential options for improving efficacy.
Carfilzomib combinations
The second generation epoxyketone, which induces irreversible proteasome inhibition, carfilzomib combines well with other agents to make active combination therapies. With an additional advantage of its lack of neurotoxicity, it proved to be a safe combination with IMiD lenalidomide and delivered good efficacy. CRD in a phase 2 trial exerted an impressive PFS of 15.4 months and resulted in high-quality responses with >VGPR rates of 22%.33 Further data from the phase 3 trial in the early relapsed setting comparing CRD versus Rd demonstrated the longest PFS reported to date in a relapsed myeloma trial with a median PFS benefit of close to 9 months of 26.3 versus 17.6 months (HR = 0.690; 95% CI, 0.570-0.834; p < 0.0001).4 Similar superior results with carfilzomib in combination with pomalidomide were seen with PR rates of 64% and >VGPR rates 26% among heavily pretreated myeloma patient population with a median of 6 prior lines of therapy. The PFS is 12 months.37 Though there are concerns about potential cardiotoxicity, data from emerging phase 3 clinical trials suggests no increase in cardiac toxicity compared with control patients.4 Among heavily pretreated myeloma patients, in combination with the kinesin spindle protein inhibitor AARY-520,44 the response rate was 35% and in combination with the HDAC inhibitors,45,46 a response rates of 50%-60% were seen, approximately one-half of which are >VGPR, supporting the use of carfilzomib with many new drugs and drug classes.
HDACIs
Vorinostat combinations
Vorinostat is a pan-deacetylase inhibitor that was evaluated in combination with bortezomib in the VANTAGE-095 trail. This trial included heavily pretreated myeloma patients and the reported ≥PR rates were seen in 17% patients suggesting activity of the combination.47 More importantly, among these bortezomib-refractory patients, addition of vorinostat regained bortezomib sensitivity. In a similar patient population, the ORR was 34.5% for bortezomib in combination with panabinostat in the PANORAMA-2 trial.41 The VANTAGE-088 that followed, randomized 637 patients to vorinostat and bortezomib or placebo and bortezomib in the early relapsed setting. Higher toxicity and the marginal clinical benefit in this trial (PFS favoring vorinostat and bortezomib combination: 7.63 vs 6.83 months; p = 0.01; HR 0.77), precluded the combination in regular use.48 Another combination trial of vorinostat with CRD as illustrated in Table 3 suggested that higher responses are possible by in heavily pretreated patients and by effectively managing the toxicities, the efficacy can be retained.
Panobinostat combinations
Panobinostat is the first HDACi that was FDA approved among myeloma patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent. This approval was based on the phase 3 randomized trial, PANORAMA-1, that randomized 768 patients to receive combination of panabinostat, bortezomib, and dexamethasone or placebo, bortezomib, and dexamethasone. The combination panobinostat arm demonstrated a significant increase in median PFS (∼4 months) compared with that of the placebo arm (median PFS: 12 vs 8.1 months; p < 0.0001) favoring the use of panobinostat. Patients who received a longer duration of treatment on the panobinostat arm demonstrated improved outcomes of higher nCR/CR rates and median PFS suggesting that by optimizing the management of adverse events by appropriate dose adjustments or interruptions and/or supportive therapy, we can derive the clinical benefit of this combination.49
ACY-1215 combinations
Rocilinostat (ACY1215) is a selective HDAC-6 inhibitor, developed in an effort to preserve the clinical efficacy of histone deacetylase inhibition while minimizing toxicity. Early trials combining rocilinostat with bortezomib and dexamethasone,50 lenalidomide, and dexamethasone51 are promising. Combination trial with pomalidomide and dexamethasone is in process. The current oral formulation and the preliminary potency, safety suggests for a potential combination regimen with PIs or IMiDS in 3 or 4 drug regimens.
Other new targets in development
KSP inhibitors
ARRY-520.
Inhibiting the spindle formation during mitosis results in cell death. ARRY-520, a kinesin spindle protein (KSP) inhibitor targets the KSP for exerting the anti-myeloma effect. Another agent where modest activity was seen as single agent, but in combination with steroids, showed good anti-myeloma activity among heavily pretreated multi-refractory myeloma patients.52 It is showing promising activity in combination with bortezomib and dexamethasone,53 and in combination with carfilzomib.54 The agent potentially may be combined with most anti-myeloma agents to improve on the efficacy to use the advantage of the alternate mechanism of additive cell death and due to its non-overlapping toxicities.
AKT inhibitors
Afuresertib.
Selective oral AKT kinase inhibitors have shown promising anti-tumor activity in the solid tumors. In combination with bortezomib at the MTD of 150 mg PO daily, increased phospho-AKT levels were seen in myeloma cells, demonstrating achievement of target inhibition and clinically impressive results were observed.55 Further trials with carfilzomib combinations are underway.
SINE XPO1 inhibitors
Selenexor is a first in class selective inhibitor of nuclear export (SINE) molecule that inhibits the nuclear export protein exportin 1 (XPO1), currently being evaluated in multiple myeloma. It demonstrated synergy in combination with dexamethasone and delivered 10% sCR and 50% PR rates among the 10 patients treated at 45 mg/m2 on the phase I trial.56 Further phase 2 trials at 80 mg dose are underway.
Immune approaches
Antibodies
Daratumumab.
Daratumumab, a humanized anti-CD38 antibody is the most active monoclonal antibody in myeloma clinical trials today and was recently granted breakthrough status by the FDA. It acts through multiple mechanisms of action by directly targeting tumor cells and also effectively mediates killing of CD38–expressing plasma cells via antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and apoptosis. The MTD and active dose was confirmed to be 16 mg/kg resulting in response rates of 29%, and one-half of which are >VGPR.57 In combination with Rd, among heavily pretreated myeloma patients that have received a median of 4 lines of therapy, response rates of 100% and three-quarters (75%) of which are >VGPR were observed. At the active dose of 16 mg/kg, in combination with Rd, the response rates were 87% and >VGPR rates seen at 50%.58 The interference of the daratumumab (an IgG kappa antibody) may also spuriously be accounted for a paraprotein in the disease assessment and possibly could explain the inconsistent complete response rates observed at the active dose of daratumumab. Nevertheless, no doubt it is an active drug and in combination with pomalidomide and dexamethasone had a VGPR rate of >50% (Table 3).38 Multiple combination trials are ongoing for its rapid approval.
Elotuzumab.
Elotuzumab is a humanized anti-SLAMF-7 antibody targeting the cell surface cell surface glycoprotein CS1 (SLAMF7) on the myeloma cell and on NK cells resulting in activation of the NK cells. It exerts anti-myeloma effect via ADCC in myeloma cell lines and myeloma cells. Although as a single agent no objective responses were observed, in combination with Rd impressive response rates of 82% were observed suggesting that lenalidomide upregulates the NK cells, which are necessary for the NK-cell-mediated cell kill by elotuzumab. It offers a novel synergistic mechanism with lenalidomide. Response rates in the phase 3 study (ELOQUENT-2) elotuzumab at a dose of 10 mg/kg in combination with Rd (ERd) versus Rd were 79% versus 66% and median PFS was 19.4 versus 14.9 months (P < 0.001), respectively.59 An ongoing phase 3 trial (ELOQUENT-1) is evaluating the efficacy and the safety of Rd with or without elotuzumab at the active dose of 10 mg/kg among newly diagnosed myeloma patients.
Checkpoint blockade
Programmed cell death protein (PD-1) or its ligand, programmed cell death-ligand 1 (PD-L1) interactions may indirectly modulate the response to tumor antigens through T cell/antigen-presenting cell (APC) interactions. PD-1 engagement may represent one means by which tumors evade immunosurveillance.60 PD-1/PD-L1 pathway is shown by multiple groups to promote progression of myeloma indirectly by undermining immune control of the malignancy.61 PD-1 blockade has been pursued as a promising therapeutic strategy to reverse immune tolerance and enhance T-cell effector function in several tumor types. The broad expression of PD-1 and its ligands in the microenvironment of myeloma and the preclinical data revealing an important role of the PD-1 pathway in immune evasion by myeloma cells, there may an immunotherapeutic potential of PD-1/PD-L inhibition in myeloma. Several PD-1 antibodies, such as nivolumab, pembrolizumab, pidiluzumab (CT-011), and PDL-1 antibodies (MEDI4736), are under investigation.
Adoptive cellular therapy
Chimeric antigen receptor (CAR) T cells.
Modifying autologous T cells with CARs against CD19 provides a promising therapeutic strategy for myeloma. CTL019 cells, consisting of autologous T cells transduced with an anti-CD19 CAR, administered following high-dose therapy (HDT) with melphalan 140 mg/m2 and autologous transplant resulted in durable remission in a heavily pretreated myeloma patient suggesting cellular therapy as a potential therapeutic option for myeloma patients.62 Another early phase trial with T cells engineered to target NY-ESO-1 antigen is ongoing.
Regulatory T cells.
Regulatory T cells (Tregs) are CD4+ T-cell population (CD4+CD25+FoxP3+ T cells) that strongly inhibit anti-tumor immune responses in myeloma patients. Treg depletion may enhance the function of tumor antigen-specific T cells. Among 10 patients undergoing ASCT, Treg depletion in myeloma patients was safe and further studies are ongoing.63
Therapeutic vaccinations
Dendritic cell (DC) vaccines.
DCs are APCs that can stimulate vigorous T-cell immune responses. Unfortunately in myeloma, because of various factors, such as down regulation of costimulatory surface receptor expression and changes in T-cell repertoire, the anti-tumor immune response fails. Novel approaches of combining DC vaccine and PD-1 antibody (CT-011), post-autologous transplant are underway.
Peptide vaccines.
PVX-410 is composed of a tetrapeptide from 3 unique regions of myeloma-associated antigens (XBP1, CD138, and CS1). The goal is to induce immunity against myeloma cells by selectively stimulating tumor-associated antigen-specific cytotoxic T lymphocytes (CTLs). The safety and tolerability of PVX-410, alone and in combination with lenalidomide, are being evaluated for the treatment of smoldering myeloma patients currently.64 Immucin, an anti-MUC1 signal peptide vaccine, induced a robust CD8+ and CD4+ specific T-cell responses in all 15 patients post-ASCT and demonstrated a marked anti-tumor humoral response.65
Conclusions
It is an exciting time for the myeloma patients with more new therapeutic options available with the availability of new drugs and understanding the potential targets. Realizing the futility of the ineffective past regimens and understanding the therapeutic efficacy of the currently available agents and the ones in pipeline, we have made a significant progress as evident by the improved survival over the past 15 years. With a better understanding of the clonal heterogeneity and the recognition of the significance of achieving the depth of response as measured by minimal residual negativity, we are in the process to start a new journey of finding the ideal non-cytotoxic combinations both among the newly diagnosed and the relapsed/refractory myeloma patients that are more effective and more tolerable too. Trials are on way, and more should be planned in pursuit of effective targeted combination therapies to provide durable long-term remissions, if not cure, with lesser toxicity and a promise for a better quality of life for the myeloma patients. With availability of next generation sequencing techniques, the identification of driver mutations that lead to myeloma progression clearly seems within reach. One such example is the BRAF V600E mutation that is seen in 4% of relapsed myeloma patients. A commercially available V600E mutation-specific BRAF inhibitor, vemurafenib, resulted in durable response in a relapsed myeloma patient.66 Although, we are in early phases of exploring and identifying the druggable targets, the potential to target an activating driver mutation is promising. The availability of future technologies for personalized immune approaches, identifying actionable mutations, and establishing biomarkers for treatment responsiveness opens the doors for personalized medicine in management of relapsed myeloma for future.
Correspondence
Sagar Lonial, MD, FACP, Professor and Executive Vice Chair, Department of Hematology and Medical Oncology, Chief Medical Officer, Winship Cancer Institute, Emory University, 1365 Clifton Rd, Building C, Rm 4004, Atlanta, GA 30322; Phone: 404-727-5572; Fax: 404-778-5530; e-mail: sloni01@emory.edu.
References
Competing Interests
Conflict-of-interest disclosures: S.L. has received research funding from BMS, Novartis, Millennium, Celgene, and Janssen, and has consulted for Onyx, BMS, Novartis, Millennium, Celgene, and Janssen; A.K.N. declares no competing financial interests.
Author notes
Off-label drug use: drugs in development for MM.