Abstract
The BCR-ABL-negative myeloproliferative neoplasms (MPNs) are clonal stem cell derived malignancies, which include polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). The MPNs are characterized by dysregulated JAK-STAT signaling pathways. PV and ET are associated with an increased risk of thrombo-hemorrhagic complications, risk of progression to MF and leukemia. Presentation of patients with PV and ET is variable and usually as a result of abnormal full blood count indices (raised hemoglobin and hematocrit, leukocytosis, and thrombocytosis). Presentation with thrombosis or splenomegaly occurs in ∼30% of patients. Historically thought of as indolent compared with MF, patients with PV and ET have significant disease symptom burden which does not directly correlate to the current clinical prognostic classifications. The mainstay of therapy is reserved for patients with high-risk disease and thus excludes a population of patients with significant symptom related morbidity impacting their quality-of-life and survival. Recent treatment strategies have aimed to incorporate disease burden assessment into the selection of therapeutic interventions such as JAK2 inhibitors and HDAC inhibitors. We will review the advances in the field of MPN symptom assessment and symptom burden experienced by ET and PV patients. We will also discuss the risk-stratified management of ET and PV patients alongside symptom assessment and the impact of potential novel therapies, for patients who fail to respond to conventional treatment.
Learning Objectives
To recognize the symptom burden in patients with PV and ET
To review the current management of symptoms in patients with PV and ET
To consider impact of novel treatments on symptom burden
Polycythemia vera (PV) and essential thrombocythemia (ET) are 2 of the 3 classical bcr-abl–negative myeloproliferative neoplasms (MPNs) characterized by clonal stem-cell proliferation and a dysregulated JAK-STAT pathway.1,2 Since the discovery of the pivotal role of the somatic JAK2 activating mutation in the pathogenesis of the MPNs there has been a decade of rapidly increasing knowledge with the discovery of additional MPL and CALR mutations 3-5 . In PV, up to 99% of patients harbor a somatic mutation in exon 12 or 14 of JAK2, and the majority have the JAK2V617F allele. In ET, 50% of patients carry the JAK2V617F allele, with up to 25% of patients with ET having the CALR mutation and ∼4% exon 10 MPLW515L/K mutations.5 The discovery of new molecular markers has led to a continual refinement and review of prognostic classifications to risk stratify treatment,6-8 alongside the expanding development of novel targeted therapies that are currently being evaluated in trials.
Patients with PV and ET are heterogeneous in their phenotypic presentation, spectra of severity and prognostic outcomes. Individual MPN subtypes are best viewed as an amalgamation of pathologies that result in a composite disease burden, specific to each patient. Within the field of BCR-ABL–negative MPNs, essential thrombocythemia, polycythemia vera, and myelofibrosis have been best studied. All 3 can be associated with splenomegaly, cytopenias, profound constitutional symptoms, and an increased risk of transformation to MF or acute leukemia. Disease complications remain the primary source of mortality. MPN patients have an estimated 5- to 7-fold increased risk of thrombosis in comparison to the general population, particularly for abdominal thrombosis.9 Clonal evolution to myelofibrosis predisposes ET and PV patients to comparable mortality rates to patients with primary myelofibrosis. Progression to acute myeloid leukemia (AML) is almost uniformly fatal within 12 months of diagnosis and observed in up to 8% of the MPN population.10
The prevalence of PV (44-57 per 100 000 persons) and ET (38-57 per 100 000 persons) is much higher than for MF in the United States.11 These patients have a longer median survival of 14 and 20 years, respectively, compared to 6-8 years for patients with MF. European data for prevalence of PV was estimated to be between 5 and 30 per 100 000 persons and ET 4-24 per 100 000 persons.12 These results were based on a review of European data sources with variability in reporting and diagnosis between 2000 and 2012, leading to an under-reporting of cases.
MPN symptom burden and assessment
The first dedicated exploration of the MPN disease burden began with a cross-sectional, internet-based symptom survey of 1179 PV, ET, and MF patients and was reported in 2007.13 Symptom assessment tools utilized were the Functional Assessment Cancer Therapy-Anaemia (FACT-An)14 and the Brief Fatigue Inventory (BFI).15 This study identified that most MPN patients face debilitating fatigue (81%), itching (53%), night sweats (50%), bone pain (44%), fevers (14%), undesired weight loss (13%), and spleen pain (6%). Patients reported the impact of these on many domains of daily living including social functioning and independence in daily tasks. Decreased levels of physical activity were noted and were primarily as a result of fatigue, dyspnea, pain, and splenic symptoms. Of note, fatigue was reported regardless of the severity of the condition and across all 3 subtypes. For example, patients with PV who lacked complications, such as thrombosis, bleeding, and/or splenomegaly and would be classified as low risk, still reported significant symptoms. This survey supported the evidence that patients with MPN, regardless of their subclassification, had a high degree of symptom burden, which impacted negatively on quality-of-life as well as life expectancy. Evidence from the use of the established European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQC30) used in 2 Nordic studies also reported significant impairment in quality-of-life (QoL) in PV and ET patients during interferon-alpha (IFNα) treatment.16,17
The BFI, FACT-An, and EORTC QLQC30 assessment tools did not capture specific symptoms reported by MF patients relating to cachexia, night sweats, and splenomegaly. Recognizing the need for a specific symptom assessment tool, the Myelofibrosis Symptom Assessment Form (MF-SAF) was developed. Successfully validated as the first MPN-designated patient reported outcome (PRO) tool, MF-SAF is an MF-specific survey instrument and was released in 2009.18 This 20-item tool was validated against the Memorial Symptom Assessment Scale (MSAS), Brief Pain Inventory (BPI) and BFI to capture the most pertinent MPN symptoms on “yes”, “no” or 0 (absent) to 10 (worst imaginable) scales. Assessed symptoms included night sweats, abdominal discomfort, abdominal pain, pruritus, weight loss, fevers, cough, inactivity, fatigue, bone pain, early satiety, and quality-of-life.
In 2012, the MF-SAF was expanded to include symptoms applicable to both ET and PV.19 Now known as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF), this 27-item survey additionally assessed microvascular-related complaints including dizziness, light headedness, insomnia, sexual dysfunction, vertigo, headaches, and numbness/tingling. The form has since been validated in multiple languages (Table 1). The form has been used in a number of important clinical trials including the COMFORT-1 and RESPONSE trials evaluating ruxolitinib in MF and PV, respectively.20,21
In 2013, the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS, also known as MPN-10) was released.22 This abridged version of the MPN-SAF is a 10-item scoring tool containing the most pertinent and representative MPN symptoms allowing for ease of administration in clinical and trial settings. Symptoms include worst fatigue, early satiety, abdominal discomfort, inactivity, itching, concentration problems, night sweats, fever, bone pain, and weight loss. The tool is available in the same languages as the MPN-SAF. A variation of this tool (MPN-SAF TSS Cytokine) was used in the RELIEF study evaluating ruxolitinib in PV.23 The MPN-10 has been recommended by the International working group (IWG) for use in clinical trials.24 Table 1 illustrates the development of the MPN PRO tools and the symptom components assessed.
In 2014, a prospective evaluation of 1470 patients was undertaken to assess whether the heterogeneity observed within MPN subtypes was indicative of distinctive MPN subclusters.25 Five distinct clusters were found within both PV and ET that differed by items such as age, gender, language, laboratory abnormalities, prior hemorrhage, spleen size, and MPN-SAF TSS scores. Notably, neither risk scores nor risk score variables correlated with degree of symptomatology for either PV or ET (Figure 1). In contrast, MF had a total of 4 clusters which, in addition to clinical variables and MPN-SAF TSS scores, demonstrated a positive correlation between risk scores (Dynamic International Prognostic Scoring System; DIPSS) and increasing degrees of symptomatology. The results of this study suggested that the heterogeneity observed within and between MPN subtypes is likely to reflect the variances in genetic and biological activity of the disease, in addition to cultural influences within the MPN population. The lack of association between symptoms and risk scores in ET and PV suggest that symptoms manifest independently of comorbidities and that current risk scoring tools should not be utilized as sole surrogates of disease severity.
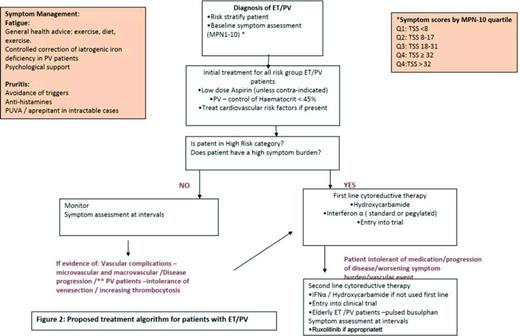
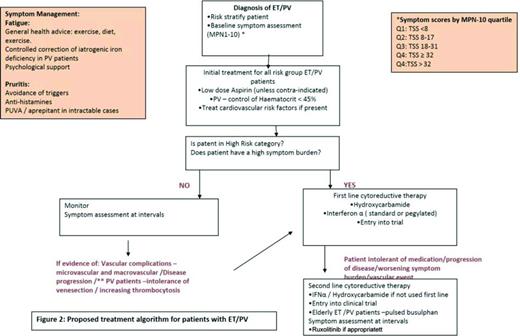
Proposed treatment algorithm for patients with ET/PV. Reprinted from Geyer et al25 with permission.
Proposed treatment algorithm for patients with ET/PV. Reprinted from Geyer et al25 with permission.
Recent focus has turned to understanding how the MPN disease burden is perceived by patients. The MPN Landmark Survey aimed to investigate how MPN patients perceived their disorders as related to symptoms, productivity, QoL and activities of daily living (ADLs).26 This survey of 813 MPN patients found that the majority were feeling anxious or worried about their condition (MF, 91%; PV, 78%; ET, 74%), and reported that MPN-symptoms reduced their QoL (MF, 81%; PV, 66%; ET, 57%). It was noted that patients had reduced work hours, increased sick days, voluntary job termination, receipt of medical disability, early retirement, and negative impact on ADLs. Of note, 25%-33% of low-risk prognostic score patients reported missing at least 1 day of work or cancelling at least 1 day of planned activities, within the previous 30 days prior to this survey being carried out.
The correlation of MPN symptoms with disease features including clinical severity, type of therapy and known MPN associated complications has become an area of increasing clinical importance. MPN-related fatigue has been particularly well studied and correlates with the degree of anemia, transfusion dependency and prior thrombohemorrhagic complications.13 A recent study discovered relationships between the severity of fatigue and education level, BMI, female gender, presence of depression, and active use of alcohol and tobacco.27,28 The role cytokines play in symptom development and severity was evaluated through an analysis of 309 MF patients, where a close relationship was demonstrated between cytokines and the MF symptom burden in JAK2 inhibitor treated patients. Cytokines implicated include VCAM1, leptin, TIMP1, B2MICG, and TNFRII.29
Patient demographics also play a role in the reporting of symptoms. A 2014 study of MPN gender differences identified that females are more likely to vocalize the complaint of fatigue despite being of younger age with lower red blood cell transfusion requirements than their male counterparts.30 Females were also found to struggle with abdominal symptoms independent of the degree of abdominal thrombosis, suggesting that microthrombosis and macrothrombosis likely operate independently. Interestingly, females demonstrate higher individual and total (PRO) scores but these do not correlate with risk category or overall QoL.
Studies evaluating MPN symptoms of insomnia and intimacy-related issues found them to correlate closely with other MPN symptoms and functional domains.31,32 Fatigue remains the most closely scrutinized symptom given its high frequency and impact (independent of MPN subtype) and numerous studies are ongoing to delineate its pathophysiology and potential therapeutic options.
Integration of the symptom assessment tools into a meaningful prognostic marker requires further evaluation. The MPN Quartile Study successfully utilized the following quartiles to predict symptomatic changes: MPN-10 Q1:<8; MPN-10 Q2: 8-17; MPN-10 Q3: 18-31; MPN-10 Q4: ≥ 32.33 Partitioning MPN-10 scores into quartiles offers quantitative objective evidence of symptom improvement or intensification.
The role of cytokines in PV symptoms
Initially noted by Sir William Dameshek in 1951, the development of MPN disorders is driven by “a hitherto of undiscovered stimulus,” since acknowledged to be a complex array of myelostimulatory mutations propagating downstream effects through a cascade of inflammatory markers. The JAK2V617F mutation, present in PV and ET was the first characterizing mutation recognized within the MPN disorders and has been found to be integral to the signaling of cytokines and growth factors, particularly those that lack intrinsic kinase activity. In ET and PV, cytokine analysis has demonstrated that these disorders are associated with significantly elevated levels of IL-4, IL-6, IL-8, IL-11, hepatocyte growth factor (HGF), granulocyte macrophage-colony stimulating factor (GCSF), interferon-y (IFN-y), monocyte chemotactic protein-1 (MCP-1), platelet-derived growth factor-BB (PDGF-BB), vascular endothelial growth factor (VEGF), and tumor necrosis factor-alpha (TNF-a), with specific patterns of elevation allowing for differentiation between the 2 disorders34,35 TNF-a and PDGF-BB have furthermore been found to correlate with the JAK2V617F allele burden in PV and ET, respectively, implying the existence of both JAK2V617F-dependent and -independent inflammatory pathways.35
The role of chronic inflammation in the development of symptoms has been a subject of recent investigation following the release of MPN-specific patient reported outcome tools (MF-SAF, MPN-SAF, MPN-10). Fatigue is recognized to be multifactorial in origin and may be linked to a variety of cytokine-associated conditions including anemia, hypocortisolism, depression, and sedentary lifestyles.35-39 Abdominal-related complaints (early satiety, abdominal discomfort/pain) has been largely attributed to structural problems such as splenomegaly, splenic infarcts, portal hypertension, and intestinal obstruction. In MF, the presence of splenomegaly has been associated with a particular array of cytokines including monokine-induced by gamma interferon (MIG), HGF, and IL-1RA.40 A recent study of JAK2V617F positive cells identified TNF-a to promote clonal expansion which may contribute to extramedullary hematopoiesis.41,42 In PV and ET, major thrombotic events have been associated with high levels of C-reactive protein and low rates of pentraxin 3, correlating to JAK2V617F allele burden.41 Constitutional symptoms produce a heavy burden in MPN patients and have been shown to relate to levels of IL-1, IL-2, TNF-alpha, and IFN. A recent investigation of MF patients demonstrated an association between elevated levels of IL-8 and constitutional symptoms. Similarly, a review of COMFORT-II trial data showed that lower levels of leptin correlated with weight loss, and high CD40L levels correlated with loss of appetite.43 Additional information is needed to understand how targeted treatments effect cytokine production and the impact these changes have upon patients/symptom burden.
Management of symptoms in PV and ET
At present there is no curative treatment for ET and PV and therapeutic goals are to control peripheral blood counts, provide adequate management of disease-related symptoms including those due to splenomegaly, reduce the risk of thrombo-hemorrhagic complications, disease progression to post-PVMF/post-ETMF, and to acute leukemia.44
Prognostic tools
A risk-stratified approach is utilized and cytoreductive therapy is usually reserved for patients who are high risk and the currently used prognostic tools are summarized below for patients with PV and ET. These are based on classical parameters of increasing age, impact of a leukocytosis, and prior history of thrombosis, which are all known to impact on survival. Currently none of these utilize symptom burden scores.
Essential thrombocythemia
Developed in 2012, the International Prognostic Scoring System for Essential Thrombocythemia (IPSET) criteria is the most widely used prognostic tool.45 IPSET has been validated to predict both median survival and the occurrence of thrombosis. The criteria utilizes leukocyte count ≥11 × 109/L (1 point), age ≥60 (2 points), and history of thrombosis (1 point) to stratify patients into low-risk (0 points, survival rate not predicted), intermediate-risk (1-2 points, 24.5 year survival), and high-risk (3-4 point, 13.8 year survival).
Polycythemia vera
A variety of prognostic scoring models are available for PV. The International Working Group for Myeloproliferative Neoplasm Research and Treatment (IWG-MRT) algorithm is most frequently employed and incorporates the variables of leukocyte count ≥15 × 10 9/L (1 point), venous thrombosis (1 point), age 57-66 years (2 points) and age ≥67 years (5 points) to assign patients as low-risk (0 points; 26 year survival), intermediate-risk (1 or 2 points, 15 year survival), and high-risk (≥3 points, 8.3 year survival).46
Symptom management
Fatigue is the most commonly reported symptom affecting PV (85%) and ET (72%) patients, is multifactorial, and does not delineate neatly into the classical risk groups as outlined above. It can be one of the most debilitating symptoms and persist despite treatment in high risk patients; in fact, phlebotomy treatment for PV and the resultant iatrogenic iron deficiency can exacerbate it. The symptom may be traced to a variety of other origins including deconditioning, microthrombosis within cardiopulmonary vasculature, depression, and even MPN therapies. In a study of 405 PV patients and 304 ET patients, the presence of anemia was found to be associated with a stepwise increase in fatigue, along with other symptoms including fever and weight loss.13 Patients find clinician acknowledgment that fatigue is a common symptom known to be associated with ET and PV to be helpful. General medical advice on diet, lifestyle, and exercise to help alleviate fatigue can be useful. In our practice, we have found working very closely with our clinical psychology and physiotherapy teams in helping those patients with extreme fatigue learn how to manage their MPN and symptoms, has been invaluable. Prescription energy stimulants, including methylphenidate and modafinil, have been utilized by up to 18% of MPN patients but solid evidence supporting their efficacy is lacking.47
Pruritus, described as generalized itching, prickling, or burning, is present in up to 65% of the PV population and is a known contributor to poor QoL despite recent studies identifying it as a favorable risk factor for survival.19,46 The symptom may be triggered by a variety of stimuli including water exposure (aquagenic pruritus), physical activity/sweating, alcohol consumption, or even temperature shifts.47 The symptom in itself may also be associated with a variety of other symptoms including emotional stimulation, anger, hostility, aggression, embarrassment, and even suicidal ideation. Although the pathophysiology of pruritus remains unknown, mast cells and basophils are thought to play a contributory role. Recent studies have identified that the number of constitutively activated and hypersensitive circulating basophils is increased in PV and further more that the presence of these cells correlates with degrees of aquagenic pruritus.48 Other studies utilizing infrared thermography have suggested that mast cell degranulation is induced by temperature changes and release greater levels of pruritogenic factors including histamine, leukotrienes, and interleukin, in comparison to patients without MPN disorders.49 Additionally, skin biopsies of PV patients have identified significantly increased mast cells in both pruritic and non-pruritic skin areas in PV patients describing this symptom.50 Therapy is required in at least 17% of the PV population and many combinations of treatments have been tried with variable measure of success. These have involved avoiding triggers such as warm showers/baths and trying to control environmental/body temperatures using various lotions and emollients. In PV, iron deficiency has been thought to play a role and replacement has been suggested to provide benefit in some populations. A retrospective study of 397 PV patients with a documented history of pruritus demonstrated that the presence of this symptom was significantly associated with a lower mean corpuscular volume (MCV) and higher leukocyte count.47 However, controlled iron supplementation for pruritus remains controversial as not all studies have demonstrated efficacy. The use of antihistamines and serotonin reuptake inhibitors, such as paroxetine, have shown benefit. In extreme cases, narrow band ultraviolet B phototherapy (PUVA) has been clinically useful for a few patients. A German study of 441 PV patients suffering from acquagenic pruritis reported patients with pruritis experience a significantly worsened QoL than those with no pruritis, (p = 0.0007) as measured using the EORTC QLQ30.37 This worsening in QoL was associated with reduced function in cognitive (p = 0.0014), emotional (p = 0.0028) and social (p<0.00010) domains with 50.5% of patients stating that their pruritic symptoms didn't benefit or were worse after PUVA therapy. Aprepitant is a potential novel therapy for intractable pruritis.38
Sexual dysfunction is an often overlooked debilitating PV symptom described by up to 57% of both male and female patients.19 Sexual-related concerns may occur within stages of arousal or relate to emotional intimacy. Critical to understanding the basis of sexuality related complaints is a recognition that male and female symptoms differ within the PV population. A recent investigations into PV gender heterogeneity determined that fatigue was a more common symptom among females than males, despite the female study population having lower transfusion requirements and being of younger age than their male counterparts.30 Females also tend to describe more abdominal discomfort, independent of the presence of abdominal thrombosis. Emerging data suggests that PV and ET patients may have unique “subtypes” or “clusters” within the disorders, characterized by sexuality-dominant complaints. Within these clusters, sexual dysfunction is reported disproportionately higher than other MPN symptoms and is expressed primarily in intermediate-risk patients with high rates of anemia.25 A recent investigation of 1908 MPN patients identified that 64% struggled with intimacy/sexuality related concerns and that symptoms were severe in 38% of the population.32 Sexuality concerns have been correlated with the presence of anemia, leukopenia, thrombocytopenia, and the need for transfusions. Furthermore, it has been found to correlate with emotional, cognitive, physical, and social functioning domains. Of particular interest is recent evidence linking sexuality-related complaints to other symptoms including insomnia, depression, night sweats, and quality-of-life. The pathophysiology appears complex and correlates to both biologic and psychologic factors. In addition to complications induced by arterial and venous thrombotic events, endothelium-dependent, flow-mediated vasodilatation has also been found to be impaired in PV patients without clinical evidence of arterial disease.53 Nitric oxide (NO)-mediated vasodilatation has been found to be impaired in PV patients. NO plays a role in both dilating vessels as well as protecting the intima from platelet aggregation and leukocyte adhesion. To date, no studies have investigated specific therapies for sexuality-related complaints.
Thrombotic risk: prevention of microvascular and macrovascular events
All patients should be assessed for cardiovascular risk factors such as diabetes, hypertension, hypercholesterolemia, and smoking. If present, these should be managed appropriately. Those patients who present with, or have developed a thrombosis post-diagnosis, should be treated with an appropriate anticoagulant. Initial management for all PV and ET patients should include daily low-dose aspirin therapy (unless contraindicated) or antiplatelet agents to prevent microvascular symptoms, such as erythromelagia, migraines, headaches, and paresthesia. Aspirin remains standard treatment for both disorders. Notably, a 2013 Cochrane analysis determined that the use of low-dose aspirin, in comparison to no treatment, is associated with a statistically nonsignificant reduction in the risk of fatal thrombotic events and all-cause mortality without increased risk of major bleeding in patients with PV without clear indication or contraindication to aspirin therapy.54 In our clinical practice, we use 75mg aspirin daily and have found that doubling the dose in patients with persistent microvascular symptoms can be beneficial but there is need to monitor for gastric and hemorrhagic side effects. Evidence from the ECLAP study55 demonstrated the efficacy of low dose aspirin in PV as primary prophylaxis (100 mg once daily). However, in ET the use of low-dose aspirin is controversial and caution should be applied in ET patients with platelets counts >1000 × 109/L who have an increased risk of bleeding due to the possibility of acquired Von Willebrand's disease. There is observational data on low-risk ET patients suggesting aspirin may not be beneficial,56 however, this has yet to be fully addressed in a randomized control trial and the final results from the PT1 trial will be informative in due course.
PV patients should additionally have hematocrit levels controlled to <45% in both males and females, as reported in the CYTO PV study.40,41 In some patients, despite carefully controlled hematocrit levels and aspirin but with persistent symptoms, we have occasionally found that venesection to a lower hematocrit level (0.43/42) may make a difference. Caution needs to be applied and patients monitored for severe iron deficiency as a contributory factor to increasing fatigue. The latter can sometimes be improved with a short course of iron supplementation, being careful to balance the need for phlebotomy requirements.
High-risk patients
PV/ET patients who fall into the high-risk categories need to be considered for cytoreductive therapy to control either the blood counts or increasing spleen size. In addition for low risk PV patients unable to tolerate phlebotomy who have progressive leukocytosis and thrombocytosis (>1500 × 109/L) or splenomegaly with persistent symptoms should be eligible for cytoreduction. First line cytoreductive therapy is controversial in PV and ET. The mainstays of treatment remain hydroxycarbamide and interferon-α (IFN-α). Anagrelide (alone or in combination) is an option for ET patients (7 and 8). In recent years, the use of IFN-α, in particular the pegylated formulation has demonstrated efficacy in patients with PV and ET, by inducing clinical responses with a reduction in venesection requirements and reduction in the JAK2V617F allele burden.58,59 Fatigue and flu-like symptoms associated with IFN-α use often lead to discontinuation by patients; however, the pegylated formulation appears to be better tolerated. It is important to note that ∼25% of patients may develop resistance or intolerance to hydroxycarbamide60 and this identifies a cohort of patients with a poor prognosis. Criteria for intolerance include leg ulcers, aphthous ulcers, acne, gastrointestinal symptoms, pneumonitis, and fever. Recurrent aphthous ulcers can be very troublesome. We have found some patients benefit from treatment with acyclovir for single episode or prophylactic acyclovir long-term in patients with recurrent oral ulceration.
International phase III trials are being conducted to evaluate the safety and efficacy of low-dose IFN-α versus hydroxycarbamide in patients with high risk ET, PV, and MF incorporating MPN QOL PROs: MPD RC112, a phase 3 randomized study of hydroxycarbamide versus Pegasys in newly diagnosed high-risk ET and PV patients and PROUD-PV with AOP IFN in PV. Results of these 2 trials are eagerly awaited to address the important questions regarding the relative benefits, efficacy, and toxicities of these 2 agents. Second-line agents used in PV and ET include busulphan (caution as increased cumulative leukemogenic risk with hydroxycarbamide) and anagrelide either as a single agent or in combination with hydroxycarbamide for ET.
In December 2014, the FDA approved the JAK2 inhibitor ruxolitinib for use in PV patients resistant to or intolerant of hydroxycarbamide based on the results of the RESPONSE trial, a phase 3 prospective study randomizing patients with phlebotomy-dependant PV and splenomegaly to ruxolitinib versus best available therapy (BAT).61 Ruxolitinib demonstrated rapid and durable clinical benefits for these patients. The composite primary end point of hematocrit control without phlebotomy from week 8 to 32 (with ≤1 venesection from week 0 to 8) and a ≥35% reduction in MRI spleen volume from baseline was reached by 21% of ruxolitinib patients compared with 1% for those in the BAT arm (p < 0.0001).The responses were also durable with 91% of ruxolitinib patients who achieved the primary endpoint maintaining the response at 48 weeks. 60% of the patients treated with ruxolitinib achieved haematocrit control without phlebotomy compared with 20% on the BAT arm. Reduction in spleen volume by ≥35% was seen in 38% of ruxolitinib treated patients compared with 1% in the BAT arm. MPN-SAF scores were evaluated at week 32 with 49% of the patients with ruxolitinib reporting a ≥50% improvement in MPN-SAF 14 item TSS compared with 5% in the BAT group. Hematologic toxicities reported grade 3/4 anaemia (0% and 1.8% in ruxolitinib and BAT arms, respectively) and grade 3/4 thrombocytopenia (5.5% and 3.6% in ruxolitinib and BAT arms, respectively).
The results of the RESPONSE trial suggest that PV patients with the features of splenomegaly, phlebotomy dependence, and resistance or intolerance to hydroxycarbamide represent the ideal candidate for JAK2 therapy. However, recent studies have identified that PV patients with even just one of these features still suffer from significant symptom burden. As a result of that symptom, an additive relationship exists between the number of features present and the degree of symptomatology expressed.62 ET and PV patients who remain symptomatic despite appropriate cell counts should be considered for clinical trials using JAK2 inhibitors or other novel agents. It should be noted that the current evidence demonstrates that patients who are intolerant of first-line therapy should benefit from ruxolitinib treatment with significant symptomatic relief but it is still too early to comment on the long-term disease-modifying effect of this agent for these patients. Recent data from a cohort of patients in the COMFORT-II study demonstrated complete molecular remissions in 3 patients treated with ruxolitinib.63
Additional novel targeted therapies are currently being evaluated in clinical trials for these poor-risk ET/PV patients, such as histone deacetylase (HDAC) inhibitors, JAK1/2 inhibitors, and heat-shock protein-90 (HSP-90) inhibitors. Time will provide evidence of their efficacy in the management of PV and ET patients with respect to symptom and disease control.
Summary
Substantial advancements have been made over the past decade in our understanding of the MPNs at a molecular level, with the identification of pathogenetic mutations (JAK2, CALR, and MPLW), poor prognostic associations with ASXL-1, EZH, IDH1, IDH2, and SRF2 mutations. Two distinct clinical phenotypes in PV patients have been identified using gene profiling in stem cells with cytokine mapping which provide an insight into the heterogenity of the disorder and may aid future treatment algorithms.64 This rapidly expanding evidence base in the field has led to the review of current risk stratification tools to help inform patient outcomes and treatment. In parallel, the development of validated MPN specific patient reported outcome tools (PRO) have been invaluable in providing objectively measurable symptom assessment. These can be utilized in practice at the time of diagnosis and throughout the patients' clinical journey. In clinical trial settings, MPN PROs have allowed for the rigorous evaluation of novel agents via objective, systematic comparisons of direct patient experiences. Data obtained from international collaboration within the MPN directed research consortia, such as the MPN-QOL Research Study Group comprising of 18 countries and over 70 researchers, is yielding a rich source of data and evidence to enable us to gain a better understanding of how to utilize and incorporate these PRO tools to benefit MPN patients. In the long-term, integration of these PRO tools within prognostic systems will lead to a more objective and holistic approach to personalized patient therapy. Data from the RESPONSE trial has offered encouraging evidence that hydroxycarbamide-resistant or intolerant PV patients with splenomegaly and phlebotomy-dependence may benefit from ruxolitinib treatment from both a symptomatic and QoL standpoint (phlebotomy independence). We eagerly await future investigations that may offer insight into the effects of targeted treatments in symptomatic lower-risk populations. Certainly, the benefits to be gained from reductions in the need for phlebotomy, either by hydroxycarbamide, interferon, targeted treatments, or others, could offer a new degree of freedom to PV patients previously facing the challenges of undergoing this frequent procedure and the symptoms induced by iron deficiency. Reflecting on the substantial achievements of the past decade, it becomes apparent that assessment of symptom burden, international collaboration with the advent of targeted treatments, genetic and cytokine profiling, will continue to be the cornerstone of success to improve the QoL and prognosis in PV and ET patients.
Correspondence
Deepti Radia, Department of Haematology, Guy's and St Thomas' NHS Foundation Trust, Haematology Department, 4th Fl, Southwark Wing, Great Maze Pond, London, UK, SE1 9RT; Phone: 00-440-20-7188-2740; Fax: 00-440-20-7188-2728; e-mail: Deepti.Radia@gstt.nhs.uk.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.