Abstract
Recent insights into the pathogenesis of microvascular occlusion downstream of the sickled red cell have revealed new therapeutic targets for sickle cell disease (SCD). After the formation of sickle cells, tissue injury spurs inflammation, which leads to receptor-mediated contacts between sickle cells, leukocytes, and vascular endothelium. Specifically, selectins decelerate sickled red cells and leukocytes in the circulation to facilitate endothelial adhesion and other cell–cell interactions, ultimately leading to vascular occlusion. Invariant NKT (iNKT) cells, activated during reperfusion, generate a broad inflammatory response, which further increases cellular adhesion and vascular occlusion. Novel therapies are in development that target selectins and iNKT cells to prevent or interrupt the vicious cycle of adhesion and inflammation. Although the therapies hold promise for the treatment of SCD, an underappreciated threat to their development is poor access to care for people with SCD. Unless the majority of people with SCD have access to consistent, high-quality care, they will not have the opportunity to participate in a clinical trial or receive any new therapy, regardless of its efficacy.
Learning Objectives
To contrast the advantages and disadvantages of novel anti-selectin and invariant NKT cell therapies for the treatment or prevention of vascular occlusion in sickle cell disease
To recognize the effect of poor access to care on the ability to examine and implement new therapies for people with sickle cell disease
Sickle cell disease (SCD) is first and foremost a disease of red cells. A single amino acid substitution in the β-globin chain produces abnormal hemoglobin which, under conditions of hypoxia, polymerizes and causes red cells to assume a sickle shape. Sickle red cells are rigid, non-deformable, and occlude the microvasculature, causing acute and chronic organ damage in affected people. In the process of vascular occlusion (VO), however, sickle cells do not act alone. Interactions with leukocytes, endothelial cells, and platelets are critical for sickle cells to cause VO.1,2 From the recognition of VO as more than just a “log jam” of sickle cells, new therapeutic targets have emerged. Of these, selectin adhesion molecules and a type of lymphocyte called an invariant NKT (iNKT) cell have been identified as particularly promising targets for SCD therapy.1,3 The objective of anti-selectin or iNKT cell therapy is to disrupt the series of events downstream of sickle cell formation, where a vicious cycle of adhesion, inflammation, and VO occur.4,5 Neither approach will stop the initial formation of a sickle cell, but they may prevent, or at least dampen, the severity of a pain episode.
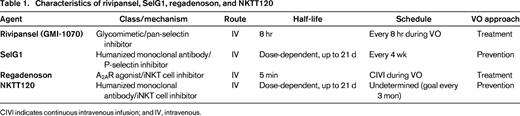
The focus of this article is on four new therapeutic agents currently under evaluation in clinical trials for the treatment of SCD. Two of the agents target selectins: rivipansel (formerly GMI-1070) and SelG1; the other 2 target iNKT cells: regadenoson and NKTT120. All of these novel therapies have generated excitement in the field of SCD based on strong preclinical and clinical data. The question on everyone's mind, of course, is whether or not any of the new therapies will work; SCD therapies have a dismal track record of success. This article provides a discussion about the rationale, current state of data, and pros and cons for each therapy. A discussion limited to the scientific merit of the therapies, however, is not sufficient to determine whether they will actually help people with SCD. Other factors are at play. Hydroxyurea, the only available treatment for SCD, is still vastly under-utilized nearly 20 years after FDA-approval.6 Why would any new therapy be expected to fare better? The realities of people who live with SCD and the challenges posed to the development of new therapies will also be discussed. If these issues are not addressed, neither anti-selectin nor iNKT cell therapy will be very impactful in clinical practice, regardless of their efficacy in clinical trials.
Selectins
Selectins are a family of adhesion molecules comprised of three subtypes (L-, P-, and E-selectin) involved in cell migration and activation.7 P-selectin (expressed on platelets and endothelial cells) and E-selectin (expressed on endothelial cells) have been shown to mediate the adhesion of sickle cells and leukocytes to endothelium, an initial step in VO.1,8 The key function of P- and E-selectin is to slow down leukocytes in the circulation and bring them into close proximity with integrin molecules, which facilitate firm adherence to the endothelium. Once stationary on the endothelium, leukocytes are able to migrate outside of the vasculature to sites of inflammation.
Although P-selectin and E-selectin both act to decelerate leukocytes, important biologic differences between the 2 selectin molecules may affect their ability to interrupt VO when blocked. Stored in Weibel-Palade bodies, P-selectin can be rapidly expressed on endothelial cells and is generally thought to be the instigator of leukocyte deceleration.9 Once expressed, P-selectin has been shown to directly bind to sickle cells, in addition to leukocytes.8 Consistent with this observation, sickle cell and leukocyte adherence to the endothelium was reduced when P-selectin was blocked in mice.10,11 Because P-selectin is also expressed on platelets, its blockage may interrupt cell–platelet interactions as well.2 In contrast to P-selectin, E-selectin is not known to directly bind sickle cells and is not preformed in endothelial cells.12 E-selectin must be transcribed, which may delay its expression on endothelial cells for several hours after activation.13 E-selectin, therefore, likely is involved in a later step in the leukocyte–endothelium interaction, when leukocyte velocity slows and they begin to adhere to the endothelium.14 The close interaction between E-selectin and leukocytes may facilitate signaling that stimulates the expression of integrin receptors on leukocytes, potentially an important step in VO. In recent studies, E-selectin has been shown to promote neutrophil expression of macrophage-1 antigen (MAC-1), an integrin that is able to bind sickle cells.12 Whether E-selectin's ability to promote MAC-1 expression on neutrophils or P-selectin's ability to be rapidly expressed on endothelial cells has therapeutic implications remains to be seen. Animal and human data support a role for both molecules in the adherence of sickle cells and leukocytes to the endothelium in VO.1,11 Two anti-selectin drugs are currently in development for people with SCD, one targeting P-selectin and the other predominantly E-selectin.
Rivipansel (GMI-1070)
Rivipansel, formerly known as GMI-1070, is a pan-selectin inhibitor (Table 1).4 Although the compound has activity against L-, P-, and E-selectin, the inhibition of E-selectin is ∼100 times stronger than for L- or P-selectin. In initial studies of SCD mice stimulated with tumor necrosis factor alpha (TNFα), rivipansel increased leukocyte rolling velocities, decreased adhesion to endothelium, and increased blood flow.4 Rivipansel's short half-life (∼8 hours) limits its use to an acute pain event, so another important finding was that the beneficial effects of rivipansel were present when it was administered after the onset of VO. One potential limitation of the work was the use of TNFα to promote VO. TNFα is a known stimulator of not only VO, but of E-selectin expression on endothelial cells.15 Thus, rivipansel's target may have been overexpressed in this particular model.
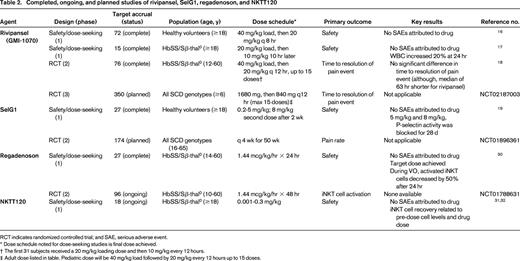
Pharmacokinetic and safety data for rivipansel was determined from studies of healthy volunteers and people with SCD (Table 2).16,17 The pharmacokinetics of the drug was similar between healthy volunteers and people with SCD. The only notable event was a 20% increase in white blood cell count 24 hours after SCD subjects were administered rivipansel. Potentially, blockage of E-selectin prevented the extravasation of leukocytes from the circulation, which could explain the increase in white blood cell count. No adverse events were associated with the increased white blood cell counts. Rivipansel was associated with a reduction in the neutrophil activation marker, MAC-1, as well as markers of endothelial activation and coagulation.17
A phase 2 trial to determine the efficacy of rivipansel in decreasing the severity of a pain event in children and adults with SCD was recently completed (Table 2).18 The primary outcome measure was time to resolution of a pain event defined as an improvement in the visual analog scale of 1.5 cm compared to pre-drug and transition to oral pain medicines, or documentation of readiness for discharge. Subjects were dosed within 24 hours of admission to the hospital and were then treated with a maximum of 15 doses or until resolution of the pain episode. The primary analysis did not show a difference in time to resolution of the pain event between the treatment and placebo groups, even though the duration of hospitalization was reduced in the rivipansel group by a median of 63 hours (P = 0.19). In secondary analyses, subjects treated with rivipansel required significantly less intravenous opioids than the placebo group.
The phase 2 trial of rivipansel highlights the difficulties of a study aimed to improve an acute pain event. Ironclad endpoints for the determination of resolution of a pain event are a huge challenge. Differences in physician practice about readiness for discharge, differences in patient's ability to transition to oral pain medicines, and psychosocial factors, such as homelessness or mental illness, may all impact outcomes. A phase 3 trial of rivipansel is planned (clinicaltrials.gov NCT02187003; Table 2).
SelG1
SelG1 is a humanized monoclonal antibody directed against P-selectin (Table 1).19 As opposed to rivipansel, which aims to treat an acute pain event, SelG1 would be administered on a scheduled basis in order to prevent pain events. The safety, pharmacokinetic and pharmacodynamic properties of SelG1 were determined in a phase 1 study of healthy volunteers (Table 2).19 In the 2 highest-dose cohorts, P-selectin activity was blocked for at least 28 days. All adverse events in the study were mild to moderate and none were attributed to the drug. Investigators have now announced a phase 2 trial, named the SUSTAIN trial, to determine the safety and efficacy of SelG1 in people with SCD (clinicaltrials.gov NCT01896361; Table 2).
iNKT cells
iNKT cells are a unique type of lymphocyte with features of innate immune cells, which are able to be rapidly activated and promote a larger immune response.20 Although iNKT cells comprise only 0.01%-1% of lymphocytes in the circulation, certain characteristics, such as an invariant T cell receptor (TCR) and stored, preformed cytokines, allow them to have an important role in the immune response, disproportionate to their small numbers. Unlike conventional T cells, which have a diverse TCR repertoire and recognize unique peptides, iNKT cells express an invariant TCR (Vα14Jα18 in mice, Vα24Jα18 in humans) and recognize the pattern of a lipid antigen, akin to innate immune cells. iNKT cells can also be activated without TCR engagement by cytokines or through toll-like receptor signals, another common feature of innate immunity.21 Within 2 hours of activation, iNKT cells release copious amounts of interferon gamma and interleukin 4, which are translated from mRNA stored in the cytoplasm, as well as other cytokines, including TNFα.22 The net effect is the activation of B cells, T cells, NK cells, neutrophils, and endothelial cells.22 iNKT cells' ability to generate inflammation was implicated in ischemia-reperfusion injury after solid organ transplant, which led to investigations in SCD.23
Data from mice and people with SCD provided preliminary evidence for a role of iNKT cells in VO.3,5 iNKT cells were more likely to be activated in mice and people with SCD compared with controls.3 In mice, interruption or depletion of iNKT cells with an adenosine A2A receptor (A2AR) agonist or an anti-NKT cell antibody decreased inflammation and tissue injury after ischemia-reperfusion (hypoxia-reoxygenation).3,5,24 When the role of iNKT cells is further investigated in people with SCD, a consideration will be the difference in iNKT cell distribution between mice and humans. As an example, iNKT cells comprise 30% of T lymphocytes in the livers of mice compared to <5% in humans.25 These differences may produce more dramatic effects in mice than people with SCD when iNKT cells are targeted. Ongoing investigations in people with SCD with the A2AR agonist, regadenoson, and the anti-iNKT cell antibody, NKTT120, will determine whether the differences in iNKT distribution between mice and humans are relevant to VO.
Regadenoson
Regadenoson is a highly specific A2AR agonist (Table 1).26 Adenosine signaling has an organ-protective effect through actions on 4 unique adenosine receptors (A1, A2A, A2B, and A3).27 After release from injured tissue, adenosine binds to receptors and promotes a slowed physiology with increased blood flow and decreased inflammation. The particular physiological response to adenosine is dependent upon the particular receptor engaged and the cell type on which the receptor is expressed. A2ARs are richly expressed in the coronary vasculature, where they induce vasodilation and cardiac hyperemia. Based on the actions of A2ARs in the coronaries, regadenoson has an FDA approval for use in myocardial imaging.26 Unlike intravenous adenosine, which acts on all of the adenosine receptors, regadenoson's specificity for the A2AR allows for vasodilation with fewer unwanted side effects (bradycardia, bronchospasm). The benefits of A2AR agonists, however, are not limited to the echo lab, as regadenoson also has potent anti-inflammatory effects. A2ARs are ubiquitously expressed on neutrophils, macrophages, leukocytes, and in particular, iNKT cells, which express high levels of A2ARs and show exquisite sensitivity to A2AR activation.28 Importantly, the anti-inflammatory effects of A2ARs are 10-100 times more potent than the cardiovascular effects.23 A low-dose infusion of regadenoson could therefore be administered during a pain event and achieve a decrease in inflammation without cardiovascular toxicity.29
To investigate the effects of A2AR activation on iNKT cells in adults with SCD, a phase 1 dose-finding and safety study of infusional regadenoson was performed (Table 2).30 The target dose of 1.44 mcg/kg/hour was achieved without cardiovascular toxicity, likely because the peak plasma concentration of regadenoson was much lower than the concentration observed for bolus dosing (2 ng/mL vs ∼16 ng/mL). In a subgroup of 6 subjects who received a 24 hour infusion of regadenoson during a pain event, the percent of highly activated iNKT cells decreased by 50% to levels similar to steady-state patients and healthy controls. A multicenter, phase 2 trial of infusional regadenoson for the treatment of an acute pain event is now underway (clinicaltrials.gov NCT01788631; Table 2). At the time this article was prepared, accrual was >50% toward goal.
NKTT120
NKTT120 is a humanized monoclonal antibody directed against the invariant TCR of iNKT cells (Table 1).31,32 Even though NKTT120 and regadenoson both target iNKT cells, they use different approaches. NKTT120 is administered prophylactically to prevent pain events, whereas regadenoson is given acutely. For NKTT120 to prevent a pain event, a longer-term reduction of iNKT cells is necessary, which means that they will need to be reduced in tissue, not just the circulation. If iNKT cell reduction is limited to the circulation, they will simply equilibrate back from the tissues to the circulation after only a few days.31 Preliminary evidence of NKTT120's ability to reduce iNKT cell number and prevent vascular occlusion was provided in mice with a similar monoclonal antibody (NKT-14).24 Mice pretreated with NKT-14 showed less lung inflammation after hypoxia-reoxygenation versus controls. Human studies of NKTT120 are now underway.
A first-in-man, phase 1 study of NKTT120 is being performed in adults with SCD (Table 2).31,32 The study is closed to accrual and preliminary results have been presented. The goal of the study was to identify a safe and efficacious dose to reduce iNKT cells in the blood and tissue to facilitate every 3 month dosing. To date, the results of 18 adults in 6 dose cohorts (up to 0.3 mg/kg) have been reported. Preliminary results show a reduction in iNKT cell number in the circulation within 6 hours for all dose cohorts.31 In general, subjects in higher dose cohorts and those with lower levels of circulating iNKT cells took a longer time to recover iNKT cells.31
The recovery of iNKT cells after NKTT120 is likely related to reduction in tissue stores. Once tissue stores are reduced and the drug is metabolized, recovery is then based on the generation of new iNKT cells. Not surprisingly, higher doses of NKTT120 were, in general, associated with longer recovery times. A slower recovery of iNKT cells, however, was also associated with a lower number of iNKT cells in circulation. This suggests a correlation between iNKT cell number in the circulation and tissue. Ideally, though, dose adjustments for NKTT120 would not be based on the number of iNKT cells in the circulation, since iNKT cell measurements are not standard. Once the phase 1 study is complete, a phase 2 study to determine NKTT120's effect on the frequency of pain events is planned.
An appraisal of downstream approaches
In theory, strategies that block targets downstream of the sickle cell itself are suboptimal because, by definition, they concede some of the sickle cells' ill-effects (hemolysis, VO, organ dysfunction). So, why bother to examine targets like selectins and iNKT cells? Why not concentrate scarce resources on curative approaches and strategies to prevent the formation of sickle cells, especially in light of recent advances in stem cell transplantation,33,34 gene therapy,35 and fetal hemoglobin regulation?36 Even though a cure (or near-cure) may be closer at hand than in the past, many challenges remain before these therapies are available. Although these therapies progress in development, hydroxyurea is the only drug available for people with SCD. Any efficacious treatment would add to the limited toolbox of options currently available to treat people with SCD.
If >1 therapy is found to be efficacious, combinations of therapies should be examined. The best treatment strategy to overcome the complex pathophysiology of SCD may be to target different points in VO. Potentially, a multimodal approach to prevent the formation of sickle cells along with a downstream therapy, such as SelG1 or NKTT120, may be more efficacious than any therapy alone.
Pros and cons of anti-selectin and iNKT cell therapies
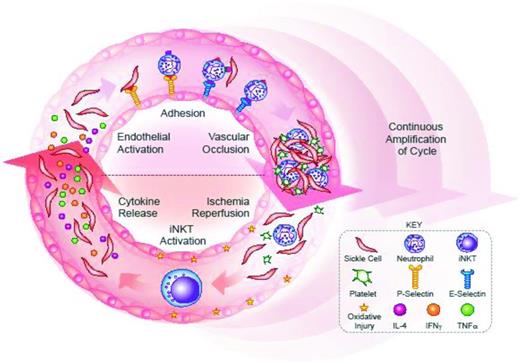
The theoretical advantage of an upstream target cannot be used to assess the likelihood of success for selectin versus iNKT cell therapies, because the exact order of events in VO is unknown. In vivo, the ability of selectins and iNKT cells to promote VO may be inter-related. Inflammation can stimulate the expression of P- and E-selectin on the endothelium.1 Because iNKT cells promote a broad inflammatory response, the blockage of iNKT cell activity may prevent selectin expression.20 Alternatively, iNKT cells are activated by ischemia-reperfusion, a process that occurs after the formation of an occlusive clot.3 If blockage of selectins prevents VO, there will be less ischemia-reperfusion and likely fewer activated iNKT cells. The process of VO may be better viewed as a cycle, as opposed to a series of events (Figure 1). With every turn of the cycle, the actions of selectins and iNKT cells promote sickle cell formation and amplify the process of VO.
VO is a vicious cycle. Formation of sickle cells is the first step in VO. Thereafter, sickle cells interact with leukocytes, endothelial cells, and platelets to form an occlusive clot. Downstream of the occlusion, hypoxia then causes the formation of more sickle cells. Once the clot is broken up, ischemia-reperfusion produces oxidative injury, which further activates cells and promotes VO. Thus begins a cycle of adhesion, inflammation, and VO, wherein more sickle cells are generated and the process of VO is amplified with each turn of the cycle. Selectins and invariant NKT (iNKT) cells are 2 critical elements of the VO cycle. (Top) P-selectin binds sickle cells and leukocytes, representing the first step of adhesion. In a later step of adhesion, E-selectin binds leukocytes and may induce activation signals to promote the capture the sickle red cells. The end result of P-selectin and E-selectin actions is an occlusive clot. (Bottom) Ischemia-reperfusion, after clot break up, activates iNKT cells, which produce numerous cytokines, including interferon gamma (IFNγ), interleukin 4 (IL-4), and tumor necrosis factor alpha (TNFα). The iNKT cell-generated cytokines activate and attract leukocytes, and may activate the endothelium to express selectins. TNFα, in particular, promotes the up-regulation of E-selectin.
VO is a vicious cycle. Formation of sickle cells is the first step in VO. Thereafter, sickle cells interact with leukocytes, endothelial cells, and platelets to form an occlusive clot. Downstream of the occlusion, hypoxia then causes the formation of more sickle cells. Once the clot is broken up, ischemia-reperfusion produces oxidative injury, which further activates cells and promotes VO. Thus begins a cycle of adhesion, inflammation, and VO, wherein more sickle cells are generated and the process of VO is amplified with each turn of the cycle. Selectins and invariant NKT (iNKT) cells are 2 critical elements of the VO cycle. (Top) P-selectin binds sickle cells and leukocytes, representing the first step of adhesion. In a later step of adhesion, E-selectin binds leukocytes and may induce activation signals to promote the capture the sickle red cells. The end result of P-selectin and E-selectin actions is an occlusive clot. (Bottom) Ischemia-reperfusion, after clot break up, activates iNKT cells, which produce numerous cytokines, including interferon gamma (IFNγ), interleukin 4 (IL-4), and tumor necrosis factor alpha (TNFα). The iNKT cell-generated cytokines activate and attract leukocytes, and may activate the endothelium to express selectins. TNFα, in particular, promotes the up-regulation of E-selectin.
There may not be a theoretical advantage in targeting selectins over iNKT cells, or vice versa, but there are potential advantages to the different therapeutic approaches. Rivipansel and regadenoson treat the acute pain event, whereas SelG1 and NKTT seek to prevent the pain altogether. The acute approaches limit the duration of exposure to the drug, which could be important because selectins and iNKT cells have important roles in immunity.7,20 Long-term blockage of P-selectin with SelG1 or iNKT cells with NKTT120 carries a potential risk of infection. In terms of SCD pathophysiology, however, the prevention of a pain event is a better approach than the treatment of an acute one. When a person with SCD presents with an acute pain event, VO is already disseminated, and adhesion molecules and inflammation are already up-regulated.30 The prevention of a pain event is likely easier than the reversal of one that has already begun. Moreover, even if rivipansel or regadenoson decreases the length of hospital stay, the patient has already begun to suffer pain, and potentially, some degree of organ damage. Nevertheless, no approach with promise should be cast aside. A therapy to reduce the severity of a pain event is still preferable to no therapy at all.
Poor access to care will be a barrier for any new therapy, regardless of efficacy
Anti-selectin and iNKT cell therapies are cause for excitement. Three multicenter clinical trials, the result of novel mechanistic insights into VO and promising early phase clinical data, are planned or already underway. Given the challenges of identifying new therapies for VO, a demonstration of efficacy in any of these trials would be a major scientific advancement. However, a discussion about the science behind these approaches is less meaningful if anti-selectin and iNKT cell therapies do not reach people with SCD. The introduction of hydroxyurea into clinical practice has exposed barriers to the effective use of therapies over the last several years.6 Although many factors have been blamed for hydroxyurea's limited use, most relate to access to care. The best way to guarantee the delivery of new therapies to people with SCD, either in a clinical trial or in practice, is to ensure access to high-quality care. Unfortunately, the medical system is inhospitable to many people with SCD, especially adults. A big reason for this is low reimbursement for hospitals and doctors, although at the individual level, other factors may include poverty, narcotics, and race.37-39
Although the case of every person is unique, the following is a common storyline for a person with SCD. Thanks to newborn screening, penicillin prophylaxis, and good supportive care, almost all children with SCD survive to become adults.40 Childhood is not easy, however, due to pain events, hospital stays, and many days of missed school.41,42 The product of these acute illnesses in childhood is often an adult who is chronically ill, undereducated, and without a steady job or income.43,44 Due to disability or poverty, many adults rely on Medicaid health insurance. Because Medicaid's rate of reimbursement is so low, the financial realities of indigent care then become apparent; many hospitals and physicians simply refuse to shoulder the financial burden.45 Poor reimbursement, however, is not the only barrier to care for adults with SCD. A lack of trust between patients and providers, often due to narcotics and/or race, can also be a major obstacle.46 Many adults with SCD require narcotics for pain. Although misuse of narcotics is a concern for any patient with pain, the situation may be worse in a minority population (African Americans are almost exclusively affected by SCD in the United States).46,47 With or without the issue of narcotics, provider distrust of people with SCD is a pervasive problem.48 The end result is that many adults with SCD, unable to find consistent care, are forced to rely on emergency departments and hospitals for treatment.45,49 These one-time interactions are not conducive to participation in clinical trials or implementation of a new therapy. Unless access to care is improved for people with SCD, the development of new therapies will continue to be a challenge. Even if efficacy is shown in a clinical trial, new therapies may not be very effective in clinical practice.
Correspondence
Joshua J. Field, Medical Sciences Institute, BloodCenter of Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI, 53226; Phone: 414-937-3848; Fax: 414-937-6811; e-mail: joshua.field@bcw.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from NKT Therapeutics and Astellas, and has consulted for NKT Therapeutics.
Author notes
Off-label drug use: Regadenoson is FDA-approved for use during myocardial imaging. I will discuss the investigational use of regadenoson for the treatment of pain episodes in sickle cell disease.