Abstract
Recent reports of recurrent mutations in childhood acute myeloid leukemia (AML) have identified potential targets for new therapeutic strategies. Acute promyelocytic leukemia (APL) is characterized commonly by a fusion between the PML gene and the RARA gene, genes targetable by arsenic (ATO) and retinoic acid (ATRA), respectively. A mutation in GATA1, common in AML of Down syndrome (ML-DS), renders cells more susceptible to cytarabine and anthracyclines, thus permitting targeted dose reductions to preserve high survival rates while reducing toxicity. In all other patients, Ras pathway mutations, KMT2A and other methyltransferase mutations, FLT3 mutations, and KIT mutations are all relatively common in childhood AML and all are potentially “druggable”. The focus of this review is on those therapies likely to be clinically available in the near future. The preclinical and clinical data providing a rationale for testing in children of specific agents in children is discussed. Whether the expression of a potential target is sufficient to predict response to a targeted therapy is an open question in childhood AML. Development of clinical trials to evaluate targeted therapies in small molecularly defined subsets of AML will be the next great challenge for all cooperative groups in North America and Europe.
Learning Objectives
To understand the potential for targeted therapies and targeted therapeutic approaches for children with AML
To understand the current clinical development status of relevant target therapies in childhood AML
To understand the complexities of clinical trial development for small molecularly defined subsets of childhood AML
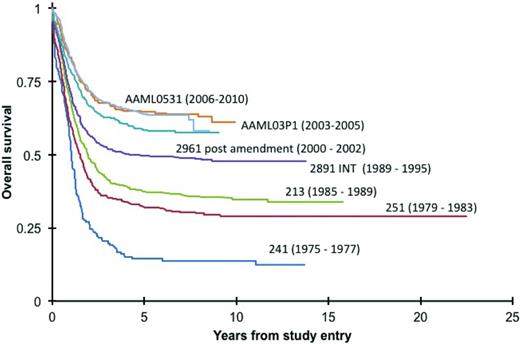
Acute myeloid leukemia (AML) of childhood accounts for ∼20% of childhood leukemia diagnoses. Cure rates are approaching 70% (Figure 1), but at the expense of life-altering long-term side effects from high cumulative anthracycline doses for all patients and bone marrow transplant for many patients. Cytogenetic and molecular events now define distinct subsets of childhood AML. With the availability of increasing specific small molecule and antibody-based targeted therapies, clinical trial development is focusing on identifying novel therapies for molecularly defined subsets of disease. The underlying goal of these efforts will be to reduce the toxicity while maintaining or improving survival.
In COG and legacy trials over the last 40 years, there is incremental improvement in overall survival for children with AML.
In COG and legacy trials over the last 40 years, there is incremental improvement in overall survival for children with AML.
Increasing awareness of the leukemogenic genetic events defining subset of AML provides new opportunities to develop strategies designed to inhibit the underlying events necessary to maintain a leukemic phenotype. However, for the vast majority of mutations, it is not sufficient to assume that target expression will equal a response to targeted therapy. However, for the vast majority of mutations, it is not safe to assume that target expression will equal a response to targeted therapy. This, as discussed below, may be the case in acute promyelocytic leukemia, but it is the exception and not the rule. As whole-genome and whole-exome sequencing become commercially available and less expensive, clinicians and researchers will face the challenge of deciphering and defining actionable data likely to yield a therapeutically relevant target. Although the pace of drug development in children lags far behind the pace of target identification, there is the added challenge of drug availability for children. To realize the potential of targeted therapies in children with AML, clinicians, researchers, industry, and the National Institutes of Health must engage in collaborative, well-controlled trails of molecularly stratified targeted therapies. Recent efforts in cooperative groups across the globe to define the AML genome have been successful and informative. Now the cooperative groups must test the hypothesis that targeted therapies are indeed effective in improving survival and reducing side effects for each molecular subset of AML.
For the purposes of this review, AML will be divided into acute promyelocytic leukemia (APL), myeloid leukemia of Down syndrome (ML-DS), and all other forms of AML. APL and ML-DS are examples of well-characterized, clearly defined molecular subtypes of acute myeloid leukemia addicted to the effect of mutant oncoproteins, thus rendering the cells susceptible to specific therapeutic approaches. With planned trials in Europe and North America in ML-DS and APL, we are moving rapidly toward highly effective therapies designed to take advantage of leukemogenic mutations resulting in unique drug susceptibilities. With an increasing focus on precision medicine, the development of specific targeting strategies in all AML subsets is a high research priority.
With the fusion of the N-terminus of the PML protein with the C-terminus of the RARA transcription factor, the PML-RARA fusion protein effectively blocks promyelocyte differentiation and leads to uncontrolled self-renewal.1 Other variant N-terminal fusion partners for RARA resulting in APL include nucleophosmin 1 (npm1), nuclear mitotic apparatus (numa), promyelocytic leukemia zinc finger (plzf), and others.2 However, with physiologic levels of RA, PML-RARA is an ineffective transcriptional activator,3 resulting in myeloid maturation arrest at the promyelocyte stage.
Historically, conventional chemotherapy approaches including daunorubicin and cytarabine, yielded complete remission rates of 75%-80%, but cure rates were only 35%-40%. As many as 17% of patients died within the first month of therapy due to bleeding complications from disseminated intravascular coagulation (DIC) triggered by the APL blasts (reviewed by Kutny et al4 ). With the introduction of all trans retinoic acid (ATRA) and arsenic trioxide (ATO) cure rates for APL now exceed 90% with greatly reduced early toxic mortality (reviewed by Wang and Chen5 ).
ATRA as a single agent is capable of inducing promyelocyte differentiation, ultimately inducing complete morphologic remission and reduced risk for early coagulopathy-associated mortality. The induction of apoptosis by chemotherapy results in the release of procoagulant and fibrinolytic factors, such as annexin A2 and tissue factor. ATRA markedly up-regulates thrombomodulin expression and decreases tissue factor release, providing a reduction in the incidence and severity of coagulopathy. The mechanism of action of ATRA is dependent on ATRA binding to PML-RARA leading to transcriptional activation of retinoic acid receptor elements, cellular differentiation, and subsequent polyubiquitination with proteolysis of the PML-RARA oncoprotein6
Whereas ATRA acts on the RARA moiety, ATO acts on the PML moiety inducing oxidation of 2 cysteine residues followed by sumoylation of a lysine residue, which ultimately leads to polyubiquitination and proteolysis.7 Together ATRA and ATO permit the critical degradation of PML-RARA through independent targeting of both the PML and RARA moieties in a manner that yields clinical efficacy suggestive of true combination synergy.8 Although not yet studied in children, survival rates in adults when ATO and ATRA are used in combination now exceed 90%. COG will soon study ATO and ATRA in children without concomitant conventional chemotherapy for those children with white blood cell counts <10 000 at diagnosis. This will be the first trial using an exclusively targeted approach to cure an acute leukemia of childhood.
Myeloid leukemia of Down syndrome
Children with Down syndrome (DS) have a 10- to 20-fold higher incidence of leukemia relative to other children.9 Children with DS <4 years of age have a 500-fold increased incidence of AML. The acute megakaryoblastic (AMKL) phenotype accounts for approximately one-half of all ML-DS. AMKL is quite rare in non-DS AML and is typically associated with the t(1;22) translocation, a fusion not seen in ML-DS.10 It is clear that ML-DS is truly unique in presentation and pathogenesis, and it is therefore not surprising that nearly all patients diagnosed prior to age 4 years share a common genetic make up. In 2002, Wechsler et al11 described mutations in GATA1, an essential transcription factor in hematopoiesis, in children with ML-DS. Each of the identified mutations introduces a stop codon resulting in a truncated but functional protein with a deficient or absent amino-terminal activation domain.11 The GATA1 mutation alone is sufficient to cause dysplastic myeloid lineage maturation but not AML, suggesting that Trisomy 21 is also required for leukemogenesis.12,13 Mutations in GATA1 are found in nearly all patients with ML-DS diagnosed prior to age 4 years, but are rare in non-DS AML and in ML-DS in children older than 4 years of age.
Directly targeting GATA1 is not a treatment strategy currently being developed in ML-DS in childhood. However, other approaches may effectively capitalize on the unique molecular and cellular landscape of ML-DS. Trisomy 21 results in increase expression of several genes located on chromosome 21 that are involved in oxidative metabolism, including cystathionine beta synthase (CBS) and zinc-copper superoxide dismutase (SOD1). In observing that small insertions, deletions, duplications, and base substitutions account for a majority of GATA1 mutations in ML-DS, Cabeloff et al proposed a model where GATA1 mutations occur as a consequence of increased constitutive oxidative stress, and disrupted folate metabolism resulting from SOD1 and CBS overexpression, respectively.14
The combination of oxidative stress and altered folate metabolism also yields deficient DNA repair mechanisms and increased sensitivity to DNA damaging chemotherapeutic agents (eg, anthracyclines and cytarabine, agents commonly used in the treatment of ML-DS) rendering ML-DS cells more susceptible to chemotherapy than non-DS AML.15 Further, it is hypothesized that the disrupted folate metabolism mediated by overexpression of CBS favors ara-C conversion to ara-CTP resulting in increased DNA incorporation and cell death when compared to non-DS AML.16 In concert with ara-C conversion to ara-CTP, mutated GATA1 inhibits degradation of ara-C indirectly through the transcriptional repression of cytidine deaminase (CDA is the enzyme responsible for ara-C degradation).17 The cumulative pharmacogenomic and leukemogenic effect of trisomy 21 is the mutagenesis of a truncated but functional GATA1 protein, and the creation of constitutive cellular conditions permissive of cytarabine toxicity while detrimental to DNA repair mechanisms.
Among 54 ML-DS patients treated with conventional anthracycline doses in the POG9421 study, 10 (17.5%) developed symptomatic cardiomyopathy (fatal in 3 patients).18 In 1992, a Pediatric Oncology Group (POG 9498) reported superior event-free survival and overall survival among ML-DS patients in comparison to non-DS AML patients. Subsequent studies have successfully reduced exposures to cytarabine and daunorubicin while maintaining event-free survival rates in ML-DS of 83%-93% among children <4 years of age (presumed to have the GATA1 mutation).19 Among 54 ML-DS patients treated with conventional anthracycline doses in the POG9421 study, 10 (17.5%) developed symptomatic cardiomyopathy (fatal in 3 patients).11 Finding the optimal dosing to improve survival and reduce toxicity in this vulnerable population is critical. The results of the Children's Oncology Group AAML0431 trial demonstrated a 3 year event-free survival of 90% despite a 25% decrease in the total anthracycline dose (compared with the predecessor Children Cancer Group A2971 trial), and a reduction in intrathecal treatments from a total of 7 to 2.20 Currently the Children's Oncology Group is developing the AAML1531 study with an 85% dose reduction in total cytarabine dosing (personal communication, AAML1531 Study Chairs, Johann Hitzler and Jason Berman). In the era of precision medicine, novel targeted therapies that enhance cure rates while reducing side effects of treatment is the ultimate goal. However, in ML-DS, the pharmacogenomic impact of leukemogenic events conspire to permit a targeted dose-reduction strategy using conventional chemotherapy while achieving the same goal of cure with fewer adverse events.
Acute promyelocytic leukemia
Acute promyelocytic leukemia has long been characterized as a unique subtype of AML with a characteristic morphology and a common reciprocal translocation between the promyelocytic leukemia (PML) gene on chromosome 15 and the retinoic acid receptor alpha (RARA) gene on chromosome 17. With the fusion of the N-terminus of the PML protein with the C-terminus of the RARA transcription factor, the PML-RARA fusion protein effectively blocks promyelocyte differentiation and leads to uncontrolled self-renewal.13 Other variant N-terminal fusion partners for RARA include NPM1, NUMA, and PLZF, to name a few.14 In normal conditions, RARA is a nuclear receptor capable of recruiting histone deacetylases and corepressors to act as a transcriptional repressor. In the presence of retinoic acid (RA), RARA becomes a potent activator of genes mediating myeloid differentiation. However, with physiologic levels of RA, PML-RARA is an ineffective transcriptional activator,15 resulting in myeloid maturation arrest at the promyelocyte stage.
APL accounts for ∼10% of all childhood AML. APL blasts can trigger DIC, fibrinolysis, proteolysis, and thrombocytopenia resulting in an early death rate as high as 10%.16 Historically, conventional chemotherapy approaches including daunorubicin and cytaraine, yielded complete remission rates of 75%-80%, but cure rates are only 35%-40%. However, as many as 17% of patients died within the first month of therapy due to bleeding complications (reviewed by Kutny et al16 ). With the introduction of ATRA and ATO cure rates for APL now exceed 90% with greatly reduced early toxic mortality (reviewed by Wang and Chen17 ).
ATRA as a single agent is capable of inducing promelocyte differentiation, ultimately inducing complete morphologic remission and reduced risk for early coagulopathy associated mortality. The induction of apoptosis by chemotherapy results in the release of procoagulant and fibrinolytic factors such as Annexin A2 and tissue factor. ATRA markedly upregulates thrombomodulin expression and decreases tissue factor release, providing a reduction in the incidence and severity of coagulopathy. The mechanism of action of ATRA is dependent on ATRA binding to PML-RARA with transcriptional activation of retinoic acid receptor elements resulting in cellular differentiation and followed by subsequent polyubiquitination and proteolysis of the PML-RARA oncoprotein.18 However, differentiation alone is not adequate for cure.
However, ATO also has a dual proapoptotic effect. Whereas ATRA acts on the RARA moiety, ATO acts on the PML moiety inducing oxidation of 2 cysteine residues followed by sumoylation of a lysine residue, which ultimately leads to polyubiquitination and proteolysis.19 Together ATRA and ATO permit the critical degradation of PML-RARA through independent targeting of both the PML and RARA moieties in a manner that yields clinical efficacy suggestive of true combination synergy.20
Acute myeloid leukemia: all other subtypes
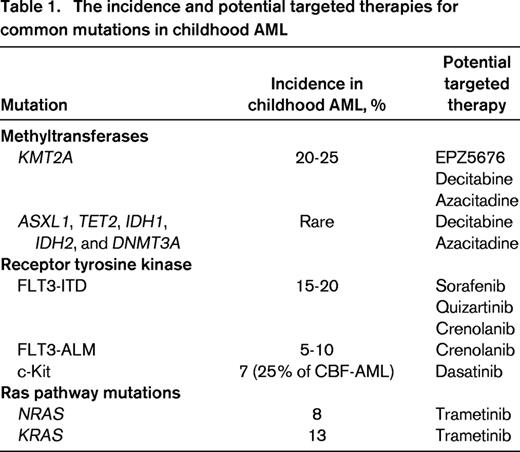
APL and DS-AML are model systems proving the hypothesis that precision medicine can drive progress toward improved survival while minimizing short and long-term toxicities in molecular subsets of AML. Through the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) initiative, the National Cancer Institute has supported next generation sequencing on samples from >800 children with AML. Ongoing data analysis has identified previously unknown molecular events that define new genotypically and phenotypically distinct subsets of AML. In the discussion below, mutations are reviewed in the context of potential targeted treatment approaches (Table 1). This is by no means an exhaustive review of the genomics of AML, rather a focused analysis of targets associated with promising targeted therapies potentially available for study in children in the near future.
Survival in AML of childhood (excluding APL and ML-DS) has improved over the past few decades through improved supportive care, through progress in bone marrow transplant, and through a reapplication of old drugs (primarily anthracyclines and cytarabine). The introduction of targeted therapies into a standard backbone is a recent strategy with mixed success. One challenge in this approach is that AML therapy is already maximally intensive. Anthracyclines doses are pushed well >400mg/m2 in many North American and European studies, and related and unrelated donor transplants are commonplace for patients with high-risk disease features. The introduction of novel therapies in the backbone of highly toxic therapy can introduce cumulative toxicities that negate any beneficial antileukemic effect. This is a challenge in drug development for childhood AML. Clinical trials introducing novel agents will need to identify an appropriate and robust study population, and will need to be tolerant of toxicity risks with the expectation that the ultimate goal is to minimize the need for BMT and reduce cumulative anthracycline doses all while maintaining and improving efficacy.
Epigenetic targeting.
Mutations involving epigenetic regulators (ASXL1, TET2, IDH1, IDH2, and DNMT3A) occur with increasing frequency with age (reviewed by Abdel-Wahab and Levine21 ), and affect a majority of patients older than 60 years with myelodysplasia and AML. These mutations are rare in children. In a recent report from COG, analysis of samples from 180 patients revealed no mutations in IDH1 or DNMT3A. TET2 mutations occurred at a similar frequency to adults, but IDH2 mutations were rare.22 Through modeling the sequential acquisition of mutations in adults with AML, it is hypothesized that the early events in leukemogenesis involve genes responsible for regulation of the epigenome.23 Although children do not exhibit the same pattern of methyltransferase mutations as adults, translocation events involving the lysine (K)-specific methyltransferase 2A gene (KMT2A, previously MLL) at locus 11q23 are reported in one-quarter of childhood AML cases.24 It is therefore tempting to assume that disordered methylation is a critical event the leukemogenesis in both children and adults, albeit through different genetic regulators. Using methylation-sensitive melting curve analysis to detect promoter methylation, Juhl-Christensen noted that promoter hypermethylation patterns fell in alignment with specific genetic mutations. Interestingly, promoter hypermethylation was most frequent in core-binding factor leukemias compared with KMT2A-associated AML.25 With only 70 patients younger than age 14 studied, further analysis is needed. Nonetheless, it appears that hypermethylation may be a significant leukemogenic event in childhood AML despite mutational patterns that differ from adults.
Decitabine and azacitidine are hypomethylating agents with activity in adults with AML and MDS harboring DNMT3A mutations.26 In children with relapsed and refractory AML, decitabine monotherapy induced complete remissions in 3 of 8 heavily pretreated patients.27 In a Phase 1 study in pediatric leukemia (ALL and AML), with a Phase 2 expansion in AML, azacitidine was combined with fludarabine and cytarabine. Thirteen patients with AML were included. The combination was well tolerated. Among the 12 evaluable patients with AML, there were 9 responses (7 CR/CRi, 1 PR, 1 SD). There are several additional Phase 1 and 2 studies ongoing that combine azacitidine priming with conventional chemotherapy. However, the role and requirement for priming is not well known. Patient-derived xenograft studies suggest that decitabine in combination with cytarabine provides an enhanced antileukemic effect compared with either agent alone and that this effect is independent of drug sequence (ie, it is the combination and not the priming that matters for efficacy). In these same animal studies, genome-wide methylation analysis confirmed that decitabine resulted in global demethylation,28 suggesting that methylation is targetable in AML. Although these studies did not identify epigenetic priming as important in the efficacy of the combination, drug sequence studies with daunorubicin may yield different results. Ongoing and planned studies of hypomethylating agents in combination with conventional chemotherapy may provide answers to their role in childhood AML.
Crucial to the leukemogenic function of KMT2A in KMT2A-rearranged AML is the DOT1L histone methyltransferase. Currently, the novel DOT1L inhibitor EPZ5676 is in Phase 1 trials in children and adults (NCT01684150). Whereas azacitidine and decitabine target global methylation, inhibitors of DOT1L may target leukemogenic events in the epigenome specifically initiated by KMT2A-associated oncoproteins.
Inhibitors of histone deacetylase (eg, vorinostat, panobinostat, and etinostat) are also of interest in childhood AML. The Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) consortium recently completed a Phase 1 dose-finding study of the pan-deacetylase inhibitor, panobinostat. Among 17 subjects enrolled, 7 had AML and 10 ALL. Accrual to 3 planned dose levels is completed with no dose-limiting toxicities.29 Activity in adults with AML is promising, but combination studies are still needed to evaluate the efficacy of this class of compounds in childhood AML.
Targeting activation of the Ras network.
Activation of signaling through the Ras signaling network is common in adult and childhood cancers. In AML, Ras network activation is often the result of gain-of-function mutations in KRAS, NRAS, or FLT3. FLT3 is discussed later in this paper. Mutations in exon 1 or 2 of KRAS and NRAS occur in 8% and 13% of children with AML, respectively. Often these mutations are identified in patients with an otherwise normal karyotype,30 but there is also evidence of a possible association with CBF leukemia.31
Ras pathway mutations pose significant challenges in drug development in children. The role of Ras pathway activation in leukemogenesis in childhood leukemia is unknown. It is unclear if these mutations represent events driving the cells toward malignancy, or secondary events upon which the leukemia is not exclusively dependent. In one study, only 79% of the identified mutation involving the Ras pathway were present both at diagnosis and relapse. Eight percent of patients gained Ras pathway mutation at relapse, whereas 13% of Ras pathway mutations present at diagnosis were lost at relapse.32 These findings suggest that Ras pathway mutations may exist in subclones, but that these subclones may not drive treatment response and relapse.
Trametinib is a highly selective allosteric inhibitor of MEK1/MEK2 with recent FDA approval for the treatment of malignant melanoma. These findings suggest that RAS pathway mutations may exist in subclones, but that these subclones may not drive treatment response and relapse. However, in vitro, NRAS mutations confer a proliferative advantage required for maintenance of the malignant phenotype in primary murine leukemias and in human AML cell lines.33
A Phase 1 trail of trametinib in children is currently underway (NCT02124772). In 57 evaluable adults with relapsed or refractory AML, 12 (21%) entered a CR with trametinib as single-agent therapy (Borthakur, ASH 2012). Trametinib is being studied in adults with AML in combination with conventional chemotherapy (NCT02016729).
Targeting receptor tyrosine kinase variants
Receptor tyrosine kinases (RTKs) are a family of membrane bound proteins with extracellular ligand binding domains whose interaction with their native ligand leads to phosphorylation and activation of their intrinsic, intracellular kinase moiety. RTKs expressed on hematopoietic stem/progenitor cells mediate stem cell differentiation and proliferation through ligand-dependent kinase-mediated activation of downstream signal transduction pathways.33 Activating mutations of FLT3 and KIT have been directly implicated in the pathogenesis of AML. Such somatic mutations lead to the loss of cytokine dependence of the activation cascade and cause autonomous, cytokine-independent kinase activation, and unregulated cellular proliferation.
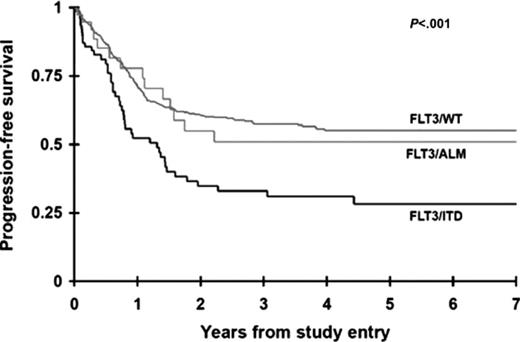
FLT3 mutations are the most common somatic mutations in childhood AML, present in ∼20% of all AML. Two distinct types of FLT3 mutations have been identified in childhood AML. One is a result of internal tandem duplication of the juxtamembrane domain-coding region (FLT3/ITD) and the second is caused by single nucleotide variation in the activation loop domain-coding sequence (FLT3/ALM). FLT3/ITD is seen in 15%-20% of childhood AML in an age-dependent manner. Although FLT3/ ITD mutations are quite rare in younger children, during the second decade of life the mutation is seen at a prevalence comparable with adults.34 Although FLT3/ITD and FLT3/ALM lead to autonomous activation of the intrinsic kinase moiety, they have significantly different clinical significance, where patients with FLT3/ITD have adverse outcome, whereas presence of FLT3/ALM is not associated with prognosis (Figure 2).35 The two FLT3 variants also exhibited differential response to kinase inhibitors, where cells harboring FLT3/ITD were exquisitely sensitive to a number of kinase inhibitors, whereas those with FLT3/ALM had a more resistant profile to the same agents.36 As a result, AML patients with FLT3/ITD were targeted for directed therapy with FLT3 inhibitors. Early phase trials showed objective but transient response to single-agent therapies, thus providing rationale for testing these agents in combination with conventional chemotherapy backbones. Such combination therapies have provided promising results.
Kinase inhibitors may bind to the active (type 1 kinase inhibitor) or the inactive (type 2 kinase inhibitor) form of the kinase. Sorafenib, a type 2 kinase inhibitor showed outstanding response in combination with Idarubicin and cytarabine, where patients with FLT3/ITD who received this combination had near uniform remission induction rate compared to significantly lower remission rates in those without FLT3.37 Unfortunately, the duration of remission was brief and majority of patients had a relapse subsequent to cessation of the drug. Nonetheless, the efficacy observed in remission induction provided the rationale for incorporation of sorafenib into numerous clinical trials. In an ongoing pediatric clinical trial, patients with high risk FLT3/ITD are treated with combination therapy that includes sorafenib followed by consolidation with allogeneic stem cell transplant (NCT01371981). Evaluation of relapse specimens from patients with prior sorafenib exposure revealed evolution of secondary mutations in the kinase domain of the FLT3 gene, linking the relapse to the gain of secondary FLT3 mutations.38 Given the promising but sub-optimal response to such type 2 inhibitors, efforts to identify more potent inhibitors have yielded a number of next generation FLT3 inhibitors. Crenolanib is a type 1 kinase inhibitor with activity against FLT3/ITD as well as FLT3/ALM.39 Such dual activity kinase inhibitor 1 if proven efficacious, would enable increase of the proportion of AML patients who can be targeted for directed therapies with FLT3 inhibitors. Crenolanib is currently in several active or planned clinical trials in adults and children, including in combination with sorafenib (NCT02270788).
Targeting KIT mutations.
Activating mutations of KIT gene have been identified in adult and childhood AML. These mutations appear to be uniquely associated with those with core-binding factor (CBF) translocations (RUNX1/RUNX1T1 and CBFB/MHY11 translocations) with ∼ 25% of those with CBF AML harboring 1 of the 2 mutations in the extracellular juxtamembrane (exon 8) or the intrinsic kinase domain (exon 17)-coding region.40 Early adult studies demonstrated significant adverse clinical impact for KIT mutations. However, more recent studies in childhood AML confirmed the high prevalence of these mutations in pediatric AML without an associated adverse outcome. Despite lack of clinical significance, KIT mutations nonetheless lead to autonomous phosphorylation and constitutive activation of the KIT receptor kinase, making it an attractive target for directed therapy. Initial in vitro studies demonstrated differential response to imatinib with potent efficacy in cells with exon 8 versus no response in those with exon 17 mutations.12,31 Dasatinib, a second generation kinase inhibitor, demonstrated more broad efficacy in AML cells with either mutation type, providing rationale in utilizing such agents in the management of CBF AML patients with KIT mutations. Although children with CBF AML have a more favorable outcome compared with their non-CBF counterparts, ∼25% of them remain at risk for relapse within 2 years from cessation of therapy. Single-agent dasatinib used in CBF patients with high-risk disease demonstrated tolerability, but disappointing response.41 Recent trials incorporating dasatinib into the treatment regimen for CBF AML patients with KIT mutations showed feasibility and tolerability of this approach, and suggested that addition of dasatinib may effectively target KIT mutations in adult AML,42 although no direct randomized trial has been performed to evaluate its efficacy in improving outcome. Given the favorable safety profile of dasatinib, this drug remains an attractive agent for inclusion into clinical management of patients with KIT mutations.
Toward targeted therapy in AML
As new mutations are reported in childhood AML, many of which predict prognosis, it is tempting to assume that the leukemic cell is addicted exclusively to the oncogenic signal initiated by the mutant gene and pathway. This may be the case in acute promyelocytic leukemia, but it is the exception and not the rule. Whole-genome and whole-exome sequencing are commercially available and becoming less expensive. Clinicians and researchers face the challenge of deciphering and defining actionable data likely to yield a therapeutically relevant target. Meanwhile, the pace of drug development in children lags far behind the pace of target identification, adding a barrier to drug availability for children. The potential for overlapping toxicities, given that current AML standard backbones approach maximal toxicity, needs to be balanced against the potential benefit to patients at high risk for relapse. To realize the promise of precision medicine in children with AML, clinicians, researchers, industry and the National Institutes of Health must engage collaboratively in well-controlled trials of molecularly stratified targeted therapies, perhaps shifting Phase 2 drug development to patients at highest risk for first relapse rather than relying exclusively on Phase 2 development in the relapsed cohort.
ML-DS and APL confirm the hypothesis that expression of a leukemogenic mutation predicts response to the targeted therapeutic approaches, which in turn leads to improvements in survival with reductions in toxicity. For all other children with AML, the relationship between target expression and response to targeted therapies is not as well established. Mutations in FLT3, KIT, NRAS, KRAS, and methyltransferases are common and targetable in de novo AML. However, it remains unknown whether targeted inhibition of these oncogenes will yield improvements in survival, and permit reductions in therapy. The study of targeted therapies in heavily pretreated relapse patients is a standard approach in drug development, but is problematic in childhood AML. Identifying appropriate study endpoints in the setting of relapse or refractory AML to predict response in an untreated de novo AML patient is difficult. Further, in the evaluation of targeted therapies in small subgroups of molecularly defined AML, patient accrual will not permit Phase 2 efficacy studies in children. Often studies in relapse and refractory patients are limited to a determination of the safety and feasibility of combining a novel therapy with conventional chemotherapy. Accordingly, as is the example with sorafenib (NCT01371981), efficacy determinations may be limited to Phase 2 evaluations in newly diagnosed patients. In such trials, we must proceed with great caution as unanticipated short- and long-term toxicities may only become apparent in larger cohorts of treated patients. The future of targeted therapies in childhood AML is bright. In the coming years we will see new agents with great potential moving forward in clinical development. It is the hope of these trials that oncogenic mutations will indeed predict response to targeted therapies.
Correspondence
E. Anders Kolb, MD, Nemours Center for Cancer and Blood Disorders, A. I. duPont Hospital for Children, 1600 Rockland Rd, Wilmington, DE 19803; Phone: 302-651-5500; Fax: 302-651-5510; e-mail: eakolb@nemours.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.