Abstract
Once suspected, the diagnosis of paroxysmal nocturnal hemoglobinuria (PNH) is straightforward when flow cytometric analysis of the peripheral blood reveals a population of glycosyl phosphatidylinositol anchor protein-deficient cells. But PNH is clinically heterogeneous, with some patients having a disease process characterized by florid intravascular, complement-mediated hemolysis, whereas in others, bone marrow failure dominates the clinical picture with modest or even no evidence of hemolysis observed. The clinical heterogeneity is due to the close, though incompletely understood, relationship between PNH and immune-mediated bone marrow failure, and that PNH is an acquired, nonmalignant clonal disease of the hematopoietic stem cells. Bone marrow failure complicates management of PNH because compromised erythropoiesis contributes, to a greater or lesser degree, to the anemia; in addition, the extent to which the mutant stem cell clone expands in an individual patient determines the magnitude of the hemolytic component of the disease. An understanding of the unique pathobiology of PNH in relationship both to complement physiology and immune-mediated bone marrow failure provides the basis for a systematic approach to management.
Learning Objectives
Learn that PNH is a heterogeneous disease and that its clinical manifestations are determined by the size of the PNH clone, the red cell phenotype, and the relationship of PNH to bone marrow failure
Understand the pathophysiology of the complement-mediated hemolytic anemia of PNH and how the hemolysis is ameliorated by blocking formation of the membrane attack complex of complement
Be able to subcategorize patients into 3 groups that will provide the basis for individualizing management
Introduction
Two features distinguish paroxysmal nocturnal hemoglobinuria (PNH) from all other hemolytic anemias. First, and most important, the abnormality that underlies the pathobiology of PNH is not confined to the erythrocyte. Rather, PNH is a disease of the hematopoietic stem cell. Second, PNH differs from all other intrinsic red cell abnormalities in that the defective process is acquired rather than inherited.
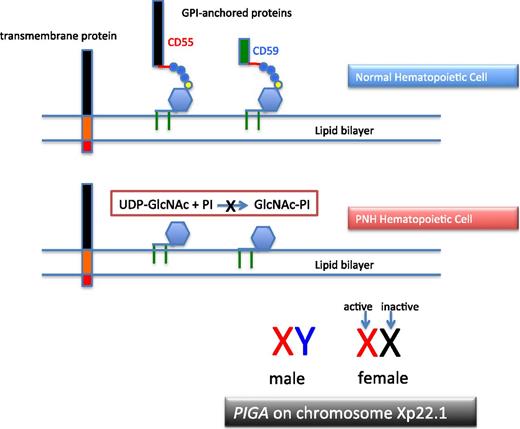
PNH arises as a consequence of somatic mutation of a gene (PIGA) whose protein product is a glycosyl transferase that is an essential component of the biosynthetic pathway that generates glycosyl phosphatidylinositol (GPI) (Figure 1).1 This moiety serves as the anchoring mechanism for a functionally diverse group of membrane-bound proteins, more than 20 of which are expressed on hematopoietic lineage cells in humans. The PIGA mutations that give rise to PNH cause loss of enzyme function (partial or total), the end result of which is near-complete or complete absence of expression of all proteins that are GPI-anchored (Figure 1). Among the cellular membrane constituents that are GPI-anchored are the complement inhibitory proteins, CD55 (decay accelerating factor [DAF]) and CD59 (membrane inhibitor of reactive lysis [MIRL]). It is the deficiency of these 2 proteins that underlies the complement-mediated intravascular hemolysis characteristic of PNH (Figure 2).2 Despite the loss of all GPI-anchored proteins (GPI-APs) from all hematopoietic lineage cells (erythrocytes, granulocytes, monocytes, lymphocytes, and platelets) derived from the PIGA mutant stem cell, there is no compelling evidence that deficiency of GPI-APs other than CD55 and CD59 contribute clinically to the pathophysiology of PNH. In addition to complement-mediated intravascular hemolysis, the other major clinical manifestations of PNH are bone marrow failure and thrombophilia.3 Although the relationship between somatic mutation of PIGA and the hemolysis of PNH is understood in detail, the relationship between somatic mutation of PIGA (and the consequent deficiency of GPI-APs) and the bone marrow failure and thrombophilia of PNH remain largely speculative.
The molecular basis of PNH. Normal hematopoietic stem cells express both transmembrane and GPI-anchored proteins (top). PNH stem cells express transmembrane proteins normally but fail to express GPI-APs because the first step in synthesis of the anchor is inactivated because the gene (PIGA) that encodes the enzyme that is required for transfer of the nucleotide sugar (UDP-GlcNAc) to phosphatidylinositol is mutant (middle). Of the more than 25 genes involved in synthesis of the GPI anchor, only PIGA is located on the X-chromosome (all others are autosomal). Location on the X-chromosome accounts for the observation that essentially all cases of PNH are due to somatic mutation of PIGA because inactivation of only 1 allele is required to produce the PNH phenotype as males have 1 X-chromosome and in females only 1 of the 2 X-chromosomes is active in somatic tissues (bottom). UDP, uridine diphosphate.
The molecular basis of PNH. Normal hematopoietic stem cells express both transmembrane and GPI-anchored proteins (top). PNH stem cells express transmembrane proteins normally but fail to express GPI-APs because the first step in synthesis of the anchor is inactivated because the gene (PIGA) that encodes the enzyme that is required for transfer of the nucleotide sugar (UDP-GlcNAc) to phosphatidylinositol is mutant (middle). Of the more than 25 genes involved in synthesis of the GPI anchor, only PIGA is located on the X-chromosome (all others are autosomal). Location on the X-chromosome accounts for the observation that essentially all cases of PNH are due to somatic mutation of PIGA because inactivation of only 1 allele is required to produce the PNH phenotype as males have 1 X-chromosome and in females only 1 of the 2 X-chromosomes is active in somatic tissues (bottom). UDP, uridine diphosphate.
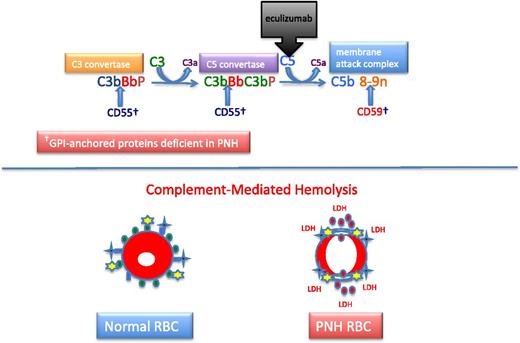
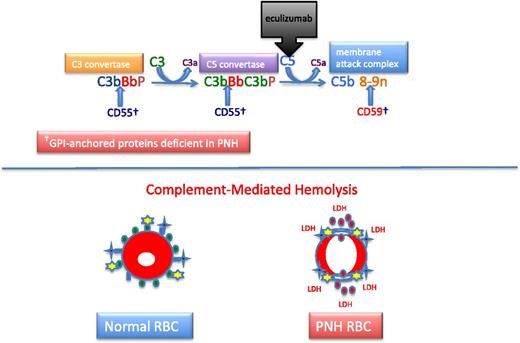
Complement and PNH. The hemolysis of PNH is due to aberrant regulation of the APC. The APC is a component of the innate immune system. Unlike the classical pathway of complement that requires a recognition factor such as antibody to activate the pathway, the APC is continuously active. Therefore safeguards have evolved to protect host cells against APC-mediated injury. In the case of erythrocytes, 2 GPI-APs, CD55 and CD59, serve this function. Two enzymatic convertases amplify the activity of the APC (top). The C3 convertase consists of activated C3 (C3b), activated factor B (Bb, the enzymatic subunit of the complexes that is proteolytically activated by factor D, a trace plasma protein that may be activated by 1 of the mannose-binding lectin-associated serine proteases), and factor P (formerly called properdin). Factor P stabilizes the C3 convertase, allowing each convertase to activate many molecules of C3, and in the process, generate the weak anaphylatoxin, C3a. The C5 convertase is similar in structure to the C3 convertase except that 2 molecules of C3b are required to position C5 for cleavage by activated factor B (Bb). Many molecules of C5 are cleaved by the C5 convertase, and this process generates many molecules of the potent anaphylatoxin and neutrophil chemo-attractant, C5a. Activated C5 (C5b) is the nidus for formation of the MAC of complement consisting of C5b, C6, C7, C8, and multiple molecules of C9. The MAC inserts into the lipid bilayer of the cell, forming a transmembrane torus that results in osmotic lysis. CD55 (DAF) blocks the formation and stability of both the C3 and C5 convertases, whereas CD59 (MIRL) blocks formation of the cytolytic MAC, primarily by inhibiting binding and multiplicity of C9. Eculizumab is a humanized monoclonal anti-C5 antibody that prevents activation of C5 by the C5 convertase. Consequently, the MAC cannot form (and C5a is not generated), accounting for the inhibition of the intravascular hemolysis of PNH. However, eculizumab does not inhibit formation of the C3 convertase, accounting for the opsonization by activation and degradation products of C3 observed in patients with PNH treated with eculizumab. Normal RBCs are protected against APC-mediated injury (black crosses represent APC C3 and C5 convertase formation; yellow stars represent MAC formation) by CD55 (blue ovals) and CD59 (green ovals) (bottom). PNH cells lacking the complement inhibitory proteins CD55 and CD59 undergo complement-mediated lysis, releasing cellular contends including hemoglobin (red circles) and LDH into the plasma.
Complement and PNH. The hemolysis of PNH is due to aberrant regulation of the APC. The APC is a component of the innate immune system. Unlike the classical pathway of complement that requires a recognition factor such as antibody to activate the pathway, the APC is continuously active. Therefore safeguards have evolved to protect host cells against APC-mediated injury. In the case of erythrocytes, 2 GPI-APs, CD55 and CD59, serve this function. Two enzymatic convertases amplify the activity of the APC (top). The C3 convertase consists of activated C3 (C3b), activated factor B (Bb, the enzymatic subunit of the complexes that is proteolytically activated by factor D, a trace plasma protein that may be activated by 1 of the mannose-binding lectin-associated serine proteases), and factor P (formerly called properdin). Factor P stabilizes the C3 convertase, allowing each convertase to activate many molecules of C3, and in the process, generate the weak anaphylatoxin, C3a. The C5 convertase is similar in structure to the C3 convertase except that 2 molecules of C3b are required to position C5 for cleavage by activated factor B (Bb). Many molecules of C5 are cleaved by the C5 convertase, and this process generates many molecules of the potent anaphylatoxin and neutrophil chemo-attractant, C5a. Activated C5 (C5b) is the nidus for formation of the MAC of complement consisting of C5b, C6, C7, C8, and multiple molecules of C9. The MAC inserts into the lipid bilayer of the cell, forming a transmembrane torus that results in osmotic lysis. CD55 (DAF) blocks the formation and stability of both the C3 and C5 convertases, whereas CD59 (MIRL) blocks formation of the cytolytic MAC, primarily by inhibiting binding and multiplicity of C9. Eculizumab is a humanized monoclonal anti-C5 antibody that prevents activation of C5 by the C5 convertase. Consequently, the MAC cannot form (and C5a is not generated), accounting for the inhibition of the intravascular hemolysis of PNH. However, eculizumab does not inhibit formation of the C3 convertase, accounting for the opsonization by activation and degradation products of C3 observed in patients with PNH treated with eculizumab. Normal RBCs are protected against APC-mediated injury (black crosses represent APC C3 and C5 convertase formation; yellow stars represent MAC formation) by CD55 (blue ovals) and CD59 (green ovals) (bottom). PNH cells lacking the complement inhibitory proteins CD55 and CD59 undergo complement-mediated lysis, releasing cellular contends including hemoglobin (red circles) and LDH into the plasma.
PNH is a clonal disease (with mutant PIGA serving as the clonal marker), but it is not a malignant disease in the sense that either acute myeloid leukemia or chronic myelogenous leukemia is a malignant clonal disease of the hematopoietic stem cell. Unlike acute myeloid leukemia or chronic myelogenous leukemia in which the mutant clone expands unrelentingly unless antileukemic therapy is initiated, the extent to which the PNH clone expands varies greatly among patients. For example, some patients have small PNH clones that can be detected only by using high-sensitivity flow cytometry that identifies GPI-AP–deficient blood cells in the peripheral blood. Other patients may have large clones in which essentially all hematopoiesis is derived from the mutant stem cell; but even when large, the PNH clone does not have malignant properties in that it does not invade sites outside of the bone marrow nor does it behave autonomously (ie, its functional properties appear to be governed by the same regulatory factors as those that control the functional properties of normal stem cells), and transformation into acute leukemia is rarely observed. In fact, an unsolved mystery of PNH is what determines the extent to which the PIGA-mutant clone expands.4-8 This question is framed by in vitro and in vivo studies demonstrating that mutant PIGA alone is insufficient to account for clonal expansion.
More than 25 proteins are involved in the synthesis of the GPI-anchor moiety. Hypothetically, somatic mutation of any of the genes that encode these proteins would produce the PNH phenotype (ie, deficiency of GPI-APs). Why then is PNH essentially always due to mutation of PIGA? The commonly agreed upon answer to this question is based on mathematical probability. Among the genes involved in GPI-AP synthesis, PIGA alone is located on the X chromosome.1 Therefore only 1 mutational event is required to produce the PNH phenotype as males have 1 X-chromosome, and in females, only 1 of the 2 X-chromosomes is active in somatic tissues (Figure 1). Because all other genes involved in GPI-AP synthesis are autosomal, 2 mutational events would be required to eliminate synthesis of the GPI-anchor and produce the PNH phenotype. Thus, based on mutational frequency (∼1.1 × 10−8 per nucleotide position per haploid genome), the probability that deficiency of GPI-APs in PNH would be due to somatically mutated PIGA is many orders of magnitude greater than the probability that the deficiency would be due to somatic mutation of both of the alleles of any of the more than 25 autosomal genes involved in GPI-anchor synthesis. Hypothetically, germ line mutation of 1 of the alleles of an autosomal gene involved in GPI-AP synthesis could predispose an individual to develop an autosomal form of PNH; a case report supports this mechanism as a fleetingly rare cause of the disease.9
Understanding the molecular basis of the hemolysis of PNH led to development of targeted therapy in the form of eculizumab (Soliris, Alexion Pharmaceuticals, Inc), and this treatment has had a major impact on the natural history the disease.10-12 Nonetheless, management of patients with PNH remains challenging because of the heterogeneous nature of the disease and its close association with bone marrow failure.13
PNH and complement
The chronic intravascular hemolysis that is the hallmark clinical manifestation of PNH is mediated by the alternative pathway of complement (APC) (Figure 2).2 The APC is a component of innate immunity.14 This ancient system evolved to protect the host against invasion by pathogenic microorganisms. Unlike the classical pathway of complement that is part of the system of acquired immunity and requires antibody for initiation of activation, the APC is in a state of continuous activation, armed at all times to protect the host. The APC cascade can be divided into 2 functional components: the amplification C3 and C5 convertases and the cytolytic membrane attack complex (MAC) (Figure 2). The C3 and C5 convertases are enzymatic complexes that initiate and amplify the activity of the APC and ultimately generate the MAC (the MAC is the common cytolytic subunit of the classical and lectin pathways of complement as well as the APC).
Because the APC is primed for attack at all times, elaborate mechanisms for self-recognition and protection of the host against APC-mediated injury have evolved. Both fluid-phase and membrane-bound proteins are involved in these processes. Normal human erythrocytes are protected against APC-mediated cytolysis primarily by DAF (CD55)15,16 and MIRL (CD59) (Figure 2).17,18 These proteins act at different steps in the complement cascade. CD55 regulates the formation and stability of the C3 and C5 convertases, whereas CD59 blocks the formation of the MAC (Figure 2). Deficiency of CD55 and CD59 on the erythrocytes of PNH is the pathophysiologic basis of the direct antiglobulin test-negative, intravascular hemolysis that is the clinical hallmark of the disease.
DAF (CD55)
In 1983, 2 groups reported that PNH erythrocytes were deficient in DAF (CD55).16,19 DAF, first identified by Hoffman in 1969 and subsequently purified by Nicholson-Weller and colleagues in 1982,15 is a 70-kD GPI-AP that inhibits the formation and stability of the C3 convertases of complement. Thus, the absence of DAF provided a plausible explanation for the greater binding of activated C3 to PNH erythrocytes that had been observed in in vitro experiments. Detailed studies, however, demonstrated that DAF does not regulate the activity of the membrane attack complex of complement. Those results implied that PNH erythrocytes were deficient in a second complement regulatory protein that was functionally distinct from DAF.
MIRL (CD59)
In 1989, Holguin et al17 reported the isolation from normal erythrocytes of an 18-kD GPI-AP called MIRL (CD59) that protected PNH red cells against complement-mediated lysis. As anticipated, PNH cells were found to be deficient in MIRL, and additional studies by those investigators and others demonstrated that MIRL inhibits complement-mediated lysis by blocking the assembly of the MAC.17,20 MIRL (CD59) may also act in concert with DAF (CD55) to control activation of the C3 convertase of the APC.21
Phenotypic mosaicism
Phenotypic mosaicism of the peripheral blood is a characteristic feature of PNH that was first recognized by Dr. Wendell Rosse in the early- to mid-1970s based on quantitative differences in complement sensitivity. In those studies, PNH I cells were defined by having normal sensitivity to complement-mediated lysis, PNH II cells were moderately complement sensitive (2-4 times more sensitive than normal), and PNH III cells were markedly complement sensitive (15-25 times more sensitive than normal). The extent of the mosaicism and the contribution of each phenotype to the mosaic pattern vary greatly among patients. Erythrocyte phenotype is clinically relevant as patients with primarily type II cells have a relatively benign clinical course, experiencing hemolytic exacerbations only during periods of brisk complement activation induced by supervening events such as trauma or infection.
By comparing expression of DAF (CD55) and MIRL (CD59) on PNH I, PNH II, and PNH III erythrocytes, the functional basis of the different complement-sensitivity phenotypes was determined.18 Those studies showed that PNH III cells are completely deficient in both DAF and MIRL, whereas PNH II cells are partially deficient, and PNH I cells have normal expression of the 2 complement regulatory proteins. Thus, the variability in sensitivity to complement-mediated lysis among the different phenotypes is explained by quantitative differences in expression of DAF and MIRL. In turn, the phenotypic mosaicism of PNH is due to genotypic mosaicism of PIGA in which the somatic mutation either partially (PNH II) or completely (PNH III) inactivates enzyme function; in an individual patient, discrete PIGA mutant clones give rise to a particular phenotype.22 As many as 4 discrete PIGA mutant clones have been identified in an individual patient, suggesting a powerful selection process based on GPI-AP deficiency had been applied to the bone marrow.
Diagnosis of PNH
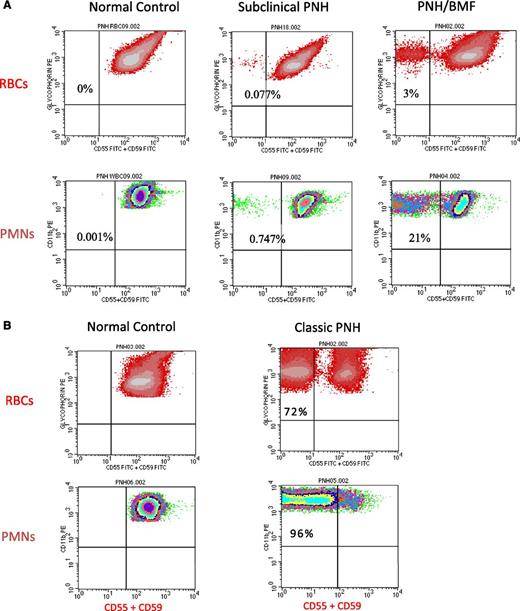
Once suspected based on clinical and laboratory data (Table 1), diagnosing PNH is straightforward because deficiency of GPI-APs on peripheral blood cells is readily demonstrated by flow cytometry (Figure 3). Analysis of both red blood cells (RBCs) and polymorphonuclear (PMN) cells is warranted; PNH clone size will be underestimated if only RBCs are examined because GPI-AP–deficient erythrocytes are selectively destroyed by complement. Recent transfusion will also affect the estimate of clone size if only RBCs are analyzed, but delineation of PNH phenotypes (ie, the percentage of type I, type II, and type III cells) requires flow cytometric analysis of the erythrocyte population.
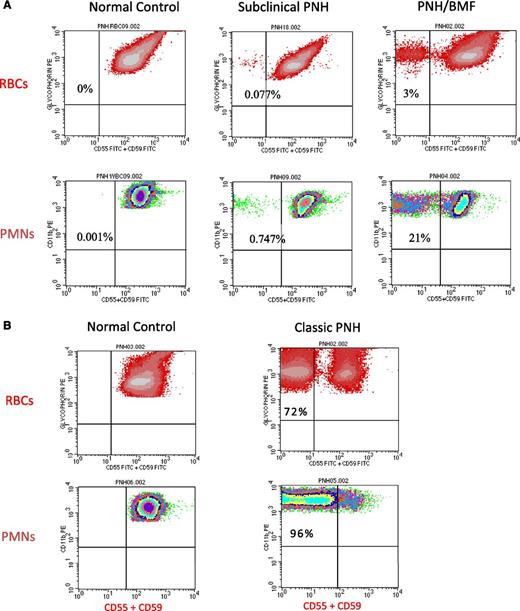
Flow cytometry of peripheral blood cells from patients with either subclinical PNH, PNH/bone marrow failure (BMF), or classic PNH. (A) In these examples, 2-color flow cytometry is used to analyze RBCs (top) and neutrophils (PMNs) (bottom). RBCs are gated on based on staining with phycoerythrin (PE)-conjugated anti-glycophorin, and GPI-APs are gated on using a combination of fluorescein isothiocyanate–conjugated anti-CD55 and anti-CD59. Neutrophils (PMNs) (bottom) are gated on using PE-conjugated anti-CD11b. (Left, top and bottom) Analysis of RBCs and PMNs from a normal volunteer (control). All the normal RBCs and PMNs express CD55 and CD59. Patients with subclinical PNH (middle) have very small clones, typically less than 1% (middle), and they have no biochemical evidence of hemolysis. Patients with PNH/BMF (right) have at least biochemical evidence of hemolysis, but clone size is generally relatively small. In the case illustrated, the clone size of 21% is at the lower limit for biochemical detection of hemolysis. (B) (Right, top and bottom) Analysis of RBCs and PMNs from a patient with classic PNH. The percentage of cells that are deficient in expression of GPI-APs is shown the inner-upper quadrant of the histograms. The percentage of deficient RBCs is invariably greater than the percentage of deficient PMNs because GPI-AP–deficient RBCs are selectively destroyed by complement-mediated lysis, whereas GPI-AP–deficient PMNs have a normal life span. For this reason, the size of the PNH clone is determined by the percentage of GPI-AP–deficient PMNs rather than by the percentage of GPI-AP–deficient RBCs.
Flow cytometry of peripheral blood cells from patients with either subclinical PNH, PNH/bone marrow failure (BMF), or classic PNH. (A) In these examples, 2-color flow cytometry is used to analyze RBCs (top) and neutrophils (PMNs) (bottom). RBCs are gated on based on staining with phycoerythrin (PE)-conjugated anti-glycophorin, and GPI-APs are gated on using a combination of fluorescein isothiocyanate–conjugated anti-CD55 and anti-CD59. Neutrophils (PMNs) (bottom) are gated on using PE-conjugated anti-CD11b. (Left, top and bottom) Analysis of RBCs and PMNs from a normal volunteer (control). All the normal RBCs and PMNs express CD55 and CD59. Patients with subclinical PNH (middle) have very small clones, typically less than 1% (middle), and they have no biochemical evidence of hemolysis. Patients with PNH/BMF (right) have at least biochemical evidence of hemolysis, but clone size is generally relatively small. In the case illustrated, the clone size of 21% is at the lower limit for biochemical detection of hemolysis. (B) (Right, top and bottom) Analysis of RBCs and PMNs from a patient with classic PNH. The percentage of cells that are deficient in expression of GPI-APs is shown the inner-upper quadrant of the histograms. The percentage of deficient RBCs is invariably greater than the percentage of deficient PMNs because GPI-AP–deficient RBCs are selectively destroyed by complement-mediated lysis, whereas GPI-AP–deficient PMNs have a normal life span. For this reason, the size of the PNH clone is determined by the percentage of GPI-AP–deficient PMNs rather than by the percentage of GPI-AP–deficient RBCs.
In addition to flow cytometric analysis, the basic initial evaluation of a patient with PNH should include complete blood count and reticulocyte count to assess the effects of the disease on production of leukocytes and platelets as well as erythrocytes; biochemical markers of hemolysis (serum concentration of lactate hydrogenase [LDH], bilirubin [fractionated], and haptoglobin); determination of iron stores; and bone marrow aspirate, biopsy, and cytogenetics. These diagnostic studies will allow classification of patients into 3 groups based on the recommendation of the International PNH Interest Group (Table 2).3
In patients with classic PNH (Table 2), the leukocyte and platelet counts are usually normal or nearly normal, whereas leukopenia, thrombocytopenia, or both invariably accompany PNH in the setting of another bone marrow failure syndrome (Table 2). The reticulocyte count is needed to assess the ongoing capacity of the bone marrow to respond to the anemia. Although the reticulocyte count is elevated in patients with classic PNH, it is consistently inappropriately low for the degree of anemia, reflecting underlying relative insufficiency of hematopoiesis that is characteristic of the disease. Serum LDH is always markedly elevated in classic PNH. Patients with classic PNH may be iron-deficient because of chronic hemoglobinuria and hemosiderinuria. Nonrandom cytogenetic abnormalities are uncommon in PNH.4
The degree of serum LDH elevation is variable in patients with PNH in the setting of another bone marrow failure syndrome; however, in a majority of patients with PNH/bone marrow failure, the clone size is smaller than 10%, with fewer than 10% of patients with PNH/bone marrow failure having a clone size larger than 50% (Table 2).23-25 Bone marrow aspirate and biopsy are needed for pathological characterization of PNH in the setting of another bone marrow abnormality.
By definition, patients with subclinical PNH (PNH-sc) have neither clinical nor biochemical evidence of hemolysis. The spectrum of bone marrow failure associated with PNH-sc parallels that of PNH/bone marrow failure (Table 2).
How I treat PNH based on disease classification
Completing the diagnostic evaluation (Table 2) will allow development of a systematic treatment plan (Figure 4) based on disease classification.
Management of PNH. A management scheme based on classification of PNH into 3 subcategories (subclinical, PNH in the setting of another bone marrow failure syndrome [PNH/BMF], and classic PNH. See Table 2 for characteristics of each category.
Management of PNH. A management scheme based on classification of PNH into 3 subcategories (subclinical, PNH in the setting of another bone marrow failure syndrome [PNH/BMF], and classic PNH. See Table 2 for characteristics of each category.
PNH-sc.
A close association exists between PNH and aplastic anemia (AA) and to a lesser extent between PNH and low-risk myelodysplastic syndromes (MDS). By using high-resolution flow cytometry, approximately 50% to 60% of patients with AA and 15% to 20% of patients with low-risk MDS have been found to have a detectable population of GPI-AP–deficient erythrocytes and granulocytes.25-29 In approximately 90% of these cases, the proportion of GPI-AP–deficient peripheral blood neutrophils is <25% of the total.25
Recent studies have investigated the natural history of PNH clones in the setting of bone marrow failure.23-25 The threshold that separates subclinical PNH from clinical PNH is reached when the neutrophil clone size is in the range of 20% to 25%, with a corresponding GPI-AP–deficient erythrocyte population of 3% to 5%.23 Longitudinal studies indicate that some degree of clonal expansion occurs in 15% to 50% of cases.23-25 In 10% to 25% of cases, the clone disappears, and in 25% to 60% of cases, the clone size persists unchanged.23-25 Importantly, available evidence indicates that patients who present with subclinical PNH do not typically progress to clinical PNH.23-25 In approximately 80% of the cases in which GPI-AP–deficient cells are detected in the setting of bone marrow failure, the proportion of GPI-AP–deficient cells is <1.0% of the total circulating granulocytes. These patients (designated PNH-sc) with very small populations of GPI-AP–deficient cells (Figure 3A, middle) have no clinical or biochemical evidence of hemolysis and require no specific treatment of PNH. The presence of PNH cells has also been observed in patients with MDS,25,28-30 with the association between PNH and MDS appearing to be confined to low-risk categories of MDS, particularly the refractory anemia variant and, to a lesser extent, the category of refractory cytopenias with multilineage dysplasia.25-29
Finding a population of GPI-AP–deficient granulocytes in patients with AA, however, may be clinically relevant, because some27,28 (but not all)31 studies suggest that these patients have a particularly high probability of responding to immunosuppressive therapy with a more rapid rate of onset of response compared with patients with AA without a population of GPI-AP–deficient erythrocytes.25
PNH in the setting of another bone marrow failure syndrome.
Patients with a bone marrow failure syndrome (AA or MDS) and a PNH clone with clinical/biochemical evidence of hemolysis are classified as PNH in the setting of another bone marrow failure syndrome (Table 2). In these patients, bone marrow failure dominates the clinical picture and hemolysis is primarily an incidental finding.23-25 The majority of patients with PNH/AA and PNH/MDS have relatively small PNH clones (Figure 3A, right), and require no specific PNH therapy; in these cases, treatment should focus on the underlying bone marrow failure syndrome (Figure 4). Among patients who present with clinical PNH in the setting of bone marrow failure, treatment of complications of PNH (eculizumab for hemolysis and anticoagulation for thrombosis) is required in approximately 50% of cases.24 There is no evidence that treatment with immunosuppressive therapy influences clonal expansion either positively or negatively.
The size of the PNH clone appears to be unaffected by treatment with immunosuppressive therapy, and the presence of a PNH clone should not deter immunosuppressive therapy if that approach to treatment of the underlying bone marrow failure syndrome is considered appropriate.23,24 In instances in which, following immunosuppressive therapy for treatment of the bone marrow failure component of the disease, the size of the PNH clone is sufficiently large so as to produce clinical symptoms, the patient can be managed using the same approach as for patients with classic PNH.
Classic PNH.
Patients with classic PNH have a large clone (>50%) (Figure 3B, right); consequently this disease subcategory is characterized by florid intravascular hemolysis as indicated by a markedly elevated serum LDH (Table 2). Patients may complain of episodic hemoglobinuria, and most experience ongoing constitutional symptoms dominated by lethargy, malaise, and asthenia that can be debilitating. The complement-mediated intravascular hemolysis of PNH can be inhibited by blocking formation of the MAC (Figure 2). The MAC consists of complement components C5b, C6, C7, C8, and multiple molecules of C9 (Figure 2). Eculizumab (Soliris, Alexion Pharmaceutics, Inc) is a humanized monoclonal antibody that binds complement C5, preventing its activation to C5b by the APC C5 convertase and thereby inhibiting MAC formation (Figure 2).12 In 2007, eculizumab was approved by both the US Food and Drug Administration and the European Medicines Agency for treatment of the hemolysis of PNH. Treatment of patients with classic PNH with eculizumab reduces transfusion requirements, ameliorates the anemia of PNH, and improves quality of life by resolving the debilitating constitutional symptoms associated with chronic complement-mediated intravascular hemolysis.32-34 Following treatment, serum LDH concentration returns to normal or near normal, with approximately one-half to two-thirds achieving transfusion independence, but mild to moderate anemia, hyperbilirubinemia, and reticulocytosis persist in essentially all treated patients.10,33,34
Because patients with congenital deficiency of complement C5 are at risk for meningococcal infections, all patients treated with eculizumab must be vaccinated prior to initiation of therapy.12 Vaccines are now available that help protect against all 3 serogroups (B, C, and Y) of meningococcal disease. In some countries, antibiotic prophylaxis is also mandated for patient treated with eculizumab.
Eculizumab is expensive (in the range of $600 000/y in the United States), and it has no effect either on the underlying stem cell abnormality or on the associated bone marrow failure. Consequently, treatment must continue indefinitely, and leukopenia, thrombocytopenia, and reticulocytopenia, if present, persist. Treatment with eculizumab may have a favorable impact on survival; a study of 79 patients treated between 2002 and 2010 showed the same survival as that of age- and sex-matched controls from the general population.10,11 The contribution of eculizumab to survival could not be quantified definitively, however, as a control patient group (PNH patients not treated with eculizumab) was not included in that study. Eculizumab appears to be safe for use in pregnancy, but whether it improves fetal-maternal outcomes is speculative because prospective, randomized studies that address this issue are lacking.35
Reasons for eculizumab failure.
The recommended maintenance dose of eculizumab is fixed (900 mg every 2 weeks ± 2 days) rather than being based on weight or body surface area. Some patients may show evidence of breakthrough intravascular hemolysis (ie, a rise in LDH and development of constitutional symptoms) near the end of a 14-day treatment cycle. In these cases, breakthrough hemolysis can be ameliorated by reducing the length of the treatment cycle to 13 days or 12 days, and, in some instances, the maintenance dose of eculizumab may need to be increased.
All patients with PNH have an element of bone marrow failure, and patients treated with eculizumab who have higher degrees of relative reticulocytopenia may remain anemic or even transfusion-dependent despite excellent control of intravascular hemolysis. Iron stores and serum erythropoietin concentration should be quantified in these patients, and if iron stores are adequate and serum erythropoietin concentration is inappropriately low, a trial of recombinant erythropoietin is warranted in patients who have symptomatic anemia or who are transfusion-dependent.
Following treatment with eculizumab, serum LDH returns to normal or near normal, but mild to moderate anemia and laboratory evidence of hemolysis persist in essentially all treated patients.33,34 A subgroup of eculizumab-treated patients experience little improvement in either anemia or constitutional symptoms. In these patients, hemolysis may be mediated by opsonization of the PNH erythrocytes by activation and degradation products of complement C3 and, when tested, the peripheral blood erythrocytes are found to be direct antiglobulin test–positive for C3 but not immunoglobulin G.36-38 The known pathophysiology of the PNH predicts that CD55 deficiency would result in ongoing extravascular hemolysis of PNH erythrocytes as a consequence of C3 opsonization as eculizumab does not block the activity of the APC C3 convertase that is unregulated on erythrocytes because of DAF and MIRL deficiency (Figure 2). Support for this hypothesis is provided by studies38 that showed that, in patients treated with eculizumab, a portion of the PNH erythrocytes (ie, the CD59-deficient population) had complement C3 bound. Those studies also confirmed the direct antiglobulin test–negative designation of PNH because no C3 was found bound to PNH erythrocytes before initiation of treatment with eculizumab, implying that PNH erythrocytes upon which complement has been activated are destroyed as a direct consequence of MAC-mediated cytolysis. Those studies provide a plausible explanation for the persistent hemolytic anemia observed in PNH patients treated with eculizumab. By inhibiting formation of the MAC, eculizumab prevents direct cytolysis of PNH erythrocytes, allowing the manifestations of DAF and MIRL deficiency to become apparent in the form of aberrant regulation of the APC C3 convertase and the consequent deposition of activated C3 on the cell surface (Figure 2).21,39 Covalently bound activation and degradation products of C3 then serve as opsonins that are recognized by specific receptors on reticuloendothelial cells resulting in extravascular hemolysis.
The extravascular hemolysis of patients with PNH receiving eculizumab does not require treatment in the absence of constitutional symptoms, symptoms of anemia, or transfusion dependence. Because the process is extravascular, splenectomy or corticosteroids may ameliorate the hemolysis in symptomatic or transfusion-dependent patients by removing or inhibiting the function of phagocytic cells (Figure 4).36,40 Responses to treatment of the C3-mediated extravascular hemolysis observed in some patients treated with eculizumab are anecdotal. Moreover, long-term use of corticosteroids is associated with significant toxicity, and concerns about both postoperative and late complications temper enthusiasm for splenectomy. Further, it is conceivable that the primary site of phagocytosis is hepatic rather than splenic. In such cases, response to splenectomy would likely be suboptimal. Based on experience in the treatment of refractory autoimmune hemolytic anemia, a trial of danazol can be considered, however, rituximab is not indicated as phagocytosis is mediated by C3 opsonization rather than opsonization by immunoglobulin G antibody. New drug development is targeting components of the C3 convertase (Figure 2). If proven safe and efficacious, such agents would provide an option for therapy for patients with PNH with clinically significant extravascular hemolysis in the setting of MAC inhibitory therapy.41
Nishimura and colleagues identified a polymorphism of C5 in 11 Japanese patients with PNH who had a poor response to treatment with eculizumab.42 An anti-C5 antibody directed against an epitope different from that recognized by eculizumab completely blocked the hemolytic activity in the sera of the patients with the C5 polymorphism. The prevalence of the polymorphism in the Japanese population appears to be in the range of 3.5%. The polymorphism was also identified in the Han Chinese population. New approaches to C5 inhibition (C5 antibodies targeting various epitopes, naturally occurring inhibitors of C5, and small interfering RNA technology targeting hepatic C5 RNA) are in development and would be expected to be effective in such cases of eculizumab resistance.41
Management of the thrombophilia of PNH.
Thromboembolic complications are the leading cause of morbidity and mortality in PNH.43 Prophylaxis against thromboembolic events in patients with PNH is an issue of active debate.3 Current estimates of risk are based on retrospective analysis, but risk may correlate with size of the PNH clone (based on flow cytometric determination of the percentage of GPI-AP–deficient PMNs), leading to the recommendation that patients with >50% to 60% GPI-AP–deficient PMNs be offered prophylactic anticoagulation.43-46
Although arterial thrombosis may be observed,43,47 thromboembolic events in patients with PNH usually involve the venous system. In the absence of an absolute contraindication (eg, hemorrhagic infarction involving the central nervous system), acute thrombotic events require anticoagulation with heparin. Systemic thrombolytic therapy, or thrombolytic therapy delivered via canalization directly to the affected site, can be considered in patients with acute onset of Budd-Chiari syndrome.48-51 If there is no contraindication, anticoagulation should continue indefinitely for a patient with PNH (even if that patient is treated with eculizumab) who has experienced a thromboembolic complication. For PNH patients on long-term anticoagulation, a vitamin K antagonist such as Coumadin has been used customarily. Whether low-molecular-weight heparins or novel oral anticoagulants such as direct thrombin inhibitors and factor Xa inhibitors are more, less, or equally efficacious has not been determined.
Thrombocytopenia often complicates PNH, and this issue must be addressed when formulating an anticoagulation management plan. Thrombocytopenia is a relative, but not an absolute, contraindication to anticoagulation, and transfusions should be given to maintain the platelet count in a safe range rather than withholding therapy. Patients with PNH who experience a thromboembolic event are candidates for indefinite anticoagulation, although recommendations for anticoagulation duration may be modified as more experience with the effects of eculizumab on the thrombophilia of PNH accumulates.43
Eculizumab appears to reduce the risk of thromboembolic complications.10,43,47 For patients being treated with eculizumab who have no prior history of thromboembolic complications, prophylactic anticoagulation may be unnecessary, although it is recommended that anticoagulation continue for those patients who experienced a thromboembolic event prior to initiating therapy with eculizumab.43
Conclusions and future directions
Systematic investigation of the molecular basis of PNH has provided a framework for management based on an understanding of disease pathophysiology and has led to development of targeted therapy that has improved the lives of patients and changed the natural history of the disease. Nonetheless, continued investigation of new approaches to therapy aimed at obviating the extravascular hemolysis that limits eculizumab efficacy in some patients is warranted.39,41 A better understanding of the pathobiology that underlies the thrombophilia of PNH is needed, and defining the complex relationship between PNH and bone marrow failure syndromes that determine clonal selection and clonal expansion may lead ultimately to therapy that targets the disease at the level of the hematopoietic stem cell.5
Correspondence
Charles J. Parker, Division of Hematology and Hematologic Malignancies, University of Utah School of Medicine, 30 North 1900 East, Room 5C 402, Salt Lake City, UT 84132; e-mail: charles.parker@hsc.utah.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.

![Figure 4. Management of PNH. A management scheme based on classification of PNH into 3 subcategories (subclinical, PNH in the setting of another bone marrow failure syndrome [PNH/BMF], and classic PNH. See Table 2 for characteristics of each category.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2016/1/10.1182_asheducation-2016.1.208/6/m_hem088419f4.jpeg?Expires=1767741586&Signature=rLgGYOjqWTGZYaX9U9gvSFUSvF29lvNWA8rvQirsZ4i7TOzw2JP4fjV7HoXBgGcJ8IyF99qJhkvIBDlUdBbREXoCzgr-E5EbffIjAd0-2S1LdfY7rJ3vW2-~gRBoMNQUTE4y6VHUrRcxB2FY-i01xoLVbQVddbsvUVoImnzuRRYE4~TjZ2NH0QgJ887RUf-G0mIMoV2B7Xl4FVCXdpeFKmUf1NLNuu6Lhqx35y0C3iyAS5qZNdKTWG1kmHJm0ztZG2PEN2S0Ks0HxiDyjPdstwWe499-K7LWyagHy9z6kG1LcmD10KcP7ROpENJdwW6YbLUNw4jKWL7FqaETSxwszA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Management of PNH. A management scheme based on classification of PNH into 3 subcategories (subclinical, PNH in the setting of another bone marrow failure syndrome [PNH/BMF], and classic PNH. See Table 2 for characteristics of each category.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2016/1/10.1182_asheducation-2016.1.208/6/m_hem088419f4.jpeg?Expires=1768161263&Signature=CoW-YOL8UgLQqSMWPpmodB~erc8Bv598XxIf7novczEGN2dwMnskjbnJiDdGHUzfCL2OKFZa1IuJ1yMJtiqogBMe~W80S-YEmEwHXMs2sDCJ8bm0F8ZapOodiY4uCqsq~VtBmFfjhn9PPsNtdf9XXEDOxJAET6DnYxFros6f4xQYSvH8gisRGY1zLyc5zROIbyyMz1DwC0Cm6CxOEmkkFLe8oTY-ncpP-Qk-qxDK5m8R4apaqVY67S2VesTOOLrM2l-LhPe0d0QMmE8e1pjUncrLUAwrQ7Gbw4KmEczuXKSrVq60FLNzQuhydsK9dZykGCF3nnBgT2AH950p74j0sw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)