Abstract
Primary chronic cold agglutinin disease (CAD) is a well-defined clinicopathologic entity in which a specific, clonal lymphoproliferative B-cell bone marrow disorder results in autoimmune hemolytic anemia. The immune hemolysis is entirely complement-dependent, predominantly mediated by activation of the classical pathway and phagocytosis of erythrocytes opsonized with complement protein C3b. Typical clinical features in CAD have diagnostic and therapeutic implications. Pharmacologic treatment should be offered to patients with symptom-producing anemia or disabling circulatory symptoms. CAD should not be treated with corticosteroids. Based on an individualized approach, rituximab monotherapy or rituximab-fludarabine in combination is recommended as first-line therapy. Rituximab-bendamustine is still an investigational therapy. Although complement-modulating agents are still to be considered experimental in CAD, therapy with the anti-C1s monoclonal antibody TNT009 seems promising.
Learning Objectives
To understand CAD as a clonal, lymphoproliferative bone marrow disorder
To evaluate and use data from prospective trials to provide effective, clonally directed therapy to patients with CAD when indicated
To understand the complement-dependent hemolysis in CAD
To gain insight in experimental data on complement inhibition and be able to evaluate future clinical studies
Background
Primary chronic cold (hem)agglutinin disease (CAD) accounts for about 15% of autoimmune hemolytic anemias (AIHAs).1 CAD is defined as an AIHA mediated by cold agglutinins (CAs), without any obvious underlying disease such as aggressive lymphoma, other overt malignancies, or specific infections.2,3 CAs are autoantibodies that are able to agglutinate red blood cells (RBCs) at an optimum temperature of 3-4°C, but can also react at higher temperatures, depending on the thermal amplitude. CAD is now regarded as a well-defined clinicopathologic entity and should be called a disease, not syndrome.3,4 The term cold agglutinin syndrome is appropriate for the still more uncommon, secondary CA-mediated hemolytic anemia occasionally complicating other specific diseases, such as Mycoplasma pneumoniae pneumonia, Epstein-Barr virus infection, or aggressive lymphoma.3
Cold hemagglutination was discovered more than 100 years ago, and the first monoclonal protein ever identified was a CA from a patient with CAD.5,6 Although characteristic electrophoretic findings were described already during the 1960s, the clonal nature of CAD has not been fully understood until the past few years.4,7-9 This insight has resulted in specific, more successful therapeutic approaches.2,3 It has also been known for decades that the complement system is involved in RBC breakdown in CAD.10 Results of recent studies on the role of complement and the evolving possibilities of pharmacologic inhibition provide a rationale for trials of therapeutic complement modulation in CAD.11,12
This review will focus on CAD as a distinct, clonal lymphoproliferative disease. Recent achievements in understanding the clinical, histopathologic, and immunologic features will be highlighted as will the entirely complement-mediated RBC destruction. The therapeutic consequences of these disease features will be discussed.
CA-associated lymphoproliferative bone marrow disease
In a multicenter descriptive study of 86 patients with primary CAD, a monoclonal serum immunoglobulin was identified by electrophoresis and/or immunofixation in 81 patients.8 In clinical practice, the frequency of positive electrophoretic findings will be somewhat lower. The immunoglobulin class and light chain restriction was immunoglobulin Mκ (IgMκ) in 71 patients (88%), whereas monoclonal IgG, IgA, “biclonal” IgA + IgG, or λ phenotype was rare to find. Flow cytometry of bone marrow aspirates in 40 patients showed a ratio >3.5 between κ and λ positive B cells in 36 patients (90%); the median ratio was 7.8 (range, 0.9-186).8
Two large studies of consecutive patients from Norway and the United States, respectively, found signs of bone marrow clonal lymphoproliferative disease (LPD) in most patients.8,9 Undoubtedly, this majority represents the same group of patients that has traditionally been diagnosed with “primary” or “idiopathic” CAD. Within each series, however, the individual hematologic and histologic diagnoses showed a striking heterogeneity. In the Norwegian cohort, lymphoplasmacytic lymphoma (LPL) was the most frequent finding (50% of the patients), whereas marginal zone lymphoma, unclassified clonal lymphoproliferation, and reactive lymphocytosis were also frequently reported.8 In the American study, 61% of the patients were classified retrospectively as having monoclonal gammopathy of undetermined significance, whereas “macroglobulinemia” accounted for 9% and “other lymphoma” 12%.9 Until a few years ago, in accordance with both studies, we presumed a considerable overlap between CAD and Waldenström macroglobulinemia (WM).13,14
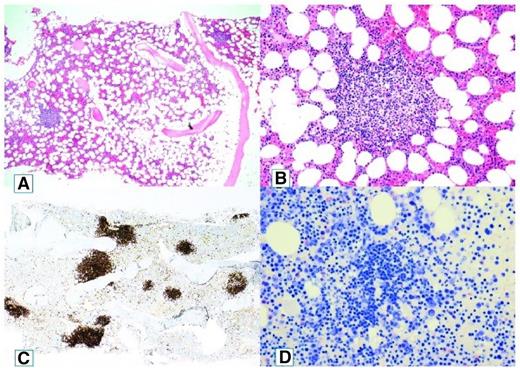
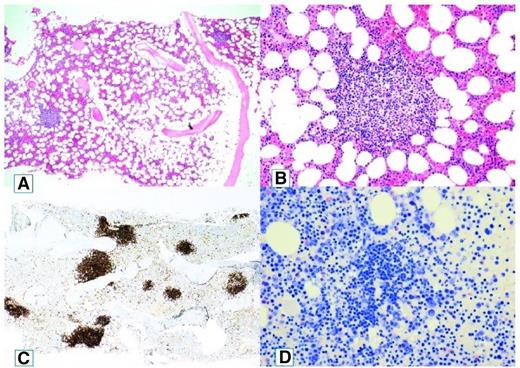
A recent, comprehensive study by our group revealed the apparent explanation for this perceived heterogeneity.4 Bone marrow biopsy samples and aspirates from 54 patients with CAD were systematically reexamined by a group of lymphoma pathologists using a standardized panel of morphologic, immunohistochemical, flow cytometric, and molecular methods. The findings were consistent with a surprisingly homogeneous bone marrow disorder, which we termed “primary CA-associated LPD” and found to be distinct from LPL/WM, marginal zone lymphoma, and other previously recognized lymphoma entities (Figure 1).
Cold agglutinin–associated lymphoproliferative bone marrow disease. Bone marrow trephine biopsy showing intraparenchymatous nodular lymphoid lesions (A-B, hematoxylin and eosin staining, original magnification ×40 and ×200, respectively). Immunoperoxidase staining for CD20 highlights intraparenchymatous nodular B-cell infiltration (C, original magnification ×200). Mast cells are not discerned around the nodular lymphoid lesions (D, Giemsa staining, original magnification ×200). Reprinted from Randen et al4 with permission.
Cold agglutinin–associated lymphoproliferative bone marrow disease. Bone marrow trephine biopsy showing intraparenchymatous nodular lymphoid lesions (A-B, hematoxylin and eosin staining, original magnification ×40 and ×200, respectively). Immunoperoxidase staining for CD20 highlights intraparenchymatous nodular B-cell infiltration (C, original magnification ×200). Mast cells are not discerned around the nodular lymphoid lesions (D, Giemsa staining, original magnification ×200). Reprinted from Randen et al4 with permission.
We found nodular B-cell aggregates in the bone marrow biopsy specimens from 40 of 54 patients with CA-associated LPD, whereas 14 patients showed only scattered B cells.4 In the former samples, median lymphoid infiltration was 10% of the intertrabecular surface (range, 5%-80%). Features associated with LPL such as paratrabecular growth, fibrosis, lymphoplasmacytoid cell morphology, or an increased number of mast cells surrounding the lymphoid aggregates were not seen. Differences between CAD and LPL were also observed by immunohistochemical and flow cytometric analysis.4 A recent British flow cytometry study also showed significant immune phenotypic differences in clonal B cells between CAD and WM.15 Although a specific immune phenotypic “signature” for CAD has recently been suggested,15 more data should be awaited.
The MYD88 L265P somatic mutation could not be detected by polymerase chain reaction in any of 17 samples from patients with CAD tested for this mutation in our study, as compared with 96% of control samples from patients with typical LPL/WM.4 In the British study, 21% of the bone marrow samples from CAD patients were polymerase chain reaction–positive for the MYD88 L265P mutation, in contrast to 70% to 100% of WM patients according to the literature.15,16 We demonstrated somatically mutated monoclonal IGHV4-34 gene rearrangement in 8/8 samples that could be tested (95% median sequence homology with germline).4 Table 1 shows that V gene usage differs between CAD and WM, supporting the conclusion that these are different entities.4,17-20
A recent study found highly homologous complementarity determining regions 3 in 9 patients with IGKV3-20 gene rearrangement.19 It is too early to extend this finding to CAD patients in general.
Complement-dependent hemolysis
Most CAs in CAD have specificity for the I carbohydrate antigen, which is almost universally present at the RBC surface in adults and children older than age 18 months.18,21 During passage through acral parts of the body, cooling of the blood allows CAs to bind to RBCs and cause agglutination. In general, the pathogenicity of CAs is more dependent on the thermal amplitude (TA; the highest temperature at which the CAs will react with the antigen) than on the titer.22 Normally occurring CAs have low thermal amplitudes. If the TA exceeds 28 to 30°C, RBCs will agglutinate in acral parts of the circulation even at mild ambient temperatures and, often, complement fixation and complement-mediated hemolysis will ensue.
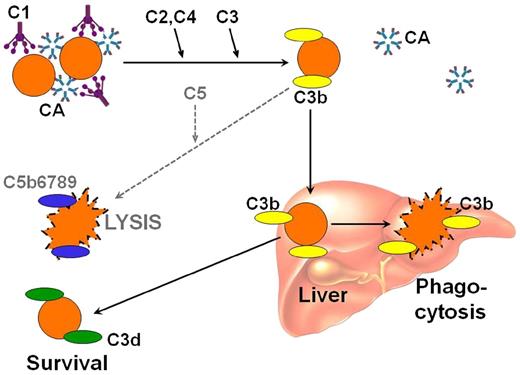
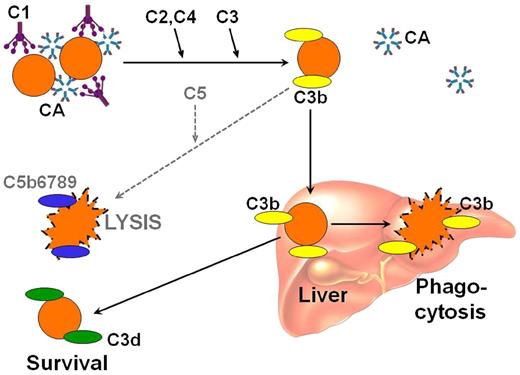
Antigen-bound IgM-CAs on the cell surface bind complement protein 1q (C1q) and thereby initiates the classical complement pathway (Figure 2).10,12,23,24 C1 esterase activates C2 and C4, generating C3 convertase, which results in the cleavage of C3 to C3a and C3b. Upon warming to 37°C in the central circulation, CAs detach from the cell, allowing agglutinated erythrocytes to separate, whereas C3b remains bound. A proportion of the C3b-coated cells is sequestered by macrophages of the reticuloendothelial system, mainly in the liver.10,25 On the surface of the surviving RBCs, C3b is cleaved, leaving high numbers of C3d molecules on the cell surface. In the standard direct antiglobulin test (DAT), the erythrocytes of CAD are positive in the polyspecific assay (that contains both anti-IgG and anti-C3); and testing with monospecific anti-IgG and monospecific anti-C3 typically shows that the cells are strongly positive for C3 only, although weak positivity for IgG is observed in up to 20% of cases.8
Complement-mediated hemolysis in cold agglutinin disease. Antigen-bound CA on the cell surface binds C1q and initiates the classical complement pathway. C1 esterase activates C2 and C4, generating C3 convertase, which results in the cleavage of C3 to C3a and C3b. Upon warming to 37°C, CA detaches from the cell, allowing agglutinated erythrocytes to separate, whereas C3b remains bound. A proportion of the C3b-coated cells is sequestered by macrophages of the reticuloendothelial system, mainly in the liver. On the surface of the surviving RBCs, C3b is cleaved, leaving high numbers of C3d molecules on the cell surface. In some situations, complement activation may proceed beyond the C3b step with cleavage of C5, resulting in activation of the terminal pathway and intravascular hemolysis. C, complement protein. Reprinted from Berentsen and Sundic24 with permission.
Complement-mediated hemolysis in cold agglutinin disease. Antigen-bound CA on the cell surface binds C1q and initiates the classical complement pathway. C1 esterase activates C2 and C4, generating C3 convertase, which results in the cleavage of C3 to C3a and C3b. Upon warming to 37°C, CA detaches from the cell, allowing agglutinated erythrocytes to separate, whereas C3b remains bound. A proportion of the C3b-coated cells is sequestered by macrophages of the reticuloendothelial system, mainly in the liver. On the surface of the surviving RBCs, C3b is cleaved, leaving high numbers of C3d molecules on the cell surface. In some situations, complement activation may proceed beyond the C3b step with cleavage of C5, resulting in activation of the terminal pathway and intravascular hemolysis. C, complement protein. Reprinted from Berentsen and Sundic24 with permission.
Complement activation may proceed beyond the C3b formation step, resulting in C5 activation, formation of the membrane attack complex (MAC) and intravascular hemolysis. Surface-bound regulatory proteins such as CD55, which has an inhibitory function at the C4-C2 level and CD59, which prevents final assembly of the MAC at the C8-C9 stage, regulate these steps. Based on early isotope studies and supported by recent in vitro experiments, is it assumed, therefore, that a major mechanism of hemolysis in stable disease is the extravascular destruction of C3b-coated RBCs.10,11,25 Undoubtedly, however, C5-mediated intravascular hemolysis does occur in severe acute exacerbations and in some profoundly hemolytic patients, as evidenced by the finding of hemoglobinuria in 15% of the patients, the occasional observation of hemosiderinuria, and the beneficial effect of C5 inhibition in a cohort of patients with CAD.8,9,26-28
Febrile infections, major trauma, or major surgery can result in acute exacerbation of hemolytic anemia.8,26,27 The probable explanation is that during steady-state chronic disease, most patients are complement-depleted with low levels of C3 and often undetectable levels of C4.18 During acute phase reactions, C3 and C4 are replete and complement-mediated hemolysis is enhanced.27
Diagnosis and clinical features
The diagnostic criteria for CAD are chronic hemolysis, positive polyspecific DAT, monospecific DAT strongly positive for C3d, CA titer ≥64 at 4°C, and no overt malignant disease by clinical (and, if required, radiological) examination.2,3 The CA titer is usually much higher than the minimum titer required for diagnosis and, as noted previously, the monospecific DAT may be weakly positive for IgG in up to 20% of patients.8 Determination of the TA is time-consuming and, in most cases, not required for diagnosis.3,18 TA determination may be useful in some situations, however, especially to rule out normally occurring CAs as a cause of false-positive findings in cases with relatively low titers.
Serum electrophoresis with immunofixation, Ig class quantification, flow cytometry in a bone marrow aspirate, and examination of a bone marrow biopsy should always be undertaken to detect and characterize the clonal LPD. Negative results do not exclude primary CAD, however, because this may be a matter of sensitivity. For serum immunoglobulin analyses and CA titration, it is of critical importance that blood specimens are kept at 37 to 38°C from sampling until serum has been removed from the clot.
The clinical presentation in CAD often provides an important clue to the differential diagnosis, making a focused history and clinical examination even more informative than in warm AHIA. Ninety percent of patients have cold-induced circulatory symptoms, which can range from slight acrocyanosis to disabling Raynaud phenomena.8 Approximately 70% have experienced exacerbation of anemia during febrile infections, as described previously. In Scandinavia, the median hemoglobin (Hb) level in CAD is 9.0 g/dL and the lower tertile 8.0 g/dL. About half of untreated patients are considered transfusion dependent for shorter or longer periods.8
Nonpharmacologic management and unspecific drug therapy
Not all patients require pharmacological therapy. The attitude toward active treatment has probably been too restrictive, however, because of the lack of effective drugs until the past 10 to 15 years and an underestimation of the clinical symptoms. In descriptive studies, 70% to 80% of patients were reported to have received drug therapy.8,9
Recommendations on warm clothing and so on have been described elsewhere.3,29 Many patients have discovered these measures by themselves far before they see the hematologist. Often, however, it is important for the specialist to provide doctors and health care professionals with adequate instructions. In the ward or outpatient department, patients with CAD should keep warm and infusion of cold liquids should be avoided. Any bacterial infection should be treated. Transfusions can safely be given when indicated, provided the necessary precautions are observed; compatibility problems typical for warm AIHA are not encountered in CAD.3,29 The patient and the extremity chosen for transfusion should be kept warm, and the use of an in-line blood warmer is recommended. Failure to observe such precautions has resulted in dismal or, very rarely, even fatal outcomes.30 Because elevated complement protein levels can exacerbate hemolysis, transfusion of blood products with a high plasma content should probably be avoided.27
Corticosteroids should not be used to treat CAD. Among 38 consecutive patients seen at the Hammersmith Hospital in London, only occasional patients responded to therapy with steroids.31 Several other groups have reported similar clinical experience.32 Studied retrospectively, 43% of unselected Norwegian patients with CAD had received steroids for shorter or longer periods.8 Only 14% of those treated achieved a significant response, and most of the few patients who did respond required unacceptably high doses to maintain the remission.
In a retrospective series on primary CAD, we identified 3 splenectomized patients, none of whom had responded to the splenectomy.8 Because clearance of C3b-opsonized RBCs mainly occurs in the liver, this observation is not surprising. Improvement after splenectomy has been occasionally reported among the rare patients with CAD mediated by IgG instead of IgM.33 Because almost all IgM is intravascular, plasmapheresis efficiently induces clinical improvement in acute situations or before surgery requiring hypothermia.3,29 Such remissions are short-lived, and concomitant specific therapy should be initiated.
Clonally directed therapies
During the past 12 to 15 years, targeting the pathogenic B-cell clone has resulted in relatively successful therapy for CAD.34-36
Rituximab monotherapy
Single-agent therapy with rituximab 375 mg/m2 weekly for 4 weeks was studied in 2 prospective, uncontrolled trials of 37 and 20 treatment courses, respectively.34,35 Table 2 shows the response criteria used in our trial. The Danish study used similar strict definitions.35 The overall response rate was 54% and 45%, respectively, in the 2 trials. With the exception of 1 CR observed in the Norwegian trial, all remissions were PRs. Ten patients were treated for relapse after previously having received rituximab therapy; 6 of them responded to a second course. In our study, the responders achieved a median increase in Hb levels of 4.0 g/dL. Median time to response was 1.5 months (range, 0.5-4.0) and median response duration 11 months (range, 2-42).34
“Real-life” descriptive studies8,9 have confirmed the essential results of the prospective trials; rituximab monotherapy is an efficient treatment of primary CAD. CR is uncommon, however; the median response duration is relatively short; and the number of nonresponders is considerable. Retreatment is feasible, and adverse events are few and tolerable.8,34,35 There are no published data on rituximab maintenance therapy.
Rituximab and fludarabine combination therapy
In a prospective, uncontrolled trial, 29 patients received rituximab 375 mg/m2 on days 1, 29, 57, and 85, and fludarabine orally, 40 mg/m2, on days 1 through 5, 29 through 34, 57 through 61, and 85 through 89.36 Growth factors, cotrimoxazole, or antiviral agents were not used routinely. We used the same response criteria as in previous studies (Table 2). Responses were observed in 22 patients (76%); 6 (21%) achieved CR and 16 (55%) achieved PR. Ten patients had previously been nonresponsive to rituximab monotherapy. In this subgroup, CR was observed in 1 patient and PR in 6. Median increase in Hb level was 3.1 g/dL in the responders and 4.0 g/dL among those who achieved CR. Median time to response was 4.0 months, and estimated median response duration was more than 66 months.
Hematological toxicity grade 3-4 was observed in 12 patients (41%).36 Seventeen patients (59%) had grade 1-3 infection, which was successfully treated. Three patients (10%) experienced herpes zoster reactivation, but Pneumocystis jirovecii pneumonia did not occur. Fludarabine-induced warm AIHA was not observed, but 3 patients (10%) experienced a transient, mild exacerbation of CAD precipitated by infection.27,36 The study was not designed to address the risk of myelodysplasia or late-occurring hematologic malignancies. Although not specific to nucleoside analogs, such late events have been reported after fludarabine-based therapy for WM.37
After rituximab monotherapy, but not after the rituximab-fludarabine combination, we also observed a few patients whose Hb levels improved without meeting the formal response criteria. In both studies, some of the nonresponders had their IgM levels reduced even though the anemia or circulatory symptoms did not improve.34,36
Comparison of nonrandomized trials must be undertaken with caution. Nevertheless, the much higher response rates, considerable frequency of CR, and very long response duration observed after the combination therapy compare favorably with the results achieved by rituximab monotherapy. Toxicity, however, was more frequent with the combination.
Other clonally directed therapies
Favorable response to bendamustine-rituximab combination therapy has been described in a case report.38 In a recent trial by our group, eligible patients received bendamustine 90 mg/m2 on days 1 and 2, 29 and 30, 57 and 58, and 85 and 86 and rituximab 375 mg/m2 on days 1, 29, 57, and 85.39 Safety and efficacy seem promising, but the data are currently being analyzed and the results have not yet been published. Response to bortezomib-based therapy has been reported in single cases, and an Italian prospective trial is ongoing.40,41 There are no published data on therapy with novel B-cell receptor pathway modulators such as ibrutinib and idelalisib, and the theoretical rationale for Bruton tyrosine kinase inhibitors is weaker in CAD than in WM.
Future perspective: complement modulation?
The monoclonal anti-C5 antibody eculizumab is a powerful therapeutic agent in paroxysmal nocturnal hemoglobinuria (PNH).42 In PNH, however, the predominant route of complement-mediated hemolysis is the intravascular cell destruction caused by activation of the terminal complement pathway, starting with C5 cleavage. Moreover, regulation of terminal complement activation by CD59, known to be deficient in PNH, is intact in CAD. Nevertheless, eculizumab therapy did produce improvement of CAD according to a recently completed, small prospective trial.28 This is the first documentation of clinical effect of complement inhibition in CAD. The findings support the hypothesis that in addition to phagocytosis of C3-opsonized RBCs, terminal complement activation contributes to hemolysis, at least in some patients and situations.
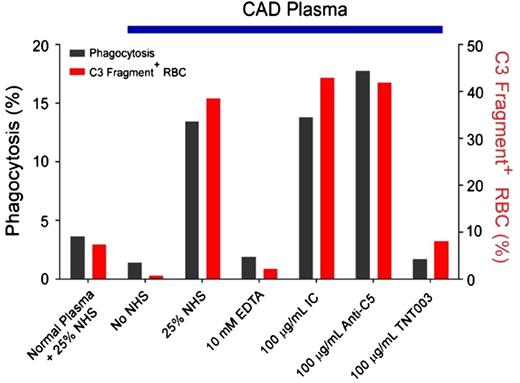
An in vitro study published in 2014 tested the effects of TNT003, a mouse monoclonal antibody targeting C1s, on CA-induced complement activity against human RBCs.11 Using CA samples from 40 patients with CAD, the authors found that TNT003 prevented CA-induced erythrophagocytosis by a phagocytic cell line (Figure 3). The monoclonal antibody also prevented deposition of C3 fragments on the RBCs at the same concentration of antibody that stopped phagocytosis. Furthermore, TNT003 blocked the CA-induced, classical pathway-driven generation of anaphylotoxins C4a, C3a, and C5a. Of importance, CA from only 1 patient sample of 40 was able to directly induce terminal complement activation and MAC-mediated hemolysis. These findings reveal a very promising potential for investigating a corresponding humanized antibody, TNT009, for the treatment of CAD. In addition, the results support the existing view that complement activity in CAD terminates before activation of the terminal pathway in most patients.11 The activity, safety, and tolerability TNT009 is now being clinically tested, and a phase 1B trial has shown favorable results.43
TNT003 prevents complement C3 deposition and phagocytosis of RBCs exposed to CAD patient plasma in the presence of normal human serum. Representative assay using a single patient sample. IC, internal control; NHS, normal human serum. Columns below blue bar represent measurements after addition of cold agglutinin (patient plasma). Reprinted from Shi et al.11
TNT003 prevents complement C3 deposition and phagocytosis of RBCs exposed to CAD patient plasma in the presence of normal human serum. Representative assay using a single patient sample. IC, internal control; NHS, normal human serum. Columns below blue bar represent measurements after addition of cold agglutinin (patient plasma). Reprinted from Shi et al.11
Small molecule peptide inhibitors of the classical complement pathway are also of potential interest.44,45 Peptide inhibitor of complement C1 is a recently described class of such molecules that targets C1q, blocking the activation of associated serine proteases (C1s-C1r-C1r-C1s) and subsequent downstream complement activation.46 These molecules have also been shown to inhibit the lectin pathway. Peptide inhibitor of complement C1 has been studied in acute hemolytic transfusion reaction in animal models but not, so far, in CA-related experiments.
Compstatin is a class of low-molecular-weight peptides that block cleavage of C3.45 Although no data have been published for CA-induced hemolysis, compstatin has been found to efficiently prevent in vitro lysis of RBCs from PNH patients.47
Taking into consideration that the complement system is part of the innate immune system, it is important to ask whether complement modulation will be too dangerous. Regarding eculizumab therapy in PNH, we know that the risk of severe infection following C5 blockade is negligible provided the patients can be efficiently protected against meningococci.42 Inhibition at the C3 level may, in theory, carry a higher risk because it will completely block complement activation beyond this level, whether initiated by the classical, alternative, or lectin pathway.47,48 Interestingly, the still more proximal blockade at the C1 level achieved by TNT009 will selectively affect the classical pathway as required for control of hemolysis in CAD, whereas the lectin and alternative pathways will remain intact. Probably, therefore, these pathways will still enable the system to generate anaphylotoxins C3a and C5a in response to microbial stimuli.11,23,48 Although this selectivity may, theoretically, reduce the risk of infection, careful studies will be required to address this issue. Temporary complement inhibition in acute situations (eg, acute phase reaction–induced exacerbation, preoperative management) may be expected to be less dangerous than long-term therapy.
Given that targeting the pathogenic B-cell clone is an effective and more causal therapeutic approach and immunochemotherapy is administered only for a limited period, do we need complement-directed therapies for CAD? First, immunochemotherapy is unsuccessful in at least 25% of the patients because of treatment failure or toxicity.2,8,36 The pathogenic B-cell clone can be small with low proliferation activity and difficult to target efficiently.4 Second, rapidly acting therapy seems required in some specific clinical settings. Examples of such situations are acute severe exacerbation induced by febrile infections, major surgery, or trauma; and, possibly, before cardiac surgery in selected patients. Anticomplement therapy might also prove useful in severe cases of secondary cold agglutinin syndrome. It should be emphasized, however, that complement inhibition cannot be expected to ameliorate the cold-induced circulatory symptoms, which are of major clinical importance in some patients with CAD.
Correspondence
Sigbjørn Berentsen, Department of Research and Innovation, Haugesund Hospital, Helse Fonna, PO Box 2170, NO-5504 Haugesund, Norway; e-mail: sigbjorn.berentsen@haugnett.no.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Mundipharma and Roche, has consulted for True North Therapeutics and Alexion, and has received honoraria from Alexion.
Author notes
Off-label drug use is discussed for fludarabine, bendamustine, bortezomib, eculizumab, and TNT009.