Abstract
Pregnancy and the postpartum period substantially increase the risk for thrombotic events. Although the absolute risk for thrombosis is low, these events comprise a significant portion of maternal morbidity and mortality. The vast majority of such events are venous, although the risk for ischemic stroke also appears to be increased in pregnancy. This review will explore the overlapping and unique risk factors for venous and arterial thrombosis in pregnancy. Diagnosis and prevention will be discussed, and treatment will be briefly touched on. The benefit of using a multidisciplinary model in caring for pregnant women who have had a thrombotic event or who are at increased risk for thrombosis is a major focus of the review. Using the experience of our own Hematology and Obstetrics/Maternal Fetal Medicine shared care model, we discuss specific examples of when the use of such an approach is particularly valuable.
Learning Objectives
Recognize the benefits and the importance of a multidisciplinary care model when treating pregnant women with thrombotic complications
Understand current guidelines and recommendations for prevention, diagnosis, and treatment of arterial and venous thrombosis in pregnancy
Introduction
Pregnancy presents a paradoxical challenge to hemostatic balance, with hemorrhage and venous thromboembolism (VTE) being 2 of the leading causes of maternal death worldwide. In most areas of the world, hemorrhage is the primary source of maternal risk1 . The hemostatic system has evolved to reduce these risks by shifting to a procoagulant state in pregnancy. Both local and systemic adaptations facilitate avoidance of hemorrhage from the pregnant uterus, but these same changes also increase the risk for thrombosis. In developed countries, where hemorrhage is better managed and safe and accessible blood bank capabilities exist, VTE becomes a leading cause of maternal mortality.1 In the United States, for example, hemorrhage and pulmonary embolism (PE) each account for 10% to 13% of maternal deaths.2
There has been an apparent increase in the United States’ maternal mortality ratio (the number of maternal deaths resulting from pregnancy-related causes while pregnant or within 42 days of pregnancy termination per 100 000 live births) during the last 20 years. Although it is controversial whether improved ascertainment of maternal deaths explains this pattern, other developed nations have not seen this increase. In fact, the US maternal mortality ratio is almost double that of the United Kingdom, which has a robust system for case ascertainment.2 Importantly, surveillance data from the United States reveals that an increasing proportion of maternal deaths are attributable to PE, cardiovascular conditions, and cerebrovascular accidents.2 A proportion of such events are preventable, and there is evidence to suggest VTE may be one of the most preventable causes of maternal mortality.3
To optimize prevention of thrombotic outcomes of pregnancy, clinicians need an understanding of pathophysiology, risk factors, diagnosis, and management of thrombosis specific to pregnancy. A comprehensive, multidisciplinary approach is essential, as the clinical scenario is often made more complex by the specific obstetric context. This review focuses on the overlapping and unique risk factors for VTE and stroke in pregnancy. Diagnosis and treatment are also discussed. We highlight the benefits of a multidisciplinary care model. At our institution, for the past decade or more, we have formally discussed shared patients during a quarterly Thrombosis and Hemostasis Program, Maternal Fetal Medicine Conference. As a result, we have built close interdisciplinary relationships between obstetric care providers and hematologists. In our experience, this multidisciplinary model has been educationally rich and valuable in providing care to our pregnant patients with a history of, and/or significant risk factors for, venous and/or arterial thrombosis. Table 1 highlights some of the benefits of our care model.
Epidemiology, pathophysiology, and risk factors
Thrombosis in the venous and arterial circulation is substantially increased in pregnancy. Despite this, the absolute risk for VTE, arterial ischemic stroke, and cerebral vein thrombosis is low. In a retrospective study of more than 9 million pregnancy-associated hospital admissions, and more than 73 000 postpartum admissions, the risk for VTE was 1.72 per 1000 deliveries, of which 80% were deep vein thrombosis (DVT) and 20% were PE.4 Arterial thrombotic events were 4 times less common than VTE, although estimates of ischemic stroke incidence in pregnancy vary.4,5 Risk for cerebral vein thrombosis is increased in pregnancy and may account for a substantial portion of all pregnancy-related strokes.6,7 This increased risk for both venous and arterial thrombosis in pregnancy adds to the accumulating evidence that VTE and arterial thrombosis have shared risk factors.
Risk for VTE in pregnancy is highest around the time of delivery and immediately postpartum. About a third of pregnancy-related DVT and half of pregnancy-related PE occur after delivery.8 A systematic review reported that the risk in the first 6 weeks postpartum was increased 21.5-fold to 84-fold compared with risk in nonpregnant, nonpostpartum women.9 Similarly, the risk for arterial ischemic stroke is greatest in the postpartum period; in a retrospective review of 145 women with pregnancy-associated ischemic stroke, 44.8% were antepartum, 2.8% occurred during delivery, and 52.4% occurred within 6 weeks postpartum.10 Importantly, a recent large, retrospective crossover-cohort study confirmed earlier reports that the risk period for venous and arterial thrombotic events persists beyond the 6-week postpartum period, although the absolute increase in risk after 6 weeks was low. The odds ratio (OR) for a thrombotic event within 6 weeks after delivery was 10.8 compared with 2.2 between 6 and 12 weeks, with no increase beyond 12 weeks postpartum.11
The pathophysiology of thrombosis risk in pregnancy is well-described.8,12,13 Normal pregnancy affects the 3 components of Virchow’s triad: hypercoagulability resulting from increases in most procoagulant factors, decreases in natural anticoagulants, and decreased fibrinolytic potential; stasis, resulting from mechanical compression of the inferior vena cava and pelvic veins by the enlarging uterus in the context of hormone-mediated increases in venous capacitance and from pregnancy-related exaggerated compression of the left iliac vein by the right iliac artery; and endothelial injury, which occurs in preeclampsia and may also result from delivery-related trauma. A multitude of pregnancy and/or delivery complications may further increase hypercoagulability, including multiple gestation, infection, cesarean delivery, and hemorrhage. Factors that increase VTE risk in nonpregnant women also play a role, including family or personal history of thrombosis, inherited thrombophilia, antiphospholipid syndrome, higher body mass index, older age, kidney disease, immobilization, smoking, and surgery. Pregnant women with hypertension, diabetes, valvular heart disease, hypercoagulable disorders, sickle cell disease, lupus, migraines, and older age and those who smoke are at increased risk for ischemic stroke.5,14,15 Importantly, maternal age at first birth is increasing in many developed countries.16,17 In the United States, the birth rate among women aged 40 to 44 years more than doubled from 1990 to 2012.18 Paired with the current obesity epidemic, this rise in maternal age translates to increased medical comorbidities. Thus, it is increasingly common for women to present in pregnancy with multiple thrombotic risk factors. Recognition of such risk factors, and application of prevention strategies, is central to avoiding VTE and arterial thrombosis in pregnant women.
Prevention
Prevention of thrombotic events requires understanding of risk factors, proper use of thromboprophylaxis medication, and on an ongoing assessment of the shifting risk landscape occurring in pregnancy. The need for ongoing risk assessment cannot be overemphasized. Complications during pregnancy or at the time of delivery can substantially increase VTE risk. Preexisting risk factors, such as thrombophilias and other medical conditions that confer increased VTE risk (obesity, systemic lupus erythematosus, sickle cell disease), should be considered in the context of obstetric-related risk factors as they evolve. Table 2 highlights the most common pregnancy-related VTE risk factors, with the American College of Chest Physicians (ACCP) designation of “major” (OR > 6) and “minor” (OR > 6 when combined) risk. Although thromboprophylaxis guidelines vary internationally, the ACCP recommends considering postpartum low-molecular-weight heparin (LMWH) prophylaxis during hospital admission in women after cesarean delivery who have 1 major risk factor or 2 minor risk factors (Table 2). Because these obstetric risks are additive, but often occur unpredictably, a shifting landscape of risk estimation results, which makes multidisciplinary patient care particularly important. We have found that the creation of a strong working relationship between our hematologists and obstetricians greatly assists in the navigation of this mutable landscape.
Outside of labor- and delivery-associated complications conferring substantial VTE risk, the most common reasons women are considered for antepartum and/or postpartum VTE prevention is because of a family and/or personal history of VTE and/or a prior diagnosis of thrombophilia. Guidelines for thromboprophylaxis in pregnancy continue to evolve, but are often inconsistent, which is likely a reflection of limited pregnancy-specific data and considerable variation in risk estimates for VTE in pregnancy in the context of individual thrombophilias.19,20 That said, current recommendations consistently highlight 3 important points: the significance of a personal history of VTE, whether or not this occurred in the context of a diagnosed thrombophilia; the need for added and continued clinical risk assessment, as there are multiple situations in which both antepartum surveillance and antepartum thromboprophylaxis may be appropriate; and recognition that the postpartum period is the highest-risk period for pregnancy-associated thrombosis.21-23 Table 3 provides a summary of areas of consensus between various current national and international guidelines for VTE prevention in pregnancy.21-23
Although a detailed examination of the thrombophilias is beyond the scope of this review, it is worth discussing a few key points, as thrombophilia is frequently the motive behind consideration of VTE prophylaxis in pregnancy. The inherited and acquired thrombophilias are associated with variable increases in VTE risk during pregnancy and the puerperium, with about half of all pregnancy-related VTE associated with thrombophilia.24 Current recommendations on thromboprophylaxis in pregnancy are based on this VTE risk, as opposed to risk for poor obstetric outcome. Pregnant women with a history of adverse pregnancy outcome should not be screened for inherited thrombophilia, nor should anticoagulation be prescribed in an attempt to reduce the risk for recurrence of placental-mediated pregnancy complications and/or recurrent miscarriage. Thus, both testing for inherited thrombophilia and anticoagulation in the presence of thrombophilia should only be considered in the context of VTE risk. One caveat to this is antiphospholipid syndrome, which is associated with poor obstetric outcomes of recurrent pregnancy loss and/or placental mediated complications.24-26 Thus, unlike the inherited thrombophilias, screening and/or anticoagulation in the context of antiphospholipid syndrome should be considered for reduction of thrombotic risk and for reduction in pregnancy complications.
Diagnosis
Although the signs and symptoms of ischemic stroke in pregnancy are not likely to be mistaken for pregnancy-related symptoms, the same is not true for VTE. VTE signs and symptoms have substantial overlap with common pregnancy symptoms. In addition, diagnostic imaging for suspected ischemic stroke is more straightforward than for suspected VTE. The following review will therefore focus on diagnosis of VTE.
DVT
Inferior vena cava compression by the gravid uterus results in lower extremity swelling, which is increasingly prevalent as gestation progresses. Furthermore, 30% to 50% of pregnant women suffer from leg cramps, especially in the third trimester.27 Proximal extension of DVT into pelvic veins may cause abdominal pain, which may be erroneously attributed to pregnancy. Providers caring for pregnant women therefore require ongoing awareness and concern for DVT, particularly in women with risk factors. Knowledge of the distinctive anatomic distribution of DVT in pregnancy can guide clinical suspicion and diagnostic imaging decisions; DVT in pregnant women is more often left-sided (85% vs 55% in nonpregnant individuals) and is much more likely to be proximal in location, with 72% in the iliofemoral veins vs 9% in nonpregnant individuals.28,29 Pregnancy-associated iliofemoral DVT is usually not associated with involvement of calf veins.29
Compression duplex ultrasonography (CUS) is the first-line imaging technique to investigate suspected DVT. In the case of a negative study and continued clinical suspicion, it is appropriate to repeat the ultrasound in 3 and 7 days. Given the frequency of iliac vein involvement in pregnancy-related DVT, additional imaging techniques (Doppler ultrasonography, venography, or magnetic resonance imaging) are useful when the clinical exam is concerning for isolated iliac vein DVT, but the CUS is negative. d-dimer should not be used in isolation during pregnancy to rule out DVT, as levels increase with pregnancy progression and usually reach thresholds considered abnormal in nonpregnant individuals.30 This, along with the unsuitability of Wells prediction rule in pregnant women, makes pretest probability assessment more difficult. Chan’s LEFt clinical prediction tool, which uses 3 variables (symptoms in the left leg, calf circumference difference >2 cm, and first trimester presentation) is predictive of positive imaging for DVT in pregnant women and may be useful in the decision-making for further imaging in the case of a negative CUS.31
Pulmonary embolism
Similar to DVT, pregnancy may complicate the identification of a PE. Dyspnea is a common complaint in pregnancy, reported in 75% of women by the third trimester. The mechanism is unclear, but is likely related to progesterone-mediated hyperventilation. In addition, heart rate increases by 15to 20 beats per minute in pregnancy, primarily in the third trimester, such that tachycardia is not uncommon in late pregnancy.12 These normal physiologic adaptations to pregnancy often make imaging decisions difficult in the case of suspected PE. In general, we recommend a low threshold to move to imaging for suspected PE in pregnancy.
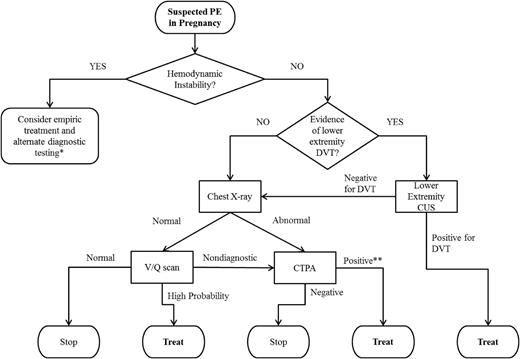
Current guidelines for the evaluation of a suspected PE in pregnancy are shown in Figure 1.32 As with DVT, d-dimer testing should not be used. Lower extremity ultrasonography should only be used as the first diagnostic imaging procedure if the patient has signs or symptoms of DVT. The ventilation/perfusion (V/Q) scan is preferred over computed tomographic pulmonary angiography (CTPA) if the chest X-ray is normal. Although these guidelines are based on limited (and at times conflicting) retrospective data comparing diagnostic test accuracy, they reflect the current understanding of risks and benefits of each testing modality. Specifically, diagnostic accuracy of the V/Q scan may be superior to CTPA in pregnancy, as CTPA is more often nondiagnostic in pregnant than in nonpregnant women (because of decreased contrast enhancement secondary to pregnancy-related physiologic alterations in body weight/surface area, plasma volume increase, and/or cardiopulmonary changes).33 Although some studies suggest comparable diagnostic accuracy, V/Q scan is the preferred test in the context of a normal chest radiograph because of the lower prevalence of indeterminate V/Q scans in pregnant women and the substantially lower radiation exposure to maternal breast and lung tissue than with CTPA. CTPA delivers slightly lower fetal radiation doses than V/Q scans (0.03-0.66 mGy vs 0.32-0.74 mGy, respectively), but higher total body maternal radiation (4-16 mSv vs 1-2.5 mSv), and particularly high doses to maternal breast tissue.34 In the scenario when a chest radiograph, V/Q scan, and CTPA are performed, the estimated additive fetal radiation exposure is substantially below the 50-mGy threshold at which the National Council of Radiation Protection and Measurements considers the risk for radiation-associated abnormalities negligible.35 That said, there is no known “safe” threshold for radiation exposure, particularly in terms of lifetime cancer risk. Thus, despite the recommendations for use of V/Q scan over CTPA in pregnancy, the American Thoracic Society’s guidelines state: “given the lack of evidence documenting clear superiority of any one diagnostic test, the values and preferences of a patient and her physician likely will and should determine the final choice and sequence of tests performed.”33 As a consequence, communication between the patient and the care team, and flexibility in decision-making based on the clinical scenario and patient preference, are paramount when PE is suspected. At our institution, when the CTPA is nondiagnostic, we frequently perform lower extremity CUS next, but decisions should be individualized, with consideration of the patient’s symptoms and risk profile.
Guidelines for the evaluation of suspected PE in pregnancy, based on the 2011 joint publication by the American Thoracic Society and the Society of Thoracic Radiology.32 *Such as echocardiography. **In the case of a symptomatic single subsegmental PE, we recommend treatment. The ACCP’s 2016 expert panel report highlights characteristics that, when present, increase the likelihood of a true positive; the presence of symptoms (ie, not an incidental finding), and clinical high clinical pretest probability are included.50 We also review the imaging to enhance confidence of interpretation.
Guidelines for the evaluation of suspected PE in pregnancy, based on the 2011 joint publication by the American Thoracic Society and the Society of Thoracic Radiology.32 *Such as echocardiography. **In the case of a symptomatic single subsegmental PE, we recommend treatment. The ACCP’s 2016 expert panel report highlights characteristics that, when present, increase the likelihood of a true positive; the presence of symptoms (ie, not an incidental finding), and clinical high clinical pretest probability are included.50 We also review the imaging to enhance confidence of interpretation.
Prophylaxis and treatment
Ischemic stroke
In contrast to the existing general consensus on primary and secondary prevention of VTE (Table 3), recommendations to reduce the risk for ischemic stroke in high-risk women during pregnancy and the puerperium are more variable. Data on stroke prevention in pregnancy are extremely limited, with no randomized trials. The 2014 American Heart Association/American Stroke Association guidelines provide a useful framework for consideration of stroke prevention in pregnant women by focusing on 2 distinct groups: those with a high-risk condition that would require anticoagulation outside of pregnancy, or women with a lower-risk situation in which antiplatelet therapy would be the treatment recommendation outside of pregnancy.36 The first group includes women with mechanical heart valves (with or without prior stroke) and women with prior stroke and thrombophilia or other high-risk condition, such as atrial fibrillation. Guidelines from different organizations for treatment in pregnant women with mechanical heart valves vary in terms of choice and timing of anticoagulant/aspirin use, although there is consistent agreement that these women are at very high risk for poor outcomes and require full anticoagulation with frequent and careful monitoring. Providers caring for such women should be knowledgeable about risks and benefits of each treatment regimen, and we refer them to available guidelines for detailed advice.21,36,37
At this time, there is insufficient evidence to support one regimen over another, particularly when both maternal and fetal outcomes are considered. A recent report from the European Society of Cardiology’s Registry of Pregnancy and Cardiac Disease illustrates this point, with evidence of decreased valve thrombosis, but substantially increased fetal loss, in women treated with warfarin in the first trimester.38 Warfarin appears to be more effective in preventing valve thrombosis in pregnant women with mechanical heart valves than heparins; however, awareness of its teratogenicity during weeks 6 to 12 of gestation requires individualized treatment planning among women with mechanical heart valves. In other high-risk women, either unfractionated heparin (UFH) or LMWH may be appropriate. The second group of women, those with a “lower-risk situation” that would require antiplatelet therapy outside of pregnancy, generally includes women with a prior noncardioembolic occlusive vascular event. Low-dose aspirin is usually indicated in this scenario. Low-dose aspirin appears to be safe in the second and third trimesters of pregnancy, based largely on preeclampsia prevention studies, which show no increased fetal/neonatal or maternal risk.39-41 The safety of low-dose aspirin in the first trimester is less clear, with inconsistent literature findings and no randomized controlled trials of first trimester aspirin exposure. Aspirin crosses the placenta, and although it does not appear to be a major risk factor for birth defects, some studies reported an increased risk for specific anomalies, with gastroschisis being the most consistently reported.42-45 Because of this, the American Heart Association/American Stroke Association 2014 guidelines suggest that “low-dose aspirin, UFH, or LMWH, or no treatment could be acceptable during the first trimester depending on the clinical context and the maternal attitude toward risk.”36 When considering first trimester secondary stroke prevention, it is important to recognize that the effectiveness of heparin in this scenario is unknown (except in the case of cardioembolic stroke), and that the risk for ischemic stroke recurrence in young women is low (and is highest postpartum).5,46
Treatment of ischemic stroke in pregnancy is similar to that outside pregnancy, with early aspirin therapy for those not receiving heparin or tissue plasminogen activator (tPA), although heparin is the treatment of choice in the setting of a thrombotic stroke. Evidence for the safe use of tPA in pregnancy and the puerperium is mounting, and both intravenous and intra-arterial tPA have been successfully used in pregnancy.10,47 Although tPA does not cross the placenta, hemorrhagic complications with thrombolysis are of particular concern near the time of delivery or in the recent postpartum setting. Discussion among neurologists, obstetricians, hematologists, anesthesiologists, and critical care physicians will help to guide treatment decisions in each individual scenario in the context of specific obstetric bleeding risk.
VTE
Although indications for the prevention of pregnancy-associated VTE were previously discussed, it is worth reiterating that although some women will clearly fall into a specific category regarding prophylaxis (Table 3), many women will require ongoing clinical vigilance and multidisciplinary decision-making as the obstetric course unfolds. For instance, although American College of Obstetricians and Gynecologists (ACOG) recommends that mechanical compression devices be placed before every cesarean delivery, they also recommend that for women undergoing cesarean delivery with “additional risk factors for thromboembolism, individual risk assessment may require thromboprophylaxis with both pneumatic compression devices and UFH or LMWH.”22 These recommendations highlight the importance of assessing individual VTE risk, but also leave room for significant practice variation. In our experience, multidisciplinary input minimizes such variation. As in the nonpregnant population, acute DVT, PE, and cerebral vein thrombosis should be treated with full-dose anticoagulation unless it is contraindicated. Women with an acute VTE while pregnant are generally treated with full-dose LMWH through the antepartum and postpartum periods, with a minimum duration of 3 months. It is currently unknown whether a lower dose of LMWH can be used after a period of full-dose treatment in women who have an event early in pregnancy. On the basis of the increased thrombotic risk going beyond the 6th postpartum week, our group continues treatment of 6 to 8 weeks after a vaginal delivery and 8 to 10 weeks after a cesarean delivery. We also treat women receiving prophylactic dosing (see following) with slightly longer postpartum therapy than the standard 6 weeks (ie, 6-8 weeks after a vaginal delivery, and 8-10 weeks after a cesarean delivery).
In terms of antithrombotic agents for prevention and treatment of VTE in pregnancy and postpartum, appropriate regimens may include warfarin, UFH, or LMWH. There are little or no data on the use of other heparin formulations, except fondaparinux, which can be used in the setting of severe adverse reactions to heparin.21 Similarly there are no data on use of the direct thrombin inhibitors or anti-Xa inhibitors in pregnancy, and they should be avoided. Warfarin crosses the placenta, and is a teratogen when administered between the 6th and 12th weeks of gestation. Except in women with mechanical heart valves, warfarin should not be used in pregnancy, although is safe to use in the postpartum period in breastfeeding women.22 Catheter-directed thrombolysis for VTE treatment confers hemorrhagic risk, particularly near the time of delivery, so generally should only be considered in the setting of life- or limb-threatening VTE.
LMWH is the agent of choice for the prevention and treatment of VTE in pregnancy, based on extrapolation of data from trials in the nonpregnant population and on a large body of observational data indicating safety and efficacy during pregnancy.28 In addition, LMWH is associated with a lower risk for heparin-induced thrombocytopenia, hemorrhage, and osteoporosis than heparin.21,48 In those receiving prophylaxis, monitoring anti-Xa levels is not recommended, and among obese women it is unclear whether alternate dosing strategies, such as weight-based dosing or fixed dose adjustment should be made.48,49 In our experience, a multidisciplinary discussion assists in making such VTE prophylaxis dosing decisions in severely obese pregnant women.
For treatment of acute VTE in pregnancy, it is uncertain whether once- or twice-daily LMWH dosing is most appropriate. Twice-daily dosing may be preferable because of increased renal excretion of LMWH in pregnancy, although no studies have shown superiority of one regimen over the other. In our practice, we use twice-daily dosing, given concern for increased clearance in pregnancy. In most circumstances, heparin level monitoring is not recommended, as there is no clear benefit. However, in certain circumstances (extremes of body weight, presence of renal disease, severe thrombophilia, and recurrent DVT), it may be appropriate to follow heparin levels.
Similar to LMWH, UFH can be used for both prophylaxis and treatment of VTE in pregnancy. However, given the risks and the activated partial thromboplastin time monitoring requirements associated with UFH, as well as the efficacy and safety of LMWH, UFH is now used less frequently in pregnant women. Notable exceptions include VTE with severe clinical manifestations, in which case intravenous UFH may be initiated; when full anticoagulation is required leading up to the time of delivery (as in the case of VTE proximate to delivery), making the reversibility and shorter half-life of intravenous UFH desirable; and to create increased flexibility for administration of neuraxial anesthesia, which subcutaneous UFH prophylaxis accomplishes. Regarding this last scenario, anesthesia guidelines generally recommend avoiding spinal and/or epidural catheter placement for 12 hours after prophylactic LMWH dosing and 24 hours after full dose treatment. Prophylactic UFH dosing (5000 U twice daily) does not usually entail this delay, and for women receiving higher UFH doses, the activated partial thromboplastin time is used to assess safety. In our practice, among women receiving prophylactic dosing, we generally transition patients from LMWH to subcutaneous UFH at 36 weeks of gestation, or earlier in cases of multiple gestation, prior preterm birth, short cervix, or other reason for high preterm birth risk. For women receiving full-dose LMWH in the third trimester, we base decisions regarding timing of discontinuation and on whether IV heparin is used on how recent the VTE was and the patient’s risk profile for recurrent VTE. Communication and shared decision-making between our obstetric providers and hematologists has been important in these complex scenarios.
Conclusions
In our current era of increasingly complex and subspecialized medicine, with ever-increasing numbers of patient hand-offs, the importance of communication between providers within and between specialties is of growing importance. The care needs of pregnant women in the context of thrombotic risk is a good example of the necessity of such communication, as different specialists bring different perspectives and experience to the same shared patient. A robust, multidisciplinary care model with clear documentation of plans not only creates the opportunity to streamline care plans for complicated obstetric scenarios but also creates strong working relationships between providers across specialties. Such relationships then allow for more flexible and immediate modifications to inpatient and outpatient care plans as the clinical situation changes. This is incredibly important in the prevention, diagnosis, and treatment of thrombosis in pregnant women because of the often rapidly changing obstetric situation, which may then substantially affect thrombotic risk. In the context of limited pregnancy-specific data, maternal and fetal safety concerns, and multiple prevention and treatment strategies, there is often no clear “right” way to care for pregnant women who are suffering a thrombotic event or who are at high risk for thrombosis. A patient-centered care model, involving experts in both hematology and obstetrics, likely supports our quest for finding the “right” plan for our pregnant patients.
Correspondence
Mary Cushman, Larner College of Medicine at the University of Vermont, 360 South Park Drive, Colchester, VT 05446.
References
Competing Interests
Conflict-of-interest disclosures: K.M. declares no competing financial interests. M.C. has received research funding and honoraria from Diadexus and has consulted for Merck.
Author notes
Off-label drug use: None disclosed.