Abstract
The clinical management of patients with acute pulmonary embolism is rapidly changing over the years. The widening spectrum of clinical management strategies for these patients requires effective tools for risk stratification. Patients at low risk for death could be candidates for home treatment or early discharge. Clinical models with high negative predictive value have been validated that could be used to select patients at low risk for death. In a major study and in several meta-analyses, thrombolysis in hemodynamically stable patients was associated with unacceptably high risk for major bleeding complications or intracranial hemorrhage. Thus, the presence of shock or sustained hypotension continues to be the criterion for the selection of candidates for thrombolytic treatment. Interventional procedures for early revascularization should be reserved to selected patients until further evidence is available. No clinical advantage is expected with the insertion of a vena cava filter in the acute-phase management of patients with acute pulmonary embolism. Direct oral anticoagulants used in fixed doses without laboratory monitoring showed similar efficacy (odds ratio [OR], 0.89; 95% confidence interval [CI], 0.70-1.12) and safety (OR, 0.89; 95% CI, 0.77-1.03) in comparison with conventional anticoagulation in patients with acute pulmonary embolism. Based on these results and on their practicality, direct oral anticoagulants are the agents of choice for the treatment of the majority of patients with acute pulmonary embolism.
Learning Objectives
To discuss the accuracy of individual predictors of death and of different scoring systems in patients with acute pulmonary embolism with specific focus in hemodynamically stable patients
To review the evidence on the efficacy and safety of different treatment strategies for acute pulmonary embolism to understand the rationale that led recent guidelines to identify direct oral anticoagulants as the treatment of choice for the majority of patients
To review the evidence on the efficacy and safety of interventional treatment approaches (percutaneous embolectomy/thrombectomy with or without thrombolysis; vena cava filters) with specific interest on their effect in mortality, recurrent venous thromboembolism, and bleeding complications
The clinical management of patients with acute pulmonary embolism has changed considerably in recent years with regard to diagnosis (widespread availability of computed tomography [CT] angiography), risk stratification (validation of prognostic algorithms, models, and scores), and treatment (new evidence on the role of thrombolysis and introduction of direct oral anticoagulants [DOACs]).
Optimal management strategies for pulmonary embolism should reduce mortality and recurrent venous thromboembolism at the cost of a low risk of bleeding complications.
We review recent strategies for risk stratification and management of patients with acute pulmonary embolism with the aim of providing the available evidence concerning their effect on the clinical course of the disease.
Clinical course and management of pulmonary embolism
According to administrative US data, the number of patients discharged from acute-care hospitals with a diagnosis of pulmonary embolism increased during the 8-year period between 1998 and 2005. In the same period, the in-hospital case fatality rates in these patients decreased from 12.3% to 8.2%, and the length of hospital stay decreased from 9.4 to 8.6 days.1 Recent European studies confirmed similar trends for reduction in mortality.2-4 In a nationwide cohort study in Denmark, mortality at 30 days after diagnosis in patients with first-time pulmonary embolism improved over time from 1980 to 2011.
This reduction in mortality was associated with a change in hospitalization practice for these patients. In the Computerized Registry of Patients with Venous Thromboembolism (RIETE), the mean length of hospital stay in patients with acute pulmonary embolism decreased from 13.6 to 9.3 days from 2001 to 2013.3 Risk-adjusted rates of all-cause mortality decreased from 6.6% in the period 2001 to 2005 to 4.9% in the period 2010 to 2013 (P = .02 for trend). Rates of pulmonary embolism–related mortality also decreased over time, with a risk-adjusted rate of 3.3% from 2001 to 2005 and 1.8% from 2010 to 2013 (P < .01 for trend).
In an Italian cohort of 2218 patients with acute venous thromboembolism included in the RIETE Registry from 2003 to 2015, 53.7% of patients with deep vein thrombosis and 17.0% of patients with pulmonary embolism were entirely treated at home, and 38.2% of deep vein thrombosis patients and 19.9% of pulmonary embolism patients were hospitalized for 5 days or fewer.5
The observed decrease in mortality over the years could be related to improvements in antithrombotic therapies as well as to increased diagnosis of minor pulmonary embolism due to the use of computed tomography.6,7 Indeed, with the improvement of CT technology, the proportion of patients diagnosed with segmental or subsegmental pulmonary embolism increased from about 5% to a rate closer to 9%; moreover, incidental asymptomatic pulmonary embolism is found in about 3% of CT performed for other reasons and not to confirm the suspicion of venous thromboembolism (eg, cancer diagnosis or staging).8 In this view, it should be noted that an inverse association has been observed between distal emboli and 30-day death or clinical deterioration (hazard ratio, 0.12; 95% confidence interval [CI], 0.015-0.97).9
The availability of low-molecular-weight heparins and fondaparinux for the initial treatment of acute pulmonary embolism could have contributed to the reduction of the duration of hospitalization in patients with acute pulmonary embolism. Indeed, these agents do not need continuous infusion, laboratory monitoring, and dose adjustment, thus improving practicality of anticoagulant treatment.
Rationale and methods for risk stratification
Pulmonary embolism may present with acute heart failure leading to sudden cardiac arrest and death shortly after hospital admission or even before hospital arrival or may be asymptomatic or associated with mild dyspnea.10,11 In between these 2 extremes, pulmonary embolism may cause a large spectrum of clinical presentations characterized by different combinations of dyspnea, chest pain, respiratory failure, hypoperfusion, and hemodynamic compromise.12 These conditions are associated with different risks for in-hospital mortality.
Vital status, right ventricle dysfunction, and myocardial injury (increased troponin) are the main predictors of short-term death in patients with acute pulmonary embolism.13 In-hospital mortality was 58.3% in hemodynamically unstable and 15.1% in hemodynamically stable patients in a registry performed in the 1990s,14 and 31.8% in hemodynamically unstable and 3.4% in hemodynamically stable patients in a more recent registry.15 Whether hemodynamic compromise in these patients is better identified by blood pressure or shock index or other signs of hypoperfusion is still undefined.
In hemodynamically stable patients, several predictors of death have been identified and several strategies have been proposed to optimize risk stratification and health-resource utilization.16 Clinical models based only on simple and rapidly available information on patients’ medical history and clinical status can be used to identify patients at low risk for death. Indeed, these models have different negative predictive values as shown in Tables 1-3. The Pulmonary Embolism Severity Index (PESI) or its simplified version (sPESI) are the most validated models.17,18 The 30-day mortality rates in patients assigned as low risk according to PESI and sPESI are 1.4% (95% CI, 1.2-1.8) and 1.0% (95% CI, 0.0-2.1), respectively.
Imaging as well as biomarkers are currently used for the assessment of right ventricle overload.27 At echocardiography, quantitative parameters, as the right-to-left end-diastolic dimension ratio, should be preferred over qualitative parameters, as free-wall hypokinesis, for the assessment of right ventricle overload. Right ventricle dysfunction at echocardiography is associated with increased risk for short-term death (OR, 2.36; 95% CI, 1.3-4.3).28 Right ventricle dilation can be assessed at CT angiography by the right-to-left ventricular diameter ratio; this measure is associated with increased mortality (OR, 1.64; 95% CI, 1.06-2.52).29 Serum levels of brain natriuretic peptide (BNP) or its precursor (pro-BNP) are highly sensitive markers of right ventricle dysfunction. Normal values of these markers are associated with high negative predictive value for death in patients with acute pulmonary embolism.28 Elevated troponin, a marker of myocardial injury, is associated with increased in-hospital mortality in patients with acute pulmonary embolism (OR, 5.90; 95% CI, 2.68-12.95), even if hemodynamically stable.30 In recent studies, increased levels of lactate were associated with increased mortality in hemodynamically stable patients.31 However, none of these predictors by itself is able to drive clinical management in hemodynamically stable patients because of low positive predictive value. Based on this evidence, several models integrating clinical evaluation and instrumental or laboratory results in 2- or 3-test strategies have been evaluated for risk stratification of patients with acute pulmonary embolism (Table 3).32 PESI has been combined with echocardiography and/or troponin and/or lower limbs ultrasonography, Hestia criteria with CT-assessed right ventricle dilation or BNP, the Prognosis in Pulmonary Embolism (PREP) score with echocardiography and BNP. Overall, adding biomarker data may help to identify higher- or lower-risk patients, but the findings on the additive value of biomarkers are inconsistent across studies, mainly concerning incremental positive predictive value.16
The real value of all of these efforts on risk stratification in hemodynamically stable patients is the identification of patients who can be safely treated at home and those who need surveillance and potential treatment upgrading. When the models are used to identify candidates for early discharge or home treatment, the definition of the acceptable rate of adverse events (death) occurring after discharge appears of critical value. When the aim is the identification of patients at high risk for death who need surveillance for potential treatment upgrading, the definition of the mortality threshold for the use of thrombolysis or revascularization is critical.
In 2014, the European Society of Cardiology proposed an updated model for risk stratification and management of patients with acute pulmonary embolism. In this model, the PESI or sPESI scores identify low-risk patients whereas shock or hypotension identify high-risk patients.23 Further stratification of intermediate-risk patients is indicated by imaging of the right ventricle and troponin (intermediate-low- and intermediate-high-risk classes). In a cohort study in 906 patients with acute pulmonary embolism, death at 30 days occurred in 22% of “high-risk” (95% CI, 14.0-29.8), 7.7% of “intermediate-high-risk” (95% CI, 4.5-10.9), 6.0% of “intermediate-low-risk” (95% CI, 3.4-8.6), and 0.5% of “low-risk” patients (95% CI, 0-1.0) as identified by the 2014 model of the European Society of Cardiology.33 As compared with the 2008 prognostic model indicated by the European Society of Cardiology, the 2014 model has similar accuracy but allows avoidance of instrumental tests for risk stratification in about 20% of the patients with acute pulmonary embolism; this is the case for patients categorized as low risk according to the sPESI score. This study also showed that risk stratification in patients at intermediate risk requires further improvement.
Evidence in favor of risk stratification
Risk stratification works when it drives decision-making and clinical management. The landmark paradigm is that in patients with acute myocardial infarction. In this setting, optimal management strategies are established based on general patients’ status (comorbidities, life expectancy, etc), hemodynamics at index event, electrocardiogram features, and troponin. A similar approach is awaited in patients with acute pulmonary embolism.
Hemodynamically unstable patients with acute pulmonary embolism, those defined as high risk according to European guidelines and massive pulmonary embolism by American guidelines, should proceed to revascularization. From the pooled results of currently available studies in patients with acute pulmonary embolism, it was determined that thrombolysis reduced mortality from 3.9% to 2.2% as compared with anticoagulant treatment (OR, 0.53; 95% CI, 0.32-0.88; number needed to treat = 59).34 However, this advantage is counterbalanced by an increase in major bleeding from 3.4% to 9.2% (OR, 2.73; 95% CI, 1.91-3.91; number needed to harm = 18) and in intracranial hemorrhage from 0.2% to 1.5% (OR, 4.63; 95% CI, 1.78-12.04; number needed to harm = 78). Moreover, the reduction in mortality seems to be mainly accounted for by studies also including hemodynamically unstable patients. The role of thrombolysis in hemodynamically stable patients at intermediate-high risk was assessed in a large placebo-controlled study; patients with right ventricle dysfunction (at echocardiography or at CT) and injury received tenecteplase (same regimen as that validated for acute myocardial infarction) or placebo on top of heparin treatment.35 Tenecteplase reduced 7-day death or clinical deterioration from 5.6% to 2.6% compared with anticoagulant treatment alone (OR, 0.44; 95% CI, 0.23-0.87). However, mortality rates reported in the Pulmonary Embolism Thrombolysis (PEITHO) study (1.2% with thrombolysis plus anticoagulants vs 1.8% with anticoagulants only) were comparable and not high enough to justify the 11.5% risk for major bleeding observed with thrombolysis plus anticoagulants vs 2.4% with anticoagulants only. In particular, the risk for hemorrhagic stroke associated with thrombolysis appeared unacceptably high (10 patients with thrombolysis plus anticoagulants vs 1 patients with anticoagulants only). Thus, based on the results of this study, thrombolysis should be avoided in hemodynamically stable patients. In this view, the value of risk stratification by imaging and troponin appears to be reduced. However, PEITHO showed that 5.6% of intermediate-high-risk patients die or deteriorate within 7 days from diagnosis. This rate is not negligible and underlines the need for close observation of these patients. Overall, the PEITHO study documents the need to improve risk stratification in patients with acute pulmonary embolism as right ventricle dysfunction and injury by themselves are probably not able to identify a high-risk group of hemodynamically stable patients. A strategy for improvement could be the selection of moderate-to-severe right ventricle dysfunction and clearly elevated troponin, as already recommended in the statement of the American Heart Association.36 Indeed, the widespread use of high-sensitivity troponins could have led to a reduction in the positive predictive value of this biomarker. Moreover, it is conceivable that markers of right ventricle dysfunction and injury should be integrated with patient status in terms of comorbidities and degree of respiratory failure or tachycardia for an effective global assessment of patient clinical severity.
On the opposite extreme of clinical severity, one randomized study and several cohort studies evaluated the feasibility of home treatment or early discharge in patients with acute pulmonary embolism at low risk for short-term mortality or complicated clinical outcome.37 Criteria for patients’ selection in these studies largely varied and until 2010 took into account almost exclusively clinical evaluation; beyond that date, the evaluation of right ventricle dysfunction or injury was usually part of the inclusion/exclusion criteria. All of the studies on this issue were largely underpowered to provide definitive evaluation of the safety of home treatment or early discharge in terms of mortality or major bleedings. In the only available randomized study, patients were included based on the PESI score.38 Fewer than one-third of the evaluated patients were eligible for the study and fewer than one-fourth were actually randomized; 8 of 172 patients assigned to home treatment (hospital stay <24 hours) were kept in the hospital for a longer period and 14 of 172 assigned to standard in-hospital care were discharged in <24 hours. At 90 days, 1 outpatient had recurrent venous thromboembolism (0.6%) compared with no inpatients (95% upper confidence limit, 2.7%; P = .011); 1 patient (0.6%) in each treatment group died (95% upper confidence limit, 2.1%; P = .005), and 3 outpatients (1.8%) but no inpatients developed major bleeding (95% upper confidence limit, 4.5%; P = .086). Overall, 5 outpatients and 1 inpatient developed adverse events at 90 days. These data show that home treatment is feasible and suggest the idea that selection criteria only based on the clinical severity of pulmonary embolism is not adequate.
The Hestia criteria is a combination of ad hoc–defined clinical signs and symptoms, comorbidities, predictors of bleeding risk, and any medical (pregnancy, need for parenteral agents) or social issue potentially raising concerns for home treatment.19 A recent study randomized patients with acute pulmonary embolism and without any of the Hestia criteria to direct discharge or to discharge based on additional N-terminal–proBNP (NT-proBNP) testing.39 In the NT-proBNP group, 12% of patients had elevated NT-proBNP values and were managed as inpatients. Thirty-day pulmonary embolism– or bleeding-related mortality, cardiopulmonary resuscitation, or intensive care admission occurred in none of the 275 patients randomized to NT-proBNP testing (95% CI, 0%-1.3%), vs in 3 of 275 patients in the direct discharge group (1.1%; 95% CI, 0.2%-3.2%) (P = .25). The safety of triaging patients based on the Hestia criteria has been confirmed by this study. However, due to the low number of patients with elevated NT-proBNP levels, this trial was unable to draw definite conclusions upon the incremental value of NT-proBNP testing over the Hestia criteria alone to select patients with acute pulmonary embolism for outpatient treatment.
In conclusion, today, evidence exists that risk stratification is useful to select patients with acute pulmonary embolism who may benefit from thrombolysis and those who are candidates for home treatment. The optimal management of patients in between remains to be defined.
Anticoagulant treatment
Anticoagulant treatment is the mainstay for the treatment of acute pulmonary embolism and should be given to all patients with suspicion of the disease, while awaiting for objective confirmation, in the absence of active bleeding.23,40 In recent years, the availability of DOACs for the treatment of acute pulmonary embolism represents a pivotal change in the treatment of the disease.
Large randomized clinical trials were conducted to compare DOACs given in fixed regimens with conventional anticoagulation in the treatment of venous thromboembolism.41-45 Rivaroxaban and apixaban were used according to the single-drug approach (<48 hours of heparin pretreatment) whereas dabigatran and edoxaban were used after 5 to 9 days of heparin pretreatment (Table 4). Overall, these studies included >10 000 patients with acute pulmonary embolism, excluded those who were hemodynamically unstable at presentation, and reported mortality rates as low as 2% to 3% at 6 months. The Einstein-Pulmonary Embolism trial focused on hemodynamically stable patients with symptomatic acute pulmonary embolism. In this study, in almost 5000 patients, rivaroxaban was noninferior to conventional anticoagulation (low-molecular-weight heparin followed by vitamin K antagonists) (hazard ratio, 1.12; 95% CI, 0.75-1.68) and was associated with a reduction in major bleeding (hazard ratio, 0.49; 95% CI, 0.31-0.79).41 About 54% of the patients had a low risk for death according to the sPESI score and <10% had a score of 2 or more. About 10% of patients in each treatment group were not hospitalized and were fully managed in the outpatient setting.
In the study by the Hokusai-VTE Investigators et al, 3392 patients with acute pulmonary embolism were randomized to edoxaban or warfarin after heparin pretreatment.45 Patients with a creatinine clearance of 30 to 50 mL per minute or a body weight of 60 kg or less or those who were receiving concomitant treatment with potent P-glycoprotein inhibitors received reduced doses of edoxaban. In a subgroup analysis, in patients with pulmonary embolism and evidence of right ventricular dysfunction (NT-proBNP level of ≥500 pg/mL), recurrent venous thromboembolism occurred in 3.3% of patients in the edoxaban group and in 6.2% of patients in the warfarin group (hazard ratio, 0.52; 95% CI, 0.28-0.98). Similar results were observed among patients with right ventricular dysfunction as assessed by means of CT (hazard ratio, 0.42; 95% CI, 0.15-1.20).
Single-drug therapy with apixaban and dabigatran was as effective as conventional therapy in dedicated clinical trials.42-44
All DOACs showed a favorable safety profile with respect to the comparator although different safety outcomes were considered (Table 4). The incidence of clinically relevant bleeding was lower in edoxaban- as compared with warfarin-treated patients (8.5% vs 10.3%; hazard ratio, 0.81; 95% CI, 0.71-0.94).45 This trend was confirmed in patients included in the study because of acute pulmonary embolism (10.1% vs 11.2%). Apixaban was associated with reduced incidence of major bleeding compared with conventional therapy (0.6% vs 1.8%; relative risk, 0.31; 95% CI, 0.17-0.55) and this advantage was confirmed in patients included in the study because of pulmonary embolism.44 In dabigatran studies, 2 safety comparisons were made: from the start of any study drug (from single-dummy period) and from the start of oral drug only (double-dummy period, after warfarin had reached therapeutic levels).43 Regardless of the calculation, pooled data from RE-COVER and RE-COVER II trials consistently showed a profile of less bleeding with dabigatran than with warfarin.
A meta-analysis of 5 randomized trials (11 539 patients) showed similar efficacy (OR, 0.89; 95% CI, 0.70-1.12) and safety (OR, 0.89; 95% CI, 0.77-1.03) of DOACs or conventional treatment in the subgroup of patients with acute pulmonary embolism.46
Limited data are currently available on the use of DOACs in patients who received thrombolytic treatment. All clinical trials with thrombolysis in patients with acute pulmonary embolism used anticoagulant treatment with unfractionated heparin. Heparin is discontinued during infusion of alteplase and started again at the end of thrombolysis. In this view, the optimal time for administration of DOACs (before or after) with respect to thrombolytic agents is unknown. Moreover, in case of bleeding complications, the half-life of heparin is quite short in comparison to that of the oral agents. Thus, while awaiting further evidence, starting of DOACs should probably be delayed at least 12 to 24 hours after thrombolysis. Similar concerns exist for patients who receive interventional treatment procedures in the acute phase.
A further breakthrough in the clinical use of DOACs will be the availability of antidotes able to obtain a complete and rapid reverse of the anticoagulant effect of dabigatran (idarucizumab) or anti-Xa agents (andexanet).47,48 Based on clinical data in about 90 patients, idarucizumab is now available for clinical use while awaiting definitive results. The use of prothrombin complex concentrates can be an option to manage major bleeding in patients on treatment with anti-Xa agents while awaiting for clinical data on andexanet.49
Interventional treatment approaches
Due to the increased risk for major bleeding and intracranial hemorrhage associated with the use of systemic thrombolysis, interest has recently arisen in procedures for local delivery of low-dose thrombolysis or percutaneous thrombectomy. Limited data are available for both strategies in terms of efficacy and safety.50 The simplest is catheter-based local infusion of thrombolytic agents close to the pulmonary emboli. Mechanical thrombus debulking via fragmentation or aspiration should be probably preferred for unstable patients who require rapid reduction of right ventricle overload if adequate expertise is locally available. In a recent prospective registry in 101 patients with massive or submassive pulmonary embolism, catheter-based therapy (mostly local fibrinolysis) significantly decreased pulmonary artery pressure and improved right ventricle overload, with no increase in major complications or bleeding.51 For those patients who are not so critically ill as to require fast resolution of pulmonary obstruction, ultrasound-accelerated fibrinolysis could be an option. In a randomized trial, reduced-dose local thrombolysis significantly reduced the right ventricle overload at 24 hours, without an increase in bleeding complications.52 A recent registry on catheter-directed mechanical or pharmacomechanical thrombectomy reported clinical success in 86% of 28 included patients with massive pulmonary embolism and in 97% of 73 patients with submassive pulmonary embolism.53 No hemorrhagic strokes were observed.
Besides not having a clear advantage in terms of mortality, the safety of interventional procedures is strictly dependent on local expertise. Local expertise is again dependent on the volume of procedures. In an era of reduced disease-related mortality, it is conceivable that only a minority of patients with acute pulmonary embolism could be candidates for interventional procedures, raising safety issues. Thus, even before discussing costs, interventional procedures should probably be reserved for hemodynamically unstable patients who have contraindication for systemic thrombolysis because of too-high risk for bleeding or as an alternative to systemic thrombolysis in high-volume hospitals with dedicated operators.
A randomized trial assessed the clinical value of a retrievable vena cava filter in 399 patients with acute symptomatic pulmonary embolism and deep vein thrombosis on top of standard anticoagulant treatment.54 The vena cava filter was removed at 3 months in about 80% of the patients who actually underwent successful filter insertion. A trend toward higher incidence of recurrent venous thromboembolism at 3 months was observed in patients randomized to filter insertion compared with patients randomized to anticoagulation alone (relative risk with filter, 2.00; 95% CI, 0.51-7.89). The role of the vena cava filter in the acute-phase management of patients with severe pulmonary embolism remains controversial. In an analysis of administrative data, unstable patients with acute pulmonary embolism treated or not with thrombolytic therapy had a lower in-hospital case fatality rate with vena cava filters than without (7.6% vs 18%, P < .0001 and 33% vs 51%, P < .0001).55 However, a recent retrospective study showed no significant reduction in 30-day risk of death by insertion of a vena cava filter among 80 697 patients with venous thromboembolism and no contraindication to anticoagulation (hazard ratio, 1.12; 95% CI, 0.98-1.28).56 This result was confirmed in patients treated for acute pulmonary embolism. These findings do not support the use of this type of filter in patients who can be treated with anticoagulation.
Pulmonary embolism management guidelines
Two international guidelines dedicated to the management of acute pulmonary embolism are currently available.23,40 Beyond treatment indications, these guidelines summarize the best evidence in terms of risk-driven patient management. The authors of the European Society of Cardiology guidelines recommend risk stratification in hemodynamically stable patients with acute pulmonary embolism (class IIa, level B recommendation). These guidelines identify 3 levels of risk for early mortality: high risk, intermediate risk, and low risk. The low-risk group is identified by means of the PESI or sPESI score. Patients with intermediate risk can be further classified into an intermediate-low-risk group if there is either no right ventricular dysfunction or injury present, or if only 1 of these factors is present. Based on this stratification, different management strategies are recommended, with different levels of evidence (Table 5).
A similar approach for risk stratification is indicated by the American Heart Association and the American College of Cardiology.36 However, in this case, the assessment of the severity of right ventricle overload is recommended to identify hemodynamically stable patients at increased risk for death. Moreover, low-risk patients are identified based on normal hemodynamics and absence of right ventricle dysfunction and injury.
The recently released CHEST–American College of Chest Physicians guidelines for the treatment of venous thromboembolism do not mention a specific role for risk stratification in hemodynamically stable patients, but identify different treatment strategies for patients with shock or hypotension and those who are hemodynamically stable.40
All of the guidelines recommend the use of thrombolytic treatment in patients with acute pulmonary embolism associated with shock or hypotension (as systolic blood pressure <90 mmHg) who do not have a high bleeding risk. Thus, although 10% or less of the patients are hemodynamically unstable at presentation, they require huge clinical efforts and intensive assistance in the acute phase aimed at reducing mortality. European guidelines and CHEST–American College of Chest Physicians guidelines also suggest considering home treatment or early discharge for patients with low-risk pulmonary embolism.40 The European Society of Cardiology and the CHEST guidelines recommend the use of DOACs for the treatment of pulmonary embolism.23,40 Based on their efficacy to safety profile and on their improved practicality, DOACs are indicated as the treatment of choice over conventional therapy with parenteral anticoagulants followed by vitamin K antagonists in the CHEST guidelines.
The reasonable choice for clinical practice
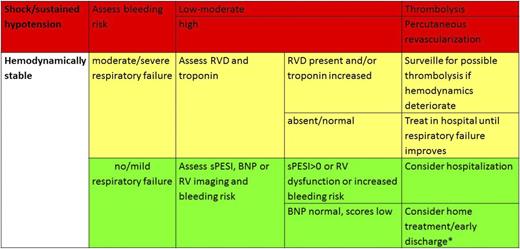
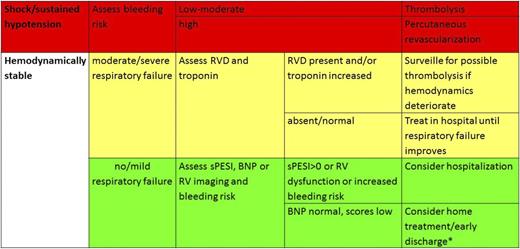
According to currently available evidence, risk stratification by means of clinical risk factors, right ventricle dysfunction, and injury allows individualized treatment of patients with acute pulmonary embolism. In the acute phase, risk stratification is essential for selecting patients for thrombolysis and home treatment or early discharge as well as for selecting the adequate level of intensity of care for each admitted patient (Figure 1). Although candidates for thrombolysis can be identified based on clinical features, it is reasonable to exclude right ventricle dysfunction (probably by BNP or CT) in addition to the assessment of clinical risk to safely identify candidates for home treatment. It should be considered that broad implementation of home treatment is controversial and varies across countries and across organization of patient care and patient pathways.
Management of pulmonary embolism. *Hestia criteria can be useful to triage for home treatment–early discharge.
Management of pulmonary embolism. *Hestia criteria can be useful to triage for home treatment–early discharge.
For all patients with acute pulmonary embolism, early after diagnosis or after hemodynamic stabilization, DOACs are the appropriate treatment of their safety profile. Pregnant women, children, and patients with severe renal failure (creatinine clearance lower than 30 mL per minute) are candidates for conventional anticoagulation. The appropriate treatment of cancer-associated pulmonary embolism is controversial. The use of low-molecular-weight heparin monotherapy is recommended in all guidelines.
Further evidence is needed on the clinical value of risk stratification in hemodynamically stable patients with acute pulmonary embolism and on the efficacy and safety of DOACs in specific subgroups of patients with acute pulmonary embolism (postthrombolysis, cancer patients, etc).
Correspondence
Cecilia Becattini, Internal and Cardiovascular Medicine, Stroke Unit, University of Perugia, Perugia, Italy; e-mail: cecilia.becattini@unipg.it.
References
Competing Interests
Conflict-of-interest disclosure: C.B. has received lecture fees from Bayer HealthCare, Bristol-Myers Squibb, and Boehringer Ingelheim. G.A. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.