Abstract
Iron-deficiency anemia is the most common hematologic problem in the world. Although oral iron is often viewed as front-line therapy, extensive published evidence has accumulated that IV iron is superior, in both efficacy and safety, to oral iron in many clinical situations and should be introduced much sooner in the treatment paradigm of iron-deficient patients. In this chapter, we will review the formulations of IV iron that allow total complete replacement doses in 1 or 2 sessions including practical tips for administration. We realize safety concerns abound and therefore will analyze evidence based overstated concerns regarding serious adverse events highlighting unnecessary interventions for minor, self-limiting infusion reactions, which infrequently occur with intravenous iron administration. Recent data for the use of IV iron in a variety of clinic situations will be reviewed including women with heavy uterine bleeding, pregnancy, bariatric surgery, inflammatory bowel disease, and restless legs syndrome. Briefly discussed is the new frontier of IV iron’s use in the prevention of acute (high altitude) mountain sickness. It is clear that in many clinical situations IV iron is a new and improved standard of care offering advantages over oral iron in efficacy, toxicity, and convenience to patients and health care providers.
Learning Objectives
Recognize the clinical pharmacology of the IV iron formulations that can safely replete iron in a short, single session
Explain the interpretation and management of minor infusion reactions associated with administration of IV iron
Define the clinical situations in which IV, and not oral, iron is the preferred replacement route
Introduction
It has been 6 years since the first Educational Session on the topic of IV iron for iron deficiency (ID) was presented at the American Society of Hematology’s annual meeting. In that session, we summarized data suggesting that IV iron is safer than most physicians realize, and is likely underutilized.
Many physicians have concerns about use of parenteral iron products which date from the era when high-molecular-weight iron dextran (HMWID)—a product frequently associated with severe infusion reactions—was widely available. Subsequently, 4 new formulations with carbohydrate shells binding elemental iron more tightly, improving adverse event profiles and enabling complete replacement doses in 15 to 60 minutes, have been approved by the Food and Drug Administration (FDA) or European Medicines Agency in recent years. These newer products include low-molecular-weight iron dextran (LMWID) (INFeD, Allergan, Parsippany, NJ), ferumoxytol (Feraheme, AMAG Pharma, Waltham, MA), ferric carboxymaltose (FCM)(InjectoFer US, Luitpold/American Regent, Shirley, NY; Ferinject Europe and Asia, Vifor, Glattbrugg, Switzerland), and iron isomaltoside 1000 (Monofer, Pharmacosmos, Holbaek, Denmark, Europe only). The characteristics of the available formulations are shown in Table 1.
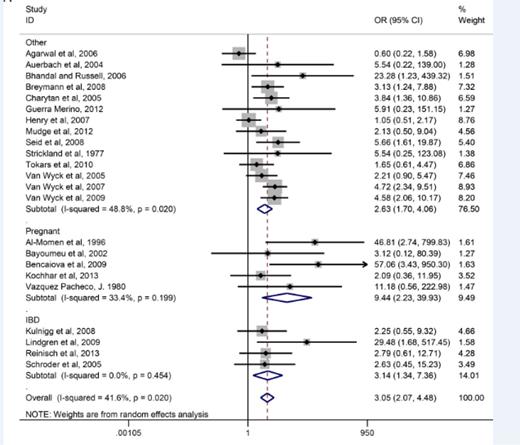
Given the availability of these safer products, IV iron has become standard front-line therapy for inflammatory bowel disease (IBD) in Europe.1 Although use of IV iron in the United States is increasing in this population, oral iron is still recommended as first-line therapy.2 However, a recent meta-analysis3 reported that 70% of those to whom oral iron is prescribed complained of significant gastrointestinal perturbation (Figure 1). IV iron is now also the recommended route of replacement for iron-deficient patients having undergone either Roux-en-Y or biliopancreatic bariatric surgery and is considered an early option for oral iron intolerance after gastric sleeve and stapling procedures.4 Although still sporadic, IV iron’s use has dramatically increased in recent years in obstetric and gynecology patients, such as those with heavy uterine bleeding (HUB), where oral iron cannot keep up with losses and in gravidas intolerant of, or unresponsive to, oral iron. Over the same time period, IV iron has also been shown to be effective and well tolerated for restless legs syndrome5,6 and may prevent mountain sickness in iron-replete mountain climbers.7 The use of IV iron has become standard in dialysis and chronic renal failure and has also been shown to markedly improve responses to erythropoiesis-stimulating agents (ESAs) in chemotherapy-induced anemia.8 Twelve of 12 studies on cancer- and chemotherapy-induced anemia report an improvement in time to target and decrements in doses of the much more expensive ESAs. Undoubtedly, this paradigm shift in the treatment of one of the most common maladies on earth has been driven by new formulations with improved adverse event profiles and infusion schedules, which allow complete replacement dosing in a brief single setting (“one-stop shopping”).
Effect of daily ferrous sulfate supplementation on the incidence of gastrointestinal side effects in IV iron-controlled randomized control trials. Reprinted from Tolkien et al3 with permission.
Effect of daily ferrous sulfate supplementation on the incidence of gastrointestinal side effects in IV iron-controlled randomized control trials. Reprinted from Tolkien et al3 with permission.
The focus of this chapter will be on practical aspects of single-dose IV iron replacement for practicing hematologists. Subsequently, the widely used iron salts, ferric gluconate and iron sucrose, whose carbohydrate shells bind iron less tightly and therefore can only be given in small doses of <250 mg at a time, will not be discussed further.
Over the last 6 years, many observational, intra-institutional retrospective, prospective open-label and double-blind studies have shown dramatic improvement in the treatment of iron-deficiency anemia (IDA) in populations in whom oral iron is either poorly tolerated or ineffective. A consistent finding in all of the trials is minimal toxicity. Nonetheless, although IV iron is clearly increasing in use, safety concerns abound, in part fueled by publications reporting differences in safety profiles based on proscribed methodologies,9 inappropriate use of antihistamines for premedication, and treatment of self-limited infusion reactions with vasopressors, which can transform a harmless reaction not in need of aggressive therapy into a serious adverse event.10
In this chapter, we will provide evidence supporting the safety of IV iron as well as its efficacy in a variety of conditions associated with iron lack. We will elaborate on approved methods of administration, while at the same time provide evidence-based practical suggestions on techniques of administration which, although off-label, are more convenient for patients and physicians.
History and safety
The earliest available IV iron formulations were associated with high levels of labile-free iron, causing serious hemodynamic toxicity. In 1954, Baird and Padmore11 introduced a solution of iron dextran whose carbohydrate core held on to the elemental iron more tightly, allowing safe intramuscular administration. As it became clear that the efficacy and safety profile of IV administration was equal to the painful and inconvenient intramuscular injections, IV iron soon gained acceptance because it was associated with rapid hematologic responses with a low incidence of adverse events.12 Nonetheless, rare instances of anaphylactic reactions and deaths resulted in admonitions to use parenteral iron only in situations where oral iron could not be used and when severe anemia was present. A few early reports described the usefulness of IV iron,13,14 and in 1964, Marchasin and Wallerstein published the results of 37 patients who received a total dose infusion of HMWID (ImFeron, Fisons, Homes Chapel, UK, no longer available).15 All experienced an erythroid response, with only one delayed reaction consisting of fever and chills without hypotension or wheezing. In 1980, Hamstra et al published the results of 471 iron-deficient patients who received a total dose infusion of HMWID. Again, all experienced an erythroid response; however, 5 patients developed signs of anaphylaxis. Although there were no deaths, it was concluded again that IV iron should be reserved only for those clinical situations where oral iron could not be used.16
With this history of danger, and the ongoing education of physicians and nurses over many years about serious risk with IV iron, it is not surprising that there continues to be resistance to its use even though safety concerns are much less with currently available products. The manufacturer of HMWID continued to market it for clinical conditions where oral iron could not be used. In 1989, recombinant erythropoietin was approved for patients undergoing hemodialysis, providing hope that the dispiriting symptoms of severe anemia would be alleviated. Yet 3 years after the release of erythropoietin, only a minority of patients undergoing hemodialysis were treated to target hemoglobin levels and many were not treated at all. In 1989 the seminal work of Eschbach and Adamson,17 and later Fishbane et al,18 revealed that iron-restricted erythropoiesis blunted the responses of erythropoietin, elucidating a need for IV iron. By the early 1990s, it was shown that IV iron, when added to erythropoietin, was associated with dramatic improvements in energy, activity, quality of life, work, sexual function, cardiac symptoms, and even survival. The doses of erythropoietin, which is more expensive than iron, were able to be reduced to achieve similar benefits,18 and IV iron became standard in the hemodialysis setting.
In 1991, a manufacturing issue resulted in the removal of the original HMWID from markets. However, LMWID was released at about the same time, and later, another HMWID (Dexferrum, American Regent/Luitpold, Shirley, NY) was released. Safety concerns continued with this new HMWID product because of reports of anaphylactic reactions. In 1999 and 2000, 2 iron salts, ferric gluconate and iron sucrose, were approved for use in the United States, ostensibly with a better safety profile than iron dextran.19 The results of comparative studies were highly statistically significant in favor of the new iron salts, but were based on retrospective spontaneous adverse event reporting rather than prospective comparative trials. Iron dextran’s use in dialysis decreased markedly and was rapidly replaced by the 2 salts.
In 2006, Chertow et al published a retrospective analysis of >30 million doses of IV iron and noted that virtually all the serious adverse events were caused by the HMWID formulation, which was subsequently removed from market. It was the conclusion of this analysis that when HMWID was avoided, the incidence of serious adverse events with IV iron were extremely rare, with an estimated incidence <1:200 000 doses.20 These conclusions are supported by prospective21-24 and intrainstitutional retrospective studies.25
Corroborating these findings, Avni et al published the results of a meta-analysis comprising 10 391 patients treated with IV iron compared with 4044 who received oral iron, 1329 with no iron, and 3335 with placebo.26 A total of 103 trials published from 1965 to 2013 were examined. Although infusion reactions were observed with IV iron, serious adverse events were not increased compared with any comparator, or there was an increased incidence of infection. A marked reduction of gastrointestinal toxicity compared with oral iron was reported. The authors concluded that IV iron formulations are safe and, unlike oral iron, were well tolerated and should be an alternative to red blood cell transfusions, which are associated with events that cause major morbidity in 1 in 21 413 components issued compared with an estimated serious adverse event incidence of <1:200 000 with IV iron (Table 2). No one formulation was found to be more or less toxic than any of the other available formulations. Consistent with these data, Wysoski et al, including investigators from the FDA, used the IMS Database (developed by a pharmaceutical marketing research company), emergency department visits, death certificates, and spontaneous adverse event reporting, and concluded that it is impossible using current monitoring systems to discern relative rates of adverse events among available formulations absent head-to-head studies.9
These conclusions were called into question by another large retrospective analysis from the FDA.27 Using a retrospective new user cohort study of IV iron recipients with 688 183 enrolled in the US fee-for-service Medicare program from January 2003 to December 2013, the investigators concluded that the adverse event of “anaphylaxis” was more likely with iron dextran compared with iron sucrose. These findings are inconsistent with the manuscript’s supplemental material (found on the Blood Web site), which reported a death rate lowest with iron dextran compared with the other formulations (Table 3). Further, in the analysis, the authors were unable to distinguish between high and LMWID, both of which were available during the period of time reviewed in the study. Cases of anaphylaxis were derived from an algorithm based on ICD-9 codes, which may have missed some cases and erroneously included others.28 Further, in this analysis, the average age of those analyzed was in the 70s, whereas younger patients frequently receive IV iron for HUB, pregnancy, bariatric surgery, and IBD.
Consensus recommendation
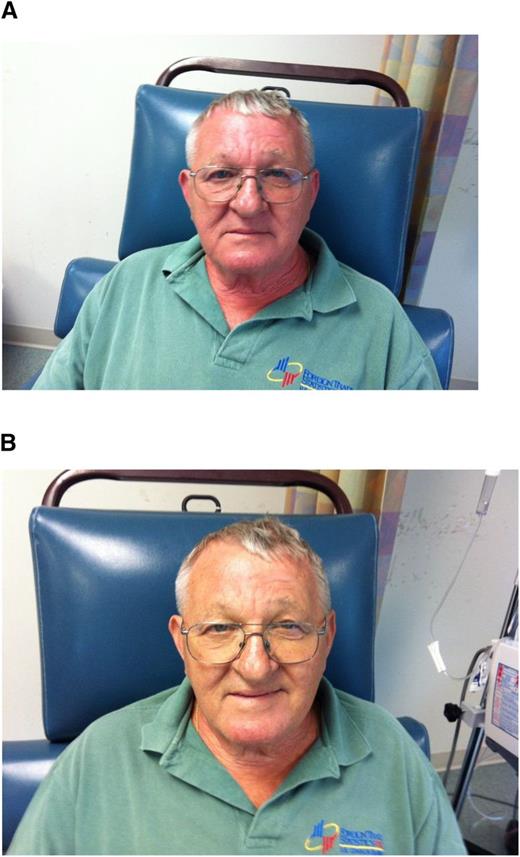
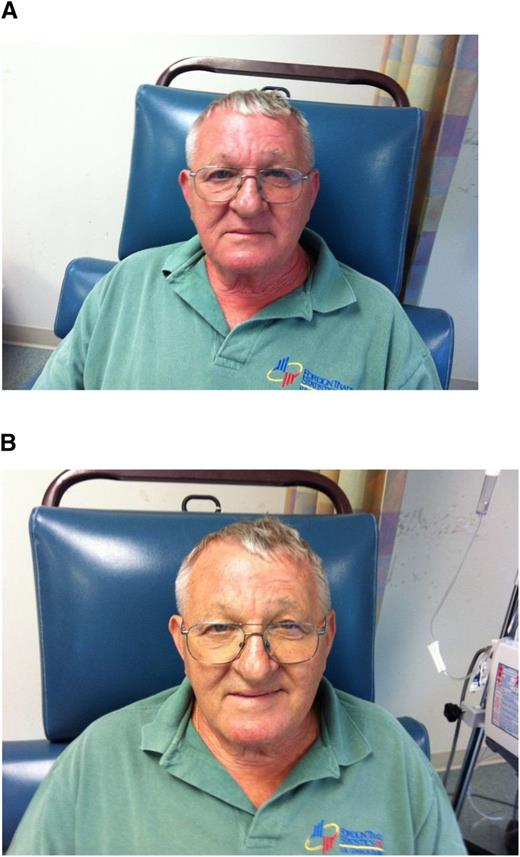
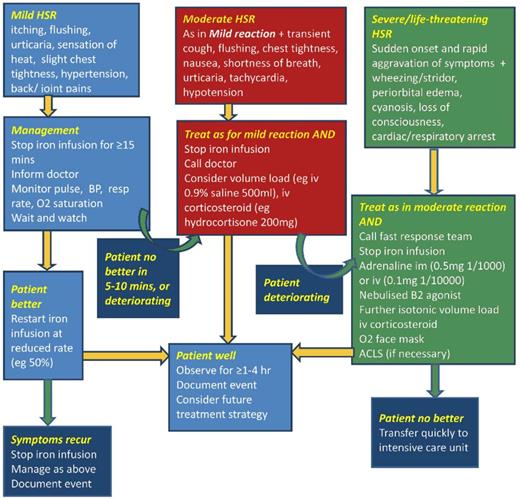
In a recent meeting report of iron management in chronic kidney disease (CKD), the conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference,29 it was emphasized that minor infusion reactions with IV iron are uncommon (∼1:200 administrations) and are almost invariably self-limited and should not be treated with antihistamines or vasopressors. Rechallenge is safe and almost never associated with a recurrence of symptoms. Premedication with antihistamines was proscribed. There are even data that the majority of perceived reactions to IV iron are caused by the premedication and mistakenly attributed to the iron.30 Physicians unfamiliar with these admonitions often treat minor infusion reactions consisting of myalgias of the chest or back or flushing of the face, without associated tachypnea, tachycardia, hypotension, wheezing, stridor, or periorbital edema, with antihistamines or vasopressors, converting a self-limited minor reaction into a serious adverse event, allegedly because of the IV iron. The patient in Figure 2A-B experienced such a minor reaction and was observed for 3 to 4 minutes, after which all symptoms abated. Methylprednisolone was administered empirically before rechallenge, after which the planned dose was completed. In that the overwhelming preponderance of published evidence reports no quantitatively significant serious toxicity with IV iron, it is possible that many serious adverse events associated with the administration of parenteral iron are iatrogenic because of inappropriate intervention for minor infusion reactions.
(A) Minor infusion reaction. (B) After (used with patient’s permission).
Recent payment bundling legislation in hemodialysis has led to decrements in ESA usage and increments in IV iron administration. This in turn has led to concerns about long-term toxicity with IV iron because of free radical formation in response to labile-free iron species. Although we do not wish to minimize the potential importance of these concerns, the preponderance of the evidence is in hemodialysis populations, where frequent small doses of IV iron are administered. It is unlikely that these data apply to populations receiving 1 or 2 large doses of IV iron. There is no data extant suggesting clinical sequelae caused by free radical generation in the clinical entities discussed in this chapter.
Overstating the toxicity of IV iron is potentially harmful. Limiting its use will dramatically increase ESA usage as well as transfusions with their associated complications.31 Essentially all interpretable evidence supports the equivalent efficacy and safety of all the currently available formulations. If a minor infusion reaction occurs, with one formulation switching to another, it is appropriate and safe.29,31
Practical aspects of administration
Iron deficits can be calculated using a variety of different published formulas. The calculated deficit is often greater than 1 gram, but there is no evidence that humans are able to use more than a gram of iron in a single setting. Andrews implied that the capacity for macrophage iron was 600 mg.32 Infused IV iron has a circulatory half-life of approximately 2 weeks. During that time, transferrin is regularly supplied with elemental iron for erythropoiesis. Therefore, it is unlikely that >1000 mg of iron can be used in a single setting, and current evidence does not support a benefit for therapeutic doses exceeding 1000 to 1500 mg. Therefore, this discussion will limit its recommendations to this dose range.
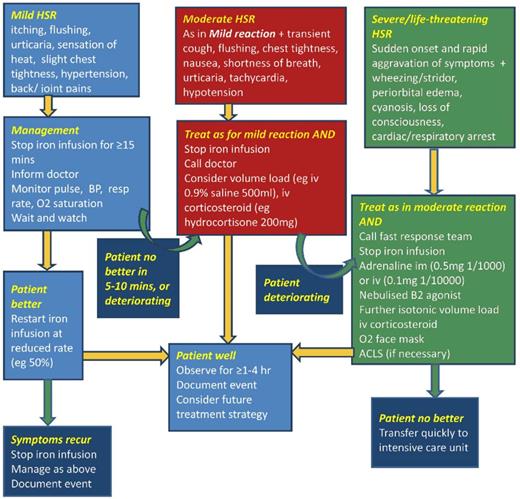
The oldest of the 4 formulations able to be administered as a complete replacement dose in a single (or sometime 2 times) session is LMWID. LMWID is FDA approved for all conditions, including pregnancy, after oral iron intolerance or in those conditions in which oral iron is ineffective or harmful. It is approved as a 100-mg bolus injection; however, extensive literature supports evidence of its safety, efficacy, and convenience when administered as a 1000-mg infusion in 1 hour.4,10,33 In our practice, we routinely administer LMWID as a 1000-mg infusion over 1 hour after a 25-mg test dose, which is a requirement per the package insert, even though there is no evidence to support use of the test dose or that it alters the therapeutic plan.34 Of note, in Europe the use of test doses has been removed with the recommendation to start all formulations slowly. The LMWID is diluted in 250 mL of normal saline and the 25-mg test dose is taken from the diluted solution. After a 10- to 15-minute observation period, if no infusion reactions are observed, the remaining solution is infused over the balance of an hour. The day after the infusion, some patients will experience stiffness, arthralgia, or myalgia that are self-limited and resolve without therapy. Nonsteroidal antiinflammatory drugs may shorten the duration of these symptoms.35 Approximately 1:200 subjects will experience a minor infusion reaction, as described before. When this occurs, we follow the recommendations in the algorithm in the review of hypersensitivity reactions with IV iron36 (Figure 3). After symptoms abate, which usually takes 3 to 5 minutes, we often premedicate with methylprednisolone before restarting the administration of the drug. Recurrence of the infusion reaction is rare. If it does recur, we switch formulations and administer the new product on the next mutually convenient day.
Treatment of iron reactions. Reprinted from Rampton et al36 with permission.
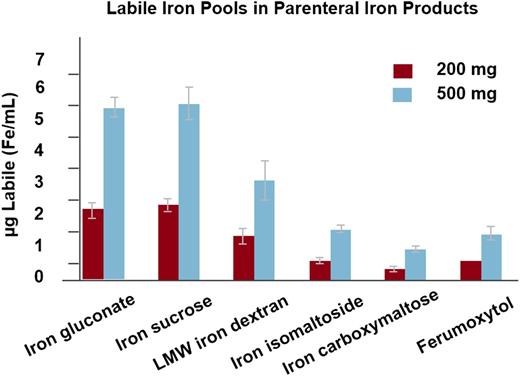
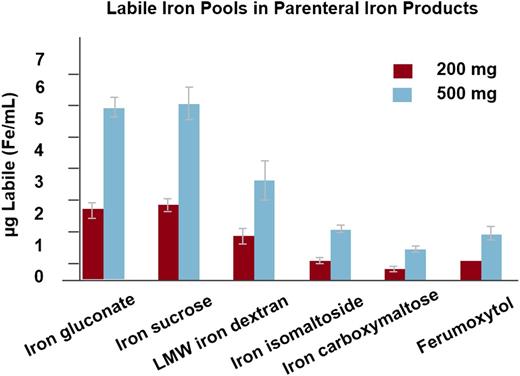
The second of the formulations released, only available in the United States, is ferumoxytol. Before describing the labeling of this very interesting product, it is worth reviewing its storied history. Ferumoxytol was originally designed as an MRI dye because it is paramagnetic. When it was learned that the labile-free iron was available for erythropoiesis, clinical trials were developed for either absolute or functional iron deficiency associated with CKD.37 The initial FDA approval was for a 510-mg bolus administered in not less than 17 seconds. This rapid infusion of a large dose was associated with expectations that dialysis providers would use the convenience of this method of administration to bring the formulation into the dialysis treatment paradigm. Unfortunately, shortly after the release of ferumoxytol in the United States, a large number of postmarketing reports of serious adverse events began to appear. On review of the adverse event report data obtained from Freedom of Information Act, it was impossible to distinguish between true hypersensitivity and intervention for minor infusion reactions. Although the chemical composition of ferumoxytol allows a rapid administration of a large dose, the labile-free iron after a bolus is ∼60% that observed with LMWID38 (Figure 4). It is our opinion that the overwhelming majority of these serious adverse events after a rapid bolus of ferumoxytol are expected side effects as a result of labile-free iron.29,39 In one of our practices (MA), the first 90 doses administered in 17 seconds, were associated with 3 episodes of hypotension which resolved rapidly with fluids without sequelae. Serum tryptase levels were all normal, suggesting that these were not anaphylactic reactions. Subsequently, upon slowing the infusion rate to 90 to 180 seconds, >2500 doses have been administered without a clinically significant adverse event. Nonetheless, the large number of SAEs reported through SAERs resulted in a black box warning, a failure to achieve a broad label for conditions other than iron deficiency associated with CKD, and a new FDA admonition to administer the 510-mg doses as a 15-minute infusion, consistent with the recommendations for the other 2 new parenteral irons (FCM and iron isomaltoside 1000).
Labile iron by iron formulation. Reprinted from Jahn et al38 with permission.
For patients with stages II-V CKD who have laboratory parameters consistent with iron-restricted erythropoiesis or absolute iron deficiency, in our practices, ferumoxytol is given as a 510-mg injection twice over a period of 1 to 3 weeks. Because of the drug’s paramagnetic qualities, the patient is always instructed to inform radiologists should an MRI be performed within the following 3 months. The presence of the drug does not impair and can potentially improve MRI interpretation. Published evidence attests to the safe and effective administration of a complete replacement dose (1020 mg) in 15 minutes, rather than two 510-mg doses.40 Although we agree that this method of administration represents improved convenience without decrements in safety or efficacy, we do not recommend administering ferumoxytol this way unless specifically approved by insurance, because denial of payment for the 1020-mg dose is frequent. In our practice, we administer ferumoxytol as a 510-mg bolus over 2 to 3 minutes because we believe that the 15-minute infusion offers no improvement in safety or efficacy. Nonetheless, in the absence of a significant experience with the administration of the 2- to 3-minute bolus injection of ferumoxytol, we believe practitioners should limit the administration to a 15-minute infusion, as recommended in the package insert, unless part of a clinical trial.
The next of the new agents able to be administered as a complete replacement dose is FCM. This formulation was actually approved in Europe before ferumoxytol was approved in the United States. In its initial filing in the United States, hypophosphatemia and concerns about hemodynamic adverse events delayed the approval for nearly 2 years. However, after a large body of prospective data concluded that the hypophosphatemia was of no clinical significance,41 FCM received a broad label approval from the FDA in 2013. The approvals differ in Europe and the United States, largely because of the vial sizes. In Europe, FCM is routinely administered as a 1000-mg 15-minute infusion. However, in the United States the vial size is 750 mg, requiring that either 750 mg or 1500 mg (2 visits) be the therapeutic dose. FCM is approved for all causes of iron deficiency, including pregnancy, in patients intolerant of, or unresponsive to, oral iron or in those conditions in which oral iron is proscribed. Although rare minor infusion reactions to FCM occur, they are infrequent and are handled identically to the minor infusion reactions with the other formulations. In our experience, we have only administered 750 mg in 15 minutes either as a single therapeutic dose or as 2 doses totaling 1500 mg. We have not observed any serious adverse events. This formulation was the first of the new IV iron compounds to show efficacy in chemotherapy-induced anemia when administered without ESAs.42 FCM is widely used in Europe and the United States for a host of conditions associated with iron lack including IBD,43 HUB,44 pregnancy,45 and in the perioperative setting.46
The last of the new formulations able to be administered as a complete replacement dose in a single setting is iron isomaltoside. Iron isomaltoside is not yet approved in the United States but was approved in Europe in 2009 for the treatment of IDA. This compound has a unique matrix structure with high stability, similar to ferumoxytol and FCM, limiting the release of labile-free iron, which allows a rapid infusion of a high dose.38 Iron isomaltoside can be administered at a maximum single dose of 20 mg/kg without a test dose. Doses up to 1000 mg can be administered in 15 minutes, but those >1000 mg should be administered over 30 minutes. This formulation has been shown in large clinical trials to be well tolerated and effective in correcting ID across a broad spectrum of diseases associated with iron lack. Examples include dialysis, non-dialysis–dependent CKD, chronic heart failure, IBD, cancer and chemotherapy-induced anemia, cardiac surgery, and postpartum hemorrhage.47 Large clinical trials are currently underway in the United States.
Clinical use of IV iron in specific disease categories
Pregnancy and heavy uterine bleeding
All of the therapeutic recommendations for iron supplementation in pregnancy apply to women with HUB, with one exception. We believe oral iron should be proscribed in women with menorrhagia because oral iron is not absorbed adequately enough to keep up with the losses. Waiting for treatment failure or intolerance is imprudent and, based on the safety and efficacy in populations with HUB, it is logical to move IV iron to the front line. We recently reported the results of 1266 infusions of LMWID administered to 888 patients intolerant of oral iron, with HUB being the commonest indication. No clinically significant adverse events were noted and a hemoglobin or hematopoietic response was seen in >85%.
In addition to the symptoms of iron deficiency in gravidas, maternal iron deficiency is also associated with adverse infant outcomes. These include delayed growth and development as well as a statistically significant increment in both cognitive and behavioral abnormalities, which persist for up to 10 years after iron repletion.48,49 We believe the current frontline standard of oral iron repletion should be revisited because a significant majority of pregnant women report gastrointestinal toxicity limiting oral iron use, the most common of which is worsening constipation.50 The recommendation is supported by a study in 1258 pregnant women who received either daily or once or twice weekly oral supplementation that did not produce a clinically important difference in birth weight or hemoglobin levels.51 Pregnant women are often constipated as a result of high progesterone levels that slow bowel transit and increasing pressure of the gravid uterus on the rectum, which is worsened by oral iron.
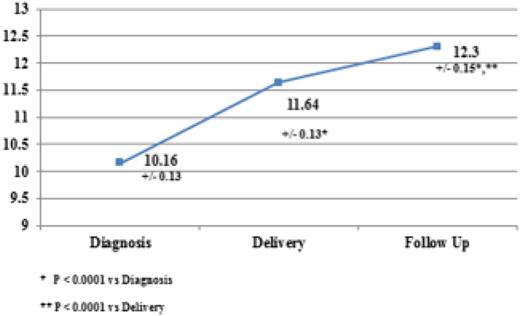
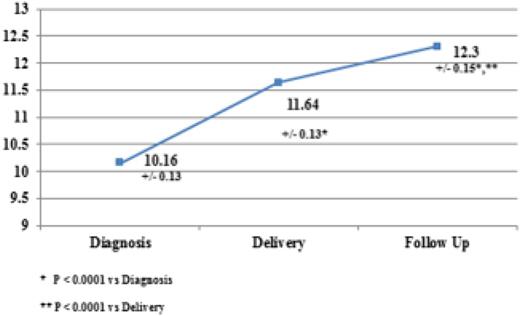
Several recent publications corroborate the safety and efficacy of IV iron in gravidas. A common theme in all of these analyses is the absence of serious adverse events. In a recent prospective study of 65 anemic gravidas, Froessler et al reported the safe effective use of FCM, administered in the second and third trimesters, without any significant adverse events noted.52 These results corroborate a previous study using LMWID as a single total dose infusion in 100 iron-deficient pregnant women.53 Once again, no clinically significant adverse events were reported. Further corroboration of these conclusions was reported in a prospective randomized trial comparing LMWID and FCM in 92 iron-deficient gravidas. The authors concluded that both preparations are effective and safe without risk of serious adverse events.45 A similar conclusion was reached in a comparison of iron sucrose and FCM in 206 pregnant women intolerant of oral iron.53 In a recent publication, Wong et al, supporting a rapid total dose infusion of 1000 mg of LMWID to iron-deficient gravida women in the second and third trimesters, all intolerant of oral iron, reported the efficacy, safety, and increased convenience of complete replacement dosing in a short, single visit.54 One hundred eighty-nine consecutive nonselected iron-deficient pregnant women were treated. Only 2% experienced transient infusion reactions, all of which abated without therapy. No serious adverse events were reported. Hemoglobin levels improved by 1 to 1.9 g/dL in 82% and >2 g in 24%. Anemia resolved in 95% (Figure 5). These results are consistent across the spectrum of published evidence. At this meeting we are reporting the results of the first American prospective study of IV iron in oral iron-intolerant pregnant women (IND 114 696, ClinTrials.gov NCT #020038023). These results mirror the published evidence supporting safety and efficacy of IV iron in anemic gravidas.
Intravenous iron in pregnancy—hemoglobin rise. Reprinted from Wong et al54 with permission.
Intravenous iron in pregnancy—hemoglobin rise. Reprinted from Wong et al54 with permission.
The preponderance of published evidence supports the convenience, safety, and efficacy of IV iron for IDA of pregnancy. We believe IV iron should be administered as soon as oral iron intolerance occurs or as front-line therapy to those in whom oral iron is known to be ineffective or harmful. Further, given the lack of reported serious adverse events and consistent safety and efficacy across published clinical trials, along with new formulations that allow the complete replacement dosing in 15 to 60 minutes, one might ask if an improved, faster, and less toxic clinical outcome could be achieved if oral iron were not part of the treatment paradigm.
Bariatric surgery
Individuals having undergone surgery for weight reduction pose a unique problem. When a person with an intact gastrointestinal tract ingests iron, it is conjugated in the stomach in the presence of acid to vitamin C, amino acids, and sugar (Figure 6), which protects the iron from conversion to ferric hydroxide in the proximal duodenum, where the massive alkaline secretions of the pancreas take place (personal communication, Dr. Jerry Spivak, Johns Hopkins School of Medicine). Ferric hydroxide cannot be absorbed and is excreted in stool. Oral iron, even if absorbed, further exacerbates the already existing gastrointestinal perturbation present in patients in whom the gastrointestinal tract has been rerouted. Regular exercise, routinely recommended as an accompaniment to the nutritional changes after surgery, is impeded by the increased fatigue that occurs with iron deficiency, even without anemia, or even more by overt IDA. Therefore, it is our contention that IV iron should be routine and front line for bariatric patients with evidence of iron lack.
Iron absorption. With permission from Dr. Jerry Spivak, Johns Hopkins University School of Medicine.
Iron absorption. With permission from Dr. Jerry Spivak, Johns Hopkins University School of Medicine.
There is even evidence that there is increased anemia and iron deficiency preoperatively in obese patients. Salgado et al reported 21.5% and 20% of preoperative bariatric patients had anemia and iron deficiency, respectively.55 Obinwanne et al reported a 51.3% incidence of patients with iron deficiency, half of whom were severely iron-deficient with ferritin levels <30 ng/mL.56 In that series, it was estimated that at least 50% of female patients would develop clinically significant iron deficiency after Roux-en-Y gastric bypass surgery. They concluded that all bariatric surgery patients should be carefully monitored and treated for iron deficiency. The recommendations are corroborated by a review by Munoz et al, reporting a high incidence of iron deficiency in the perioperative period as well as nonadherence with oral iron.57 They concluded that IV, and not oral, iron is safe, effective, and associated with 100% adherence, pointing out the ease of newer formulations able to be administered as a complete replacement dose in a single setting. These conclusions are corroborated by a prospective, randomized, open-label study of 281 oral iron–intolerant, iron-deficient bariatric patients who received 1000 mg of FCM or iron sucrose.58 Similar efficacy and safety was observed with a response rate approaching 100%. The single infusion of FCM added convenience for physicians and patients. These results are consistent with our previously described series of 888 patients, 90 of whom had undergone weight loss surgery, who received 1000 mg of LMWID in 1 hour.33 Supporting this conclusion was the superiority of ferumoxytol compared with placebo in oral iron–intolerant iron-deficient bariatric patients.59
We prefer IV iron for most patients who have undergone gastric resection, Roux-en-Y, biliopancreatic diversion, or similar procedures because it ensures adequate delivery and avoids gastrointestinal toxicities, which are especially burdensome to these patients.3 Although some patients having undergone minimally invasive procedures such as gastric banding or stapling may tolerate oral iron, given the litany of gastrointestinal adverse events, IV iron simplifies care.
Inflammatory bowel disease
Iron deficiency, nearly ubiquitous in IBD, complicates a host of disabling symptoms from the underlying disease. In addition, the inflammation and subsequent upregulation of hepcidin inhibit iron utilization in this population. Published evidence suggests that oral iron causes severe adverse events and worsens the underlying pathology.1,60 These data are supported by a randomized comparison of oral ferrous fumarate and IV iron sucrose, which reported that oral ferrous fumarate, but not IV iron sucrose, increased clinical disease activity in IBD.61 A recent publication by Lee et al reported worsening gut flora with oral iron compared with IV iron.62
A number of studies present comparative evidence demonstrating the superiority of IV iron over oral iron in both efficacy and toxicity.61,63 With the advent of 4 new formulations that allow safe and rapid complete replacement dosing in 15 to 60 minutes, the failure to routinely administer IV iron to anemic patients with IBD may represent an unmet clinical need. All 4 of the formulations have been shown to be safe and effective when ID accompanies IBD.
Nonetheless, as of today, European and American guidelines differ. European guidelines state that the preferred route of iron supplementation in IBD is IV, even though some patients may respond better to oral iron. IV iron is more effective and better tolerated and improves the quality of life to a greater extent than oral iron (Grade A).1 American guidelines state “oral formulations are more convenient and less expensive and may be used as a first-line option for patients whose IBD activity and anemia are mild”64 . The European recommendations are supported by a recent publication demonstrating not only safety and efficacy with a single infusion of FCM, as early as 2 weeks after therapy, with >80% achieving a complete response at 12 weeks, but statistically significant improvements in parameters of quality of life as well.65
The results of the preponderance of published evidence support a larger and earlier role for IV iron in the treatment paradigm of ID associated with IBD. Once again, one could surely ask if improved and less expensive clinical outcomes could be achieved were oral iron dismissed from the treatment paradigm. We believe the United States should adopt the European guidelines.
Restless legs syndrome
Restless legs syndrome (RLS) occurs in approximately one third of patients with IDA. The syndrome is characterized by brief, rapid, involuntary movements at rest and while awake and semi-rhythmic movements in sleep. The sleep disturbances are severe in >70% of those with the syndrome and can cause marked fatigue and decreased performance.66 Although the precise mechanism is unknown, significant decrements in iron concentrations in the substantia nigra have been documented using MRI.67,68 Peripheral iron status correlates with RLS severity69 and both oral and IV iron significantly reduce RLS symptoms compared with placebo.5
In 2014, Mehmood et al published the first consecutive case series evaluating the effects of IV iron therapy on RLS occurring with IDA.70 RLS–IDA patients were evaluated before and 7 to 12 months after a 1000-mg infusion of LMWID using validated questionnaires and standardized telephone interviews with subsequent classification for response and nonresponse for RLS improvement. In 70% of 60 consecutive, nonselected RLS-IDA patients, the symptoms were reduced in 76% (32/42), with 47% (20/42) showing an extended response lasting >6 months. The responses were independent of age or gender. To date, no statistically significant comparisons of oral vs IV iron have been published. To answer that question, we are currently performing a randomized double-blind study of oral iron and ferumoxytol in iron-deficient subjects with RLS using validated criteria for both diagnoses.
Mountain sickness
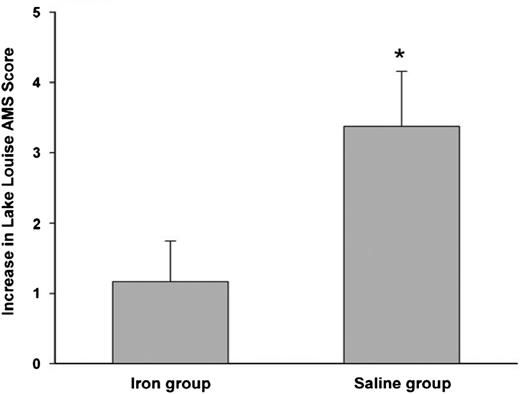
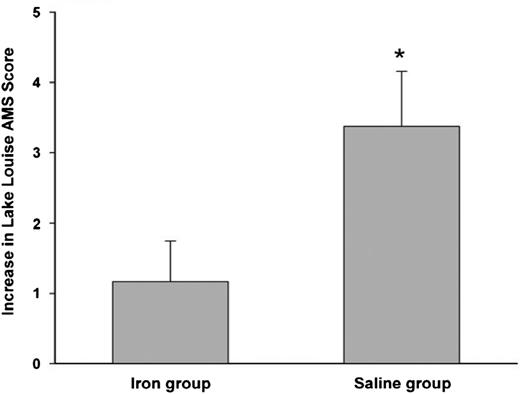
We close our discussion on specific disease categories with a relatively unknown yet interesting condition that may benefit from IV iron: acute high-altitude sickness. Twenty-four healthy sea-level residents were randomly selected to receive IV iron (4 doses of iron sucrose) or no treatment (placebo), before rapidly ascending to Cerro de Pasco in Peru at 3400 meters. The Lake Louise scoring system, the current standard, was used to assess acute mountain sickness before and after the first day.7 IV iron protected the occurrence of acute mountain sickness (Figure 7), and the Lake Louise scores, independent of hemoglobin levels, were significantly higher with placebo than patients who received IV iron. Although the mechanism is unknown, the differences were believed to be based on IV iron’s ability to influence cellular oxygen-sensing pathways that are independent of hemoglobin, because oxygen-sensing dioxygenase enzymes that regulate hypoxia inducible factor are highly sensitive to varying iron availability.71
Protective effect of IV iron in preventing acute mountain sickness. Increase in Lake Louise score—a marker of severity of mountain sickness after rapid ascent to 4340 meters. Before ascent, the iron group received 200 mg iron sucrose and placebo normal saline. Difference between groups is significant (P < .05). Reprinted from Talbot et al7 with permission.
Protective effect of IV iron in preventing acute mountain sickness. Increase in Lake Louise score—a marker of severity of mountain sickness after rapid ascent to 4340 meters. Before ascent, the iron group received 200 mg iron sucrose and placebo normal saline. Difference between groups is significant (P < .05). Reprinted from Talbot et al7 with permission.
These data are supported by work from Robbins et al,72 which reported that healthy subjects subjected to a hypoxia chamber for 8 hours and then given IV iron or desferoxamine have reduced pulmonary artery pressures. In iron-replete individuals, the administration of IV iron abolished the elevation in pulmonary artery pressure that accompanies hypoxia, induced by the hypoxic challenge as well as the accompanying increase in acute hypoxic vasoreactivity. These data suggest that the failure to address the use of IV iron in mountain climbers may be yet another unmet clinical need.
Conclusion
Increased use of IV iron is a new paradigm in treating multiple iron-deficient states. There are now 4 formulations that allow complete replacement of iron in 1 to 2 administration sessions, and there are increasing data regarding safety of IV iron and the realization that many minor infusion reactions were converted to major ones by unnecessary interventions.
Although the superiority of IV iron in the setting of anemia of CKD and cancer is well known, we have provided evidence of efficacy in several other common clinical situations. In the setting of HUB and pregnancy, IV iron is more effective and better tolerated than oral iron. Given the issues of gastrointestinal intolerance and impaired absorption, IV iron is the treatment of choice for patients having undergone bariatric surgery. In the setting of IBD, oral iron is often harmful and probably should not be used. Many patients with RLS require iron replacement, which can most effectively be achieved with IV iron. Finally the effectiveness of IV iron in prophylaxis of acute mountain sickness demonstrates the many multifaceted derangements caused by ID. In light of these findings, IV iron should be a key part of the repertoire of every practicing hematologist.
Correspondence
Michael Auerbach, private practice, Baltimore, MD 21237; e-mail: mauerbachmd@abhemonc.com.
References
Competing Interests
Conflict-of-interest disclosures: M.A. has received research funding for data management from Pharmacosmos and has consulted for Pharmacosmos, AMAG Pharma, and American Regent/Luitpold. T.D. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.