Abstract
Nontransplant therapeutic options for acquired and constitutional aplastic anemia have significantly expanded during the last 5 years. In the future, transplant may be required less frequently. That trilineage hematologic responses could be achieved with the single agent eltrombopag in refractory aplastic anemia promotes new interest in growth factors after years of failed trials using other growth factor agents. Preliminary results adding eltrombopag to immunosuppressive therapy are promising, but long-term follow-up data evaluating clonal evolution rates are required before promoting its standard use in treatment-naive disease. Danazol, which is traditionally less preferred for treating cytopenias, is capable of preventing telomere attrition associated with hematologic responses in constitutional bone marrow failure resulting from telomere disease.
Learning Objectives
Upon completion of this activity, participants will be able to:
Describe the standard nontransplant treatment of severe aplastic anemia
List the options for salvage therapy in patients with severe aplastic anemia
Define the likely mechanism of action for eltrombopag in the treatment of aplastic anemia, and when to provide the treatment in the course of the disease
In patients with inherited bone marrow failure resulting from a telomere disease, describe the nontransplant therapy that is efficacious, and why
Introduction
During the last 3 decades, patients with aplastic anemia (AA) have benefited from restoration of effective hematopoiesis via allogeneic hematopoietic stem cell transplantation or intensive immunosuppressive therapy (IST) with horse antithymocyte globulin and cyclosporine. Although successful engraftment with donor hematopoietic stem cells (HSC) is the only definitive curative treatment of AA, it is not available to many patients because of unavailability of a matched donor, undue toxicity of HSC transplantation (HSCT) associated with older age and comorbidities, or excessive lead time to identify a suitable donor. The outcomes for a majority of patients treated with IST are excellent, and this approach revolutionized outcomes for an otherwise fatal disease.1,2 However, attempts to improve IST response rates and/or decrease relapse via more intensive IST or addition of hematopoietic growth factors such as erythropoietin-stimulating agents or granulocyte colony-stimulating factor have thus far been ineffective.3-5 Treatment of patients with refractory AA has also been challenging, often resulting in referral to transplant. Recommendations for initial allogeneic stem cell transplantation vs IST has depended on age, comorbidities, count severity, and donor availability.
In this chapter, we summarize the very encouraging new findings regarding the use of eltrombopag, a small-molecule oral thrombopoietin receptor agonist, in the treatment of patients with both refractory and treatment-naive AA. During the last decade, major insights have also occurred into the prevalence and pathophysiology of constitutional marrow failure syndromes. We present our recent findings regarding the ability of androgens to lengthen telomeres and improve hematopoiesis in patients with constitutional AA linked to abnormalities in the function of telomere maintenance components.
Diagnosis
Pancytopenia and a core bone marrow biopsy hypocellular for age is a diagnostic requirement for AA, and criteria for severe disease include 2 of 3 blood lineages meeting thresholds of absolute neutrophil count lower than 500/μL, absolute reticulocyte count lower than 60 000/μL, and platelet count lower than 20 000/μL.6 Treatment is always indicated for severe disease. Unfortunately, the diagnosis of AA can be delayed because of unfamiliarity with the disease, leading to exhaustive attempts to exclude other causes of cytopenias and futile attempts “watching and waiting” for spontaneous count recovery. Urgency in the diagnosis and treatment is particularly important for those patients with very severe AA, characterized by having an absolute neutrophil count lower than 200/μL, or those with frequent infectious complications.7
Distinguishing constitutional AA from acquired disease is discussed in a companion review (see Shimamura, in this book8 ), but careful attention to extrahematopoietic abnormalities is required, as the treatment algorithms and prognosis will differ. All patients with AA should undergo evaluation for Fanconi anemia, GATA2 deficiency, and telomere disease (also referred to as dyskeratosis congenita), consisting of a careful family history, physical examination, and screening with relevant functional assays.
The delineation between hypocellular myelodysplastic syndrome (MDS) and AA can be difficult, and our understanding of the shared pathophysiology of these 2 diseases is currently undergoing significant revision.9 In individuals lacking overt features consistent with World Health Organization criteria for MDS, a diagnosis of MDS should not be assumed on the basis of age: AA can occur in both toddlers and the elderly. Somatic mutations in genes commonly mutated in MDS/acute myeloid leukemia (AML) may be present,10,11 especially in older patients, as can PIGA mutations and copy-neutral loss-of-heterozygosity, but these genetic abnormalities are currently of unclear diagnostic utility in AA.12 At present, it is not appropriate to dictate the initial approach to therapy on the basis of the presence or absence of specific somatic mutations in the absence of dysplasia or MDS-defining cytogenetic abnormalities.
Initial therapy for severe AA
Severe AA
The standard IST regimen for severe AA (SAA), horse antithymocyte globulin (hATG), and cyclosporine (CSA) was established nearly 3 decades ago. The initial research and development of ATG was not obvious: a few reports of patients achieving count recovery after graft rejection suggested a therapeutic role for the immunosuppressive agents administered for conditioning. Large studies worldwide reported consistent hematologic response rates of 60% to 70% in patients receiving hATG and CSA.13-17 Response is conventionally defined at our institution as any improvement in blood counts sufficient to no longer satisfy criteria for SAA. Most patients respond by 3 to 6 months after initiation of IST, with virtually all responders achieving full transfusion independence, and response strongly correlates with long-term survival.18
Aiming to improve response rates with IST, a series of trials was performed. Additional immunosuppressive agents were added to the hATG/CSA platform without benefit: sirolimus,19 mycophenolate mofetil,20 and high-dose corticosteroids.21 More potent immunosuppressive agents were administered frontline, such as rabbit ATG,17 cyclophosphamide,22 and alemtuzumab,17 but either they failed to enhance response or, in the case of cyclophosphamide, toxicity was unacceptable and did not prevent late events, despite suggestions to the contrary in initial reports.23 Rabbit ATG showed particular promise because of its more potent lymphocytotoxicity compared with horse ATG24 and success in salvaging some patients refractory to or relapsing after treatment with initial hATG.25,26 Retrospective studies that compared outcomes between rabbit and horse ATG showed mixed results,27,28 but the definitive randomized controlled trial comparing both formulations at the National Institutes of Health (NIH) demonstrated horse ATG to be far superior. Response rate was lower with rabbit ATG (37%) compared with horse ATG (68%); rabbit ATG was more toxic, and fewer patients survived when treated with rabbit ATG.17
Late complications of IST include relapse in a third of IST responders,18 typically identified as a rapid or steady decline in blood counts after CSA is discontinued. Expansion of paroxysmal nocturnal hemoglobinuria clones to cause clinically significant hemolysis requiring therapy occurs in ∼5% of patients.29,30 The development of cytogenetic abnormalities, termed “clonal evolution,” occurs in 10% to 15% of patients when followed long-term.1,31 Although some cytogenetic abnormalities are of unclear clinical significance, loss or partial loss of chromosome 7 is the most concerning late event and was associated with substantial risk of developing AML or high-risk MDS, and with the need for HSCT.30,31 We follow all responders with serial bone marrow evaluation at 6 and 12 months after treatment with IST, and then yearly to monitor for evolution.
Pretreatment factors are predictive of outcomes. Multivariate analysis of data at our institution identified younger age, and higher baseline absolute reticulocyte count and lymphocyte counts positively correlate with hematologic response at 6 months.32 Long-term survival is excellent in pediatric patients treated with IST, with survival rates among responders of about 90%.33,34 Both very severe neutropenia before IST and comorbid invasive fungal infections were independently predictive of poor survival,35 but this is no longer the case in more recent years, with developments in supportive care and improvements in survival.36 Detection of a paroxysmal nocturnal hemoglobinuria clone by flow cytometry of peripheral blood at baseline may indicate an immune-responsive disorder and correlates with response to IST in some studies, but not in others.29,32,37-39 Shorter pretreatment telomere lengths, as measured in peripheral blood leukocytes, identify patients at higher risk for relapse who are more likely to develop cytogenetic abnormalities, as well as associates with worse overall survival.11,36 Monosomy 7, the cytogenetic abnormality most commonly associated with the development of MDS and AML from AA, is preceded by marked progressive telomere attrition in myeloid cells indicative of a proliferative stress on a reduced stem cell population from which aneuploidy may emerge.40
High-throughput sequencing has revealed that hematopoiesis is clonal in up to 50% of patients with AA, and one-third of all patients harbor acquired genetic mutations that were also found recurrently mutated in myeloid cancers.10 Some of these mutations, or sets of gene mutations, may eventually inform treatment allocation, but should be approached in the context of clinical trials and not be used solely in treatment decision-making. Mutations in PIGA, the gene somatically mutated in paroxysmal nocturnal hemoglobinuria, and BCOR and BCORL1 mutations correlate with a better response to IST and clinical outcome.10 Mutations In DNMT3A, ASXL1, and other genes also found in AML or MDS are being studied for their unfavorable association with outcome,10,11 but at this time, the magnitude of their contribution to risk is unclear, and further studies are required to evaluate their effect on prognosis.
Salvage immunosuppressive therapies
Most relapse occurs in the first 2 to 3 years after IST, and typically at discontinuation or tapering of CSA. Review of risk factors for relapse in a retrospective cohort of pediatric patients treated with IST identified a low rate of relapse with a slower cyclosporine taper.41 Scheinberg et al performed a study at the NIH designed to evaluate whether a gradual CSA taper could prevent relapse.42 Patients responding to IST had CSA tapered starting at 6 months. Although the rate of relapse at 5 years was 33%, similar to our historic experience (30%-40%) when CSA is discontinued at 6 months, the time to relapse was prolonged (∼1 year). Kinetics of relapse suggested a dose threshold of CSA to maintain response, and further investigation of CSA, using a lower, long-term dose of CSA, is underway.
In relapsing patients for whom reintroducing CSA at full dose is ineffective, or for patients who fail to respond by 6 months to IST, alternative treatment is indicated. We refer the reader to recent reviews on the topic detailing algorithms generally aimed at stratifying patients based on age, comorbidities, and availability of histocompatible donors for transplant.1,43 A repeat course of IST is an effective option. Initially, rabbit ATG was shown to be capable of rescuing blood counts in two-thirds of relapsing patients and one-third of patients refractory to an initial course of hATG/CSA.25 In addition, alemtuzumab (without cyclosporine) has also been shown to be effective as a salvage agent in both relapsed and refractory settings, with similar salvage rates to rabbit ATG. Alemtuzumab is particularly attractive for patients unable to tolerate CSA.44,45
Eltrombopag
Although the exact pathophysiology for most AA cases is still unclear, evidence deduced from clinical and laboratory studies clearly implicates a progressive immune-mediated destruction of the bone marrow compartment, and in particular of the hematopoietic stem and progenitor cells (HSPC) as the principle mechanism responsible for AA-associated cytopenias.2 The severely reduced HSPC pool may explain persistent pancytopenia in some patients, despite repeated IST. Thus, pharmacological stimulation of HSPC self-renewal and proliferation could be a rational approach for these patients with otherwise limited therapeutic options. However, the addition of hematopoietic growth factors such as granulocyte colony-stimulating factor or erythropoietin have not resulted in higher response rates compared with IST alone in previous studies.3-5
In 2008, the thrombopoietin (TPO) receptor agonist eltrombopag (Promacta) was approved by the US Food and Drug Administration for the treatment of thrombocytopenia in patients with chronic immune thrombocytopenic purpura. In contrast to the other available TPO receptor agonist, romiplostim, eltrombopag is a synthetic and orally bioavailable molecule that activates the TPO receptor, c-MPL, by dimerizing its 2 receptor chains at the transmembrane domain, not competing with endogenous TPO.46 A number of lines of evidence support a pleiotropic role for TPO in hematopoiesis beyond its role as the primary endogenous factor controlling platelet production from megakaryocytes. C-MPL is not only expressed on very primitive HSPC but is also crucial for maintaining their number and multipotent phenotype in vivo. In patients and animal models, genetic defects leading to a loss of function of TPO/c-MPL signaling result in a significant reduction of HSPC numbers and function.47-51 Furthermore, in vitro HSPC expansion can be stimulated by TPO, either alone or in combination with other cytokines.52 TPO levels are already very elevated in SAA,53,54 arguing against efficacy of stimulating this pathway, unless very high levels of TPO agonist stimulation could be achieved.
On the basis of these observations, we conducted a phase 2 study to investigate whether eltrombopag can improve platelet counts in patients with SAA refractory to at least 1 course of standard IST; 36/43 (84%) patients who completed the protocol had 2 or more prior therapies. Eltrombopag was initiated at a daily starting dose of 50 mg, which is the effective dose in patients with immune thrombocytopenic purpura, and then escalated biweekly to a maximal dose of 150 mg. Seventeen (40%) of the 43 patients had a hematologic response at 3 to 4 months (primary endpoint), all at the 150-mg dose level. Responding patients continued eltrombopag until they fulfilled protocol criteria for robust response (platelets, >50 × 103/mL; hemoglobin, >10 g/dL; and neutrophils, >1 × 103/mL for at least 8 weeks or until they plateaued during a period of 6 months). The majority of responders had improvement of 1 blood lineage at assessment, but multilineage responses were also observed (Figure 1). Baseline absolute reticulocyte count was the only predictor of response. Continued exposure to eltrombopag further improved peripheral blood cell counts in the majority of responding patients, with protocol relevant trilineage improvements in 7 patients (Figure 1).55,56 To evaluate durability, eltrombopag was discontinued in 9 patients with robust responses. To date, all but 1 patient remains off drug with continued hematologic remission, suggesting eltrombopag is expanding the operational HSPC pool.
Responses to eltrombopag by lineage in patients with SAA refractory to IST. These Venn diagrams show the numbers of patients with unilineage and multilineage responses at response assessment (A) and best response at follow-up (B). Reprinted from Desmond et al55 with permission.
Responses to eltrombopag by lineage in patients with SAA refractory to IST. These Venn diagrams show the numbers of patients with unilineage and multilineage responses at response assessment (A) and best response at follow-up (B). Reprinted from Desmond et al55 with permission.
Despite higher dosing compared with doses used in immune thrombocytopenic purpura, eltrombopag was very well tolerated in the NIH SAA study. Other than infrequent and reversible transaminitis, no dose-limiting toxicities were reported. Of potential concern is the rate of clonal evolution observed during eltrombopag administration and the theoretical risk of stimulating dormant genetically aberrant HSPC. In the NIH study, 8 patients (19%) developed cytogenetic abnormalities detected while receiving eltrombopag, the majority of whom were nonresponders having failed 2 or more prior therapies. Loss or partial loss of chromosome 7 occurred in 5 patients without evidence of bone marrow dysplasia: 4 were successfully transplanted and 1 died of progressive cytopenias. Deletion of 13q was detected in 2 responding patients, and trisomy 8 in 1 nonresponder; none of these patients had dysplastic bone marrow morphology, and all were successfully transplanted. Repeated cytogenetic studies 8 months after a chromosome 7 abnormality was detected revealed a normal karyotype in 1 untransplanted patient. Patients on study were monitored with weekly blood counts and bone marrow biopsies after 3 months and at least every 6 months thereafter. Therefore, it is possible that clonal events were detected ahead of clinical manifestations, making a comparison with historical and retrospective data on clonal evolution difficult. Recently, Platzbecker et al investigated the safety and tolerability of eltrombopag in thrombocytopenic patients with high-risk MDS or AML.57 Bone marrow blast counts or proportions of peripheral blasts were similar between the treatment and control groups in this randomized, placebo-controlled, double-blind, multicenter study.57 In addition to randomized studies, modern genetic testing using next-generation sequencing techniques of candidate gene panels, or even the whole genome/exome, may help to better stratify risk of patients with AA for clonal evolution in the future. Until then, we recommend restricting eltrombopag to patients who are refractory to at least 1 round of IST and including serial monitoring for cytogenetic abnormalities.
Encouraged by the favorable response rates in the refractory setting, in 2012, we opened a study to investigate the addition of eltrombopag to IST in treatment-naive SAA at the NIH. If eltrombopag is capable of expanding the hematopoietic stem cell pool, perhaps administration early in disease course, before profound loss of HSC, could increase the likelihood of hematologic recovery and promote clonal diversity, subsequently limiting clonal evolution to MDS. In this phase 1/2 study, eltrombopag was administered concurrently with hATG/CSA at a fixed dose for 6 months. An interim analysis of 92 patients presented at the last annual meeting of the American Society of Hematology58 reported significantly increased overall (86%) and complete (37%) response rates after 6 months for all treatment groups combined. More strikingly, up to 94% of the patients in the cohort receiving eltrombopag without delay, on day 1 with ATG, achieved a response at 6 months of which 60% were complete. The median time for neutrophil recovery (absolute neutrophil count >200/μL) was only 8 days in this treatment group, and transfusion independency for platelets was achieved after 32 days (median). Elevated transaminases and bilirubin levels (grade 2-3) were observed in about 10% of patients, and 2 patients experienced severe cutaneous reactions necessitating termination of eltrombopag. No other dose-limiting toxicities were observed. Abnormal cytogenetics were detected in 7 (8%) of 92 patients, with 5 patients exhibiting chromosome 7 abnormalities. The median follow-up to date has been 19 months; thus, further follow-up is required to estimate the effect of eltrombopag on late events. The European Group for Blood and Marrow Transplantation sponsored randomized, placebo-controlled, RACE trial (Randomized ATG, CSA, and Eltrombopag) comparing standard IST with or without eltrombopag will also help to quantify the effect of the combined regimen on long-term events.
Inherited AA
Nontransplant therapy for inherited AA is typically supportive. Patients with telomeropathies have been regarded as unlikely to respond to IST, but we have observed count recovery in some adults with inherited mutations in the telomerase reverse transcriptase (TERT) gene and the telomerase RNA component (TERC) at our institution (D.M.T. and N. S. Young, unpublished data). Extrapolation from outcome data in acquired AA suggests these patients may be at increased risk for relapse and clonal evolution after IST. Further long-term studies are needed to definitively assess this risk in the telomere diseases.
Androgens
Androgens, such as oxymetholone or danazol, have been used to treat cytopenias in AA since the mid-20th century, with variable success.59-61 However, in patients with inherited bone marrow failure associated with a telomere disease, sex hormones are particularly efficacious.62 Some responding patients in initial studies of androgens in AA, before the use of IST, likely had telomere deficits. In vitro data showing that sex hormones directly regulate TERT to increase telomerase activity in CD34+ hematopoietic cells,63 and hematologic improvement in a mouse model of telomere dysfunction,64 have illuminated the probable mechanism of action.
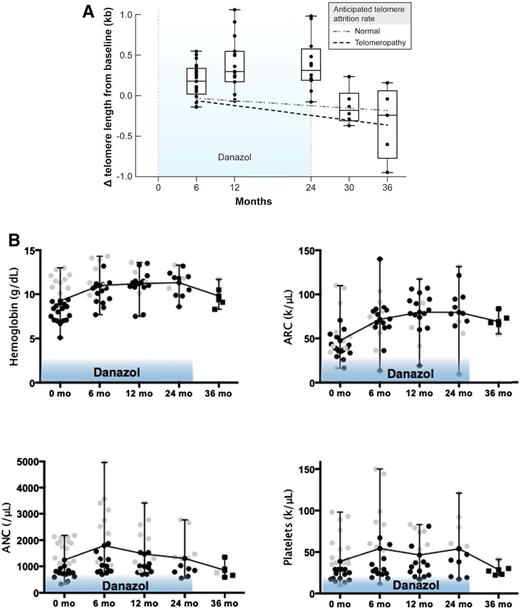
In the telomere diseases, germline mutations in genes responsible for telomere elongation and repair cause accelerated telomere attrition.65 Patients develop bone marrow failure, liver cirrhosis, pulmonary fibrosis, and increased risk for cancer. Telomere elongation during androgen therapy was reported to occur in a patient with a mutation in the TERT gene,66 and in a retrospective analysis of mostly pediatric telomere diseases, 11/16 (69%) patients had clinically significant hematologic responses when receiving androgen therapy.67 In a phase 1/2 study at the NIH, long-term danazol was prospectively administered to a cohort of adults with a telomeropathy. The study was designed to evaluate the effects of danazol on telomere length, hematopoiesis, and liver and lung dysfunction. The primary goal of treatment was to attenuate accelerated telomere attrition.62 Conducted over the course of 4 years, the study was halted early, after enrolling 27 patients, as telomere loss was prevented in all patients at 2 years, and unexpectedly, 11/12 (92%) had a gain in telomere length accompanied by hematologic improvement in all blood counts (Figure 2). Hematologic responses also occurred in the majority before 2 years: 19/24 (79%) at 3 months, and 10/12 (83%) at 24 months. The maximum dose of danazol was administered (800 mg/day) and likely contributed to a high frequency of known low-grade adverse effects to danazol: elevated liver enzymes and muscle cramps in 41% and 33%, respectively.
Danazol improves telomere length and peripheral blood cell counts. (A) Box plot shows changes in telomere length as measured from peripheral blood leukocytes by quantitative polymerase chain reaction at landmark visits as compared with baseline. The light dashed line represents the anticipated rate of telomere attrition with age in healthy persons (60 bp per year), and the bold dashed line represents the anticipated rate of attrition in patients with telomere diseases (120 bp per year). The line within each box indicates the median; the top and bottom edges the 75th and 25th percentiles, respectively; and the I bars the range. Patients were treated for 24 months (light blue shaded areas), but followed for at least 36 months. (B) In analogy, different blood cell lineages during (circles) and after (squares) danazol treatment. Black symbols indicate protocol qualifying low prestudy values. Gray symbols represent counts higher than the protocol required threshold. Patients with unilineage or multilineage cytopenias were enrolled. Reprinted from Townsley et al62 with permission.
Danazol improves telomere length and peripheral blood cell counts. (A) Box plot shows changes in telomere length as measured from peripheral blood leukocytes by quantitative polymerase chain reaction at landmark visits as compared with baseline. The light dashed line represents the anticipated rate of telomere attrition with age in healthy persons (60 bp per year), and the bold dashed line represents the anticipated rate of attrition in patients with telomere diseases (120 bp per year). The line within each box indicates the median; the top and bottom edges the 75th and 25th percentiles, respectively; and the I bars the range. Patients were treated for 24 months (light blue shaded areas), but followed for at least 36 months. (B) In analogy, different blood cell lineages during (circles) and after (squares) danazol treatment. Black symbols indicate protocol qualifying low prestudy values. Gray symbols represent counts higher than the protocol required threshold. Patients with unilineage or multilineage cytopenias were enrolled. Reprinted from Townsley et al62 with permission.
Multilineage hematological responses after androgen therapy have also been reported in retrospective analyses of patients with inherited bone marrow failure resulting from Fanconi anemia.68,69 Historically, the most commonly used androgen is oxymetholone, but synthetic androgenic hormones such as danazol also have also showed some activity.69,70 Improvement in blood counts typically occurs within 3 to 4 months of initiating treatment and are most pronounced for red blood cells and platelets; long-term stabilization of blood counts has been described in some patients. Androgens should be considered in patients with worsening cytopenias who lack matched donors for HSCT, but only HSCT is curative. Improvement in blood counts typically occurs within 3 to 4 months of initiating treatment. Adverse effects from androgens may limit adherence, and evolution of AA to MDS/AML occurs frequently in Fanconi anemia, demanding vigilant monitoring.
Conclusions
In the last 5 years, we have witnessed tremendous progress in nontransplant therapies for patients with AA. Horse ATG/CSA should be considered as the standard immunosuppressive regimen, whereas rabbit ATG and alemtuzumab are equipotent salvage agents in patients relapsing or not responding to initial IST. Eltrombopag is the first drug to be approved by the US Food and Drug Administration for the treatment of AA in nearly 3 decades, and it is now labeled for treatment of patients with SAA refractory to IST. The addition of eltrombopag to standard IST appears a promising regimen for treatment-naive SAA, and may eventually become the standard regimen, but further long-term follow-up is required to assess late events. A prototypical bench-to-bedside approach led to the discovery that patients with telomeropathies or very short telomeres may benefit from the treatment with androgens.
Correspondence
Danielle M. Townsley, Clinical Center, National Institutes of Health, Building 10-CRC, Room 3-5216, 10 Center Dr, Bethesda, MD 20892; e-mail: townsleydm@nhlbi.nih.gov.
References
Competing Interests
Conflict-of-interest disclosures: D.M.T. has received research funding from Novartis and GlaxoSmithKline. T.W. has received research funding from Novartis.
Author notes
Off-label drug use: Eltrombopag, Alemtuzumab, Danazol.