Abstract
The preferred treatment of idiopathic aplastic anemia (AA) is allogeneic hematopoietic stem cell transplantation (HSCT) from a human leukocyte antigen (HLA)–identical sibling donor. Transplantation from a well-matched unrelated donor (MUD) may be considered for patients without a sibling donor after failure of immunosuppressive therapy, as may alternative transplantation (mismatched, cord blood or haplo-identical HSCT) for patients without a MUD. HSCT may also be contemplated for congenital disorders in cases of pancytopenia or severe isolated cytopenia. Currently, HSCT aims are not only to cure patients but also to avoid long-term complications, notably chronic graft-versus-host disease (GVHD), essential for a good quality of life long term. This paper summarizes recent advances in HSCT for idiopathic and inherited AA disorders. The effect of age on current transplantation outcomes, the role of transplantation in paroxysmal nocturnal hemoglobinuria, and the prevention of GVHD are also discussed. Emerging strategies regarding the role of up-front unrelated donor and alternative donor HSCT in idiopathic AA, along with advances in the treatment of clonal evolution in Fanconi anemia, are also examined.
Learning Objectives
Understand the indications for HSCT and issues informing choice of donor and treatment regimen for inherited and acquired marrow failure
Recognize evolving strategies to minimize GVHD and long-term complications of HSCT for AA
Describe experimental approaches addressed in clinical trials for AA. Upfront matched unrelated HSCT and alternative HSCTs (mismatched unrelated, cord blood or haplo-identical) in AA are experimental procedures best tested within clinical trials
Introduction
Aplastic anemia (AA) is usually diagnosed in the setting of pancytopenia and hypocellular bone marrow (BM). An initial careful diagnostic assessment aims to distinguish between idiopathic (acquired) and inherited AA, the latter being mainly represented by Fanconi anemia (FA) and dyskeratosis congenita (DC).1
For idiopathic AA, allogeneic hematopoietic stem cell transplantation (HSCT) from an human leukocyte antigen (HLA)–matched related donor (MRD) is the preferred treatment of young patients.2 For patients without an HLA-identical sibling donor, immunosuppressive therapy (IST) is preferred.3 However, 30% to 40% of patients will eventually have relapse or disease will prove to be refractory to IST and those patients will therefore be considered for HSCT using a matched unrelated donor (MUD). Other alternative stem cell sources, including HLA-mismatched UD (MMUD), unrelated cord blood (CB), or haploidentical (haplo) familial donors are considered experimental (alternative HSCTs). HSCT may also be contemplated for congenital disorders to correct BM failure but not the underlying disease. Currently, HSCT aims not only to cure patients but also to restore a good quality of life long term, free from chronic graft-versus-host disease (GVHD) and its consequences.
This paper summarizes recent advances in HSCT for idiopathic and inherited AA disorders and discusses the effect of age on current transplantation outcomes, the role of transplantation in paroxysmal nocturnal hemoglobinuria (PNH), and the prevention of chronic GVHD. Emerging strategies regarding the role of up-front unrelated-donor and alternative-donor HSCT in idiopathic AA, along with advances in the treatment of clonal evolution in FA are also examined.
Current status of HSCT in aplastic anemia
Idiopathic aplastic anemia
HSCT as first-line treatment.
For young patients, the initial preferred treatment is HSCT.2 A recent major study by the European Group for Blood and Marrow Transplantation (EBMT) Severe Aplastic Anemia Working Party (SAAWP) examined outcomes for 1448 SAA patients receiving a MUD (n = 508) or a matched-related (n = 940) HSCT between 2005 and 2009. Best predictors for survival were age (<20 years), interval diagnosis to HSCT (<180 days), use of BM as a stem cell source, and use of antithymocyte globulin (ATG) in the conditioning regimen.4 Regarding post-transplantation GVHD prophylaxis, a prospective randomized trial showed that the combination of cyclosporine A (CsA) and short-course methotrexate (MTX) was superior to CsA alone and should be considered the standard post–HSCT IST.5 Early BM HSCT after cyclophosphamide (CY), with an ATG conditioning regimen, and a GVHD prophylaxis regimen combining CsA and MTX thus promotes excellent engraftment (95%) and overall survival (OS) (90% at 2 years) (Figure 1; Table 1).2,6-15 At the Hôpital Saint-Louis (Paris, France), we analyzed the long-term follow-up of all consecutive patients receiving this approach over 20 years.7 We reported low risk of both acute and chronic GVHD (only 1 patient death caused by GVHD). Avascular osteonecrosis was the most frequently encountered complication (cumulative incidence of 20% at 72 months). The other nonmalignant complication was metabolic and endocrine dysfunctions, which have been largely underestimated to date. The cumulative incidence of such complications reached 20% at 72 months, split among dyslipidemia, thyroid dysfunction (hypothyroidism), diabetes, and gonadal dysfunction. Another important observation was the extremely low rate of secondary malignancy (only 1 patient, with Hodgkin lymphoma) probably caused by the total body irradiation (TBI)-free regimen. Moreover, we and others showed that patients with AA conditioned with a non-TBI regimen were likely to preserve their ability to become pregnant or father normal children.7,16,17 These excellent results compare favorably with IST, where patients remain exposed to the risk of nonresponse, relapse, PNH expansion, and long-term clonal evolution. However, the age limit for this approach is still being debated (discussed later).
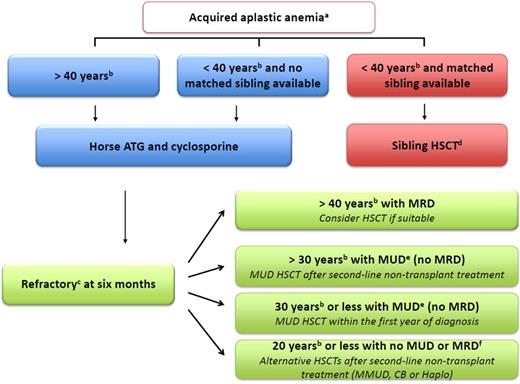
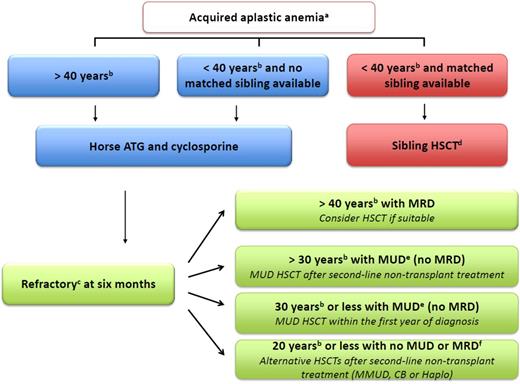
Algorithm to manage idiopathic aplastic anemia. (a) Indications for treatment are severe AA (2 in 3 blood counts, including absolute neutrophil count <500/μL, absolute reticulocyte count <60 000/μL, and platelet count <20 000/μL) or, in cases of moderate AA, where the patient needs transfusion support or has infectious complications as a result of low neutrophil count. (b) For age limit, stated cutoff ages are recommendations and are thus discussable according to institution and patient specificities. (c) For refractory patients, first carefully reassess the diagnosis to eliminate a clonal evolution such as myelodysplastic syndrome (MDS), as well as to exclude constitutional BM failure.1 (d) Early BM HSCT after cyclophosphamide (CY) with an ATG-conditioning regimen and a combination of cyclosporine (CsA) plus methotrexate (MTX) as GVHD prophylaxis is recommended. (e) Centers match either for A, B, C, and DRB1 at allelic level, looking for 8/8 matched donors or for DQ looking for a 10/10 match. BM HSCT after FLU, CY, low-dose TBI, and an ATG conditioning regimen is recommended ideally in the first year between diagnosis and HSCT. GVHD prophylaxis should combine CsA with MTX. The consensus is now that an additional low-dose TBI is beneficial for engraftment and long-term survival in adults, because many studies agree that 2 to 3 Gy TBI appears both safe and effective.8-11,13 However,the addition of low-dose TBI in young patients <14 years does not seem crucial.9 ATG has been part of the conditioning regimen since the early 1970s2 and also continues to be a positive predictor of survival in the UD setting.4 CY doses are higher (30 mg/kg for 4 days) than in the original protocol (300 mg/m2 for 4 days) to minimize rejection. Others recommend a dose of 50 mg/kg,12 and all agree that 150 mg/kg may be hazardous in these patients.14 Of note, the effect of CY dose is obviously greatly affected by other agents in the preparative regimen. EBMT, Blood and Marrow Transplant Clinical Network, as well as Japanese publications are thus not comparable regarding CY dosage because of other agents’ different dosage, brand, or schedule.9-14 For refractory patients aged ≥30 years, MUD HSCT is possible after only 1 course of IST but should be discussed on an individual patient basis, according to comorbidities at the respective transplant center. (f) Donor/recipient CMV seronegative status, absence of donor-specific antibodies, no comorbidities, >4 × 107 frozen nucleated cells/kg for CB HSCTs, and no kidney dysfunction are all favorable factors to help physicians make decisions. The recommended conditioning regimen is FCC for MMUD, a combination of FLU, CY, low-dose TBI, and ATG (5 mg/kg total dose maximum) for CB and the Baltimore protocol regarding haplo HSCT.15 ATG, anti-thymocyte globulin; CB, cord blood; Haplo, haplo-identical family donor; HSCT, hematopoietic stem cell transplantation; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Algorithm to manage idiopathic aplastic anemia. (a) Indications for treatment are severe AA (2 in 3 blood counts, including absolute neutrophil count <500/μL, absolute reticulocyte count <60 000/μL, and platelet count <20 000/μL) or, in cases of moderate AA, where the patient needs transfusion support or has infectious complications as a result of low neutrophil count. (b) For age limit, stated cutoff ages are recommendations and are thus discussable according to institution and patient specificities. (c) For refractory patients, first carefully reassess the diagnosis to eliminate a clonal evolution such as myelodysplastic syndrome (MDS), as well as to exclude constitutional BM failure.1 (d) Early BM HSCT after cyclophosphamide (CY) with an ATG-conditioning regimen and a combination of cyclosporine (CsA) plus methotrexate (MTX) as GVHD prophylaxis is recommended. (e) Centers match either for A, B, C, and DRB1 at allelic level, looking for 8/8 matched donors or for DQ looking for a 10/10 match. BM HSCT after FLU, CY, low-dose TBI, and an ATG conditioning regimen is recommended ideally in the first year between diagnosis and HSCT. GVHD prophylaxis should combine CsA with MTX. The consensus is now that an additional low-dose TBI is beneficial for engraftment and long-term survival in adults, because many studies agree that 2 to 3 Gy TBI appears both safe and effective.8-11,13 However,the addition of low-dose TBI in young patients <14 years does not seem crucial.9 ATG has been part of the conditioning regimen since the early 1970s2 and also continues to be a positive predictor of survival in the UD setting.4 CY doses are higher (30 mg/kg for 4 days) than in the original protocol (300 mg/m2 for 4 days) to minimize rejection. Others recommend a dose of 50 mg/kg,12 and all agree that 150 mg/kg may be hazardous in these patients.14 Of note, the effect of CY dose is obviously greatly affected by other agents in the preparative regimen. EBMT, Blood and Marrow Transplant Clinical Network, as well as Japanese publications are thus not comparable regarding CY dosage because of other agents’ different dosage, brand, or schedule.9-14 For refractory patients aged ≥30 years, MUD HSCT is possible after only 1 course of IST but should be discussed on an individual patient basis, according to comorbidities at the respective transplant center. (f) Donor/recipient CMV seronegative status, absence of donor-specific antibodies, no comorbidities, >4 × 107 frozen nucleated cells/kg for CB HSCTs, and no kidney dysfunction are all favorable factors to help physicians make decisions. The recommended conditioning regimen is FCC for MMUD, a combination of FLU, CY, low-dose TBI, and ATG (5 mg/kg total dose maximum) for CB and the Baltimore protocol regarding haplo HSCT.15 ATG, anti-thymocyte globulin; CB, cord blood; Haplo, haplo-identical family donor; HSCT, hematopoietic stem cell transplantation; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
HSCT as salvage treatment.
For older patients, or in the absence of an MRD, the preferred treatment is IST combining horse ATG and CsA.3,6 However, 30% to 40% of patients have disease that proves refractory to this treatment and one-third of responders will eventually have relapse.
In older patients with an MRD and confirmed AA refractory status, HSCT should be discussed in the absence of significant comorbidities (Table 1).8,15 In younger patients with a MUD and refractory or relapsed AA, HSCT is recommended: results of MUD HSCT have improved to such an extent that OS of MUD HSCT for idiopathic SAA is not statistically inferior to sibling transplants.4 This improvement has been largely attributed to better donor selection through allele matching, progress in supportive care and prophylaxis of GVHD, incorporation of fludarabine in conditioning regimens, and the addition of low-dose TBI (Table 1; Figure 1).2,4,8-15 Moreover, the risk of Epstein-Barre virus (EBV) lymphoproliferative disorder justifies the use of prophylactic rituximab9 and pre-emptive treatment in patients showing an increase in EBV-DNA, above a given cutoff. In the latter case, pre-emptive treatment is indicated in our institution >4 log or 100 000 EBV copies per mL of total blood and is successful in the majority of the patients.18 Patients undergoing MUD grafts, however, remain at greater risk of acute and chronic GVHD (twice that of MRD HSCT), which may significantly alter their quality of life and thus favor first-line IST. Transplantation from a MUD is now considered after failure to respond to 1 course of IST, better the first year between diagnosis and HSCT.19 The age effect and the role of up-front MUD and alternative HSCTs in idiopathic AA are discussed later.
Inherited aplastic anemia
Indication and assessment of patients for HSCT should be performed at specialized centers, making use of the latest molecular testing and clinical expertise.1 BM HSCTs are recommended to avoid secondary chronic GVHD, which is particularly toxic in patients with FA and DC.20-23 It is also essential that all potential MRDs are tested for the gene defect responsible for the disorder identified in the index patient to avoid using a silent carrier-donor in whom BM failure or leukemia will eventually develop. Long-term follow-up is the new challenge, because secondary cancer (mostly in FA) and organ toxicity (mostly in DC) are the leading long-term causes of death.20-24
Fanconi anemia.
When an MRD is available, the optimal timing for HSCT besides clonal evolution (see specific section) is when severe isolated cytopenia or severe bone marrow failure (BMF) occurs, ideally before the need for transfusion.25 A specific conditioning regimen with a low dose of CY should be used to avoid excessive regimen-related toxicity using the same conditioning regimen as other BMFs. In recipients of HLA-MRD HSCTs, excellent outcomes have been reported using a low-dose CY-based regimen alone26 or in combination with FLU.27 In cases involving MUD HSCTs, drastic improvements have been reported since 2000 because of better HLA typing and the use of FLU-based reduced-intensity conditioning (RIC) regimens (improving engraftment, decreasing acute GVHD, and eventually being associated with better OS), with or without T-cell depletion.20,28 BM from HLA-MUD is the preferred source of stem cells if no matched family donor exists. MacMillan et al recently reported 130 patients with FA who underwent MUD HSCTs between 1995 and 2012.28 Patients without a history of opportunistic infection or transfusions before HSCT, and who received conditioning with TBI 300 cGy, CY, FLU, and ATG, had a 5-year survival probability of 94% (Table 1).8,15
The long-term follow-up of patients with FA is mandatory because of the increased risk of malignancy post HSCT. There is a 4.4-fold higher rate of squamous cell carcinomas in patients with FA undergoing HSCT compared with those of the same age who have not received HSCT.29 Although patients with FA are inherently prone to develop tumors, chronic GVHD is also a key factor in their development post HSCT.20,30 Clonal evolution was also identified as an independent risk factor for secondary malignancies, with age >10 years at the time of HSCT, peripheral blood stem cells as the stem cell source, and previous chronic GVHD when considered as a time-dependent covariable. Thus, patients with FA at a particularly high risk of secondary cancers post HSCT that are highly associated with mortality and regular screenings for malignancies should therefore form part of long-term patient care. Such patients are systematically seen every 6 months at our clinic by gynecologists and stomatologists to enable early cancer detection and prompt surgery.31
Dyskeratosis congenita.
BM failure occurs in 80% of patients and is the main indication for HSCT. Until recently, HSCT results had been very disappointing, mainly because of severe late effects, including graft failure, GVHD, sepsis and, more importantly, the propensity to develop organ toxicity, including pulmonary fibrosis, hepatic cirrhosis, and veno-occlusive disease, among others.22,23,32 These patients are now being considered again for HSCT because factors associated with improved survival have been identified. First, MRDs remain the preferred donors, because mismatched related or MUDs are associated with poorer outcomes.22,23 Second, hypersensitivity to irradiation and chemotherapy, and possible preexisting organ dysfunction, result in poor survival rates after conventional myeloablative conditioning.22,23 It is hoped that reduced-intensity conditioning bone marrow–matched related donor HSCTs will lead to better outcomes (Table 1).8,15
Current issues
Effect of age in patients with idiopathic AA
The risks of morbidity and mortality arising from HSCT increase with age, hence the desire to avoid this procedure in older people. However, as with transplantation, post-IST survival is associated with age, with lower OS for older patients.6,33
Regarding MRD HSCT, a major Center for International Blood and Marrow Transplant Research (CIBMTR) study of 1307 patients identified 3 age cutoffs with survival differences: <20 years, 20 to 40 years, and >40 years, with a 10% to 20% absolute difference in the adjusted 5-year OS among these age groups (82%, 72%, and 53%, respectively).34 Thus, 40 years of age appears as the age cutoff, not only because of OS concerns but also a higher reported risk of GVHD in older patients.34 The decision to offer an MRD HSCT to patients >40 years who are at higher risk must be weighed carefully against the benefits of IST therapy (sustained remission but associated with risk of relapse and late clonal abnormalities). One study suggested that a FLU-based conditioning regimen may reduce the negative impact of age in older patients (>30) receiving sibling HSCT,35 but this was not confirmed elsewhere34 (Table 1).8,15
Most MUD transplants are offered to children and young adults in clinical practice. The median age of patients was ∼21 years in the latest CIBMTR study, and 17 and 14 years in the Japanese and European reports, respectively.9,10 Age >20 and >16 were significantly associated with worst OS in the Japanese10 and European9 studies, respectively. The French Society for Stem Cell Transplantation reported 139 consecutive patients with AA (median age, 23 years) who received a MUD HSCT in France since 2000. In an adjusted multivariate model, age ≤30 years, time from diagnosis to transplantation >12 months, and 9 of 10 mismatched unrelated donors were risk factors significantly worsening OS, confirmed by an independent cohort from the EBMT database of 296 patients.19 The current age limit for MUD HSCT in patients refractory to 1 course of IST thus appears to be 30 years. After this age, HSCT should be discussed on an individual patient basis, according to comorbidities at the respective transplant center.
PNH and HSCT
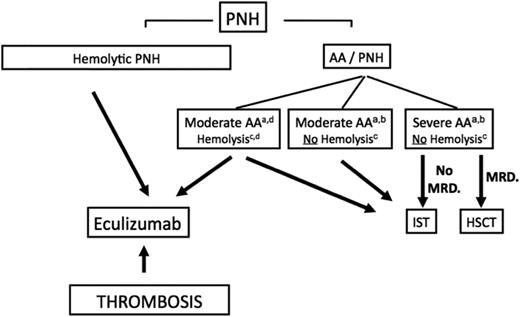
The most important factor for decision making involves the patient’s PNH clinical symptoms (hemolytic PNH, thrombosis, AA PNH syndrome) (Figure 2). The introduction of eculizumab (Ec), a humanized monoclonal antibody directed against the terminal complement protein C5, has had a major impact on the management of PNH (hemolytic form and thrombosis), preventing the occurrence of disease complications, including thrombosis, which favor the use of Ec over HSCT,36,37 and improving long-term OS.36,37 However, in the major EBMT retrospective study, 64 patients transplanted because of recurrent hemolytic crisis had an excellent 5-year OS of 86% post HSCT (30% risk of chronic GVHD). Improved OS regarding the PNH natural evolution (median OS of 22 years before the Ec era)38 thus outweighs the risk of chronic GVHD in this situation, with HSCT remaining a valuable option for patients in countries that cannot afford Ec. No consensus has yet emerged about whether we should give Ec to patients <18 years of age without thrombosis with recurrent hemolytic crisis and an available MRD. Ec does not eradicate the PNH clone, and must be given for life. HSCT offers a chance for cure but, although mortality and morbidity rates may be low for young patients transplanted from MRD, they are not fully predictable.
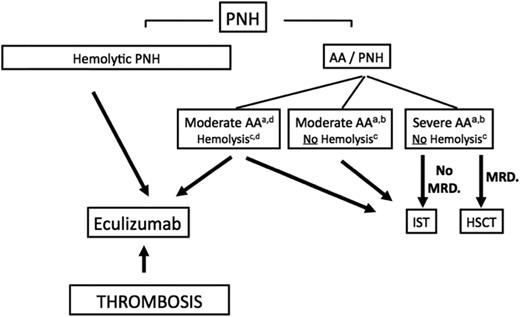
Algorithm to manage patients with PNH. (a) Indications for treatment are severe AA (2 in 3 blood counts, including absolute neutrophil count <500/μL, absolute reticulocyte count <60 000/μL, and platelet count <20 000/μL) or in cases of moderate AA, where the patient needs transfusion support or has infectious complications because of a low neutrophil count. (b) The presence of a PNH clone in this setting highlights the underlying autoimmune-mediated process in favor of an idiopathic AA and not an inherited disorder, and should also make physicians think about thrombosis upon suggestive clinical signs, because PNH is a known predisposition to thrombosis complications. Clearly, complement inhibitory therapy has no effect on the BM-failure component of the disease and should not be used in this situation. (c) The level of hemolysis is indicated by lactate dehydrogenase (LDH). Significant hemolysis is considered >2 times LDH. (d) Exceptional cases of AA-PNH syndrome with significant intravascular hemolysis may require HSCT or IST treatment of BM failure and eculizumab for the hemolysis, and should be discussed on a case-by-case basis. AA, aplastic anemia; ATG, antithymocyte globulin; HSCT, hematopoietic stem cell transplantation; IST, immunosuppressive therapy; MRD, matched related donor; PNH, paroxysmal nocturnal hemoglobinuria.
Algorithm to manage patients with PNH. (a) Indications for treatment are severe AA (2 in 3 blood counts, including absolute neutrophil count <500/μL, absolute reticulocyte count <60 000/μL, and platelet count <20 000/μL) or in cases of moderate AA, where the patient needs transfusion support or has infectious complications because of a low neutrophil count. (b) The presence of a PNH clone in this setting highlights the underlying autoimmune-mediated process in favor of an idiopathic AA and not an inherited disorder, and should also make physicians think about thrombosis upon suggestive clinical signs, because PNH is a known predisposition to thrombosis complications. Clearly, complement inhibitory therapy has no effect on the BM-failure component of the disease and should not be used in this situation. (c) The level of hemolysis is indicated by lactate dehydrogenase (LDH). Significant hemolysis is considered >2 times LDH. (d) Exceptional cases of AA-PNH syndrome with significant intravascular hemolysis may require HSCT or IST treatment of BM failure and eculizumab for the hemolysis, and should be discussed on a case-by-case basis. AA, aplastic anemia; ATG, antithymocyte globulin; HSCT, hematopoietic stem cell transplantation; IST, immunosuppressive therapy; MRD, matched related donor; PNH, paroxysmal nocturnal hemoglobinuria.
The results of a matched-comparison EBMT SAAWP study with patients not transplanted and treated before Ec era suggest that HSCT can no longer be considered as a standard of care for thrombotic complications as an indication for HSCT caused by an unacceptable toxicity.38
In patients with overt AA-PNH syndrome (typically AA with a “small” PNH clone), besides thrombosis and without hemolysis, the standard treatment should be similar to that of patients with AA and no PNH (discussed before).
GVHD prophylaxis
Survival after matched-sibling or MUD HSCT for AA is currently ∼80% or more in the latest studies.4,7,9,12 Because AA is a nonmalignant hematologic disorder, the risk of relapse is low post HSCT. GVHD thus remains a major cause of morbidity and mortality, emerging as a risk factor for nearly all long-term complications. Aside from their direct involvement on target organs (eg, skin, lungs), treatments of GVHD with steroids enhance the likelihood of delayed complications, such as cataracts or musculoskeletal disease.7,30 Moreover, GVHD is the main driver for secondary cancer, especially in patients with FA who have a poorer prognosis. Pretransplant factors known to be associated with GVHD should thus be avoided. The recommended stem cell source is BM for all AA subtypes (idiopathic and inherited)4,20 to decrease this risk. The use of ATG is associated with a reduced risk of both acute and chronic GVHD in AA.4 An alternative to ATG is alemtuzumab (anti-CD52 monoclonal antibody, Campath). A high incidence of graft failure was first associated with the use of alemtuzumab given both pre- and peritransplantation, and reduced when given only pretransplantation.39 When patients receive alemtuzumab pretransplantation only, rejection rates are similar to ATG as shown in a retrospective multicenter study in the UK.40 Alemtuzumab, combined with FLU and low-dose cyclophosphamide (FCC regimen) thus produced a low incidence of both acute (grades II-IV, 14%) and chronic GVHD (4% with only limited presentation) as well as very encouraging OS rates (81% at 5 years with related but also MUDs) without exposing patients to a higher risk of rejection than an ATG-based conditioning regimen.40 A recent retrospective study (EBMT SAAWP) also showed significant benefits of using alemtuzumab over ATG regarding GVHD.41 Low toxicity and low risk of GVHD and infections make FCC a particularly attractive regimen for older patients (Table 1).8,15 The low incidence of GVHD and no need for TBI are also major advantages in specific situations including inherited AA. Concerns have been raised that the use of alemtuzumab may increase the risk of viral infections post HSCT. However, the UK group reported low incidence of viral infections and observed only 2 cases of EBV PTLD.40 As such, FCC is a homogeneous conditioning regimen suitable for use in either idiopathic or inherited AA, from related and unrelated donors, that calls for urgent evaluation through clinical trials (Table 1).8,15
Emerging strategies
Up-front matched unrelated transplantation
Although pediatric patients respond better to IST, the long-term risks of relapse, CsA dependence, and clonal evolution are high.6 UK investigators reported the outcome of consecutive children with idiopathic SAA who received IST or MUD HSCT. The 6-month cumulative response rate after rabbit ATG/CsA (IST) was 32.5% (n = 43). The 5-year estimated failure-free survival (FFS) after IST was 13.3%. In contrast, in 44 successive children who received a 10-antigen (HLA-A, -B, -C, -DRB1, -DQB1) MUD HSCT, there was an excellent estimated 5-year FFS of 95%. Forty of these children had failed IST previously. HSCT conditioning was a FCC regimen.42 Because of those excellent results of FCC regimens in UDs, up-front UD HSCT became an attractive first-line option after the temporary withdrawal of horse ATG (lymphoglobulin) in Europe in the last decade in children. In 2005 to 2014, a UK cohort of 29 consecutive children with idiopathic SAA thus received UD HSCTs (5 mismatched transplants) as first-line therapy (none had an MRD but they did not receive IST). Results were excellent, with low GVHD rates and only 1 death (from idiopathic pneumonia).43 This cohort was then compared with historical matched controls who had received: (1) first-line MRD HSCT, (2) first-line IST with horse ATG and CsA, and (3) MUD HSCT post-IST failure as second-line therapy. Outcomes for the up-front unrelated cohort were similar to MRD HSCT and superior to IST and UD HSCT post IST failure.43 Clearly, these results should be treated with caution. The design is retrospective, the excellent up-front UD HSCT may arise from the use of the FCC regimen (alemtuzumab is not easily available worldwide), and there was no formal quality-of-life assessment. Moreover, this strategy is highly dependent on donor identification (Caucasian patients have the highest likelihood of having a MUD) and donor availability, with the risk of infections/complications caused by unexpected donor delays. In the absence of a prospective, randomized controlled trial comparing children undergoing UD HSCT with those receiving MRD HSCT or IST as first-line therapy, this strategy should only be used for highly selected patients, using the FCC regimen, because nothing is known of the use of other regimens in this setting (Table 1).8,15 The standard approach is to initiate a donor search in all younger patients and to pursue MUD HSCT upon donor availability should IST be ineffective, usually at 3 to 6 months post IST.
Alternative donor transplantation in idiopathic AA
Alternative HSCTs—using alternative donor sources (ie, mismatched MMUD, CB, and haplo family donors)—are possible for individuals with no suitable MUD, along with a second course of IST, an alternative immunosuppressive drug, or novel agent.3 Alternative HSCTs may be curative, but the risks of graft rejection, infectious complications, and GVHD are higher than those for MRD or MUD HSCT. Patient age, comorbidities, and alternative HSCT specificities are thus important issues in transplantation decision.
Age and comorbidities are the first barriers to this type of procedure. Most published series with a meaningful number of patients (reporting >50 alternative HSCTs) only involve pediatric cohorts. The Japanese Marrow Donor Program reported on the role of HLA matching using BM/peripheral blood stem cells in nonmalignant disorders in a cohort of 301 patients (median age, 17 years),44 whereas a similar NMDP/CIBMTR study included 663 patients (median age, 9 years).45 The EBMT SAAWP unrelated cord blood transplantation study comprised 71 patients (median age, 13 years).46 Estimated survival in the NMDP/CIBMTR study was 57% at 5 years in 7 of 8 matched HSCT.45 The Japanese study reported better rates of OS; however, possible race-related differences in HLA haplotypes and matching could be important. Three-year OS in a CB study was 38%.46 We must also not forget that 5-year OS in refractory patients only receiving supportive care has improved significantly over time (23% in 1989-1996 vs 35% in 1996-2002 and 57% in 2002-2008).47 Consequently, today’s best supportive care gives similar results to published alternative HSCTs that, outside scientific evidence, make patients over 20 years of age or with comorbidities less eligible to this risky procedure (assuming that toxicity-related HSCT is lower in younger patients) (Figure 1).4,9-14
Alternative HSCTs expose patients to a higher risk of graft failure, GVHD, and infectious complications that lead to morbidity and mortality:
The risk of rejection justifies adding a small dose of TBI for all alternative HSCTs.13 Donor-specific antibodies should be screened before HSCT.13 For CB, 1 or 2 CB units may be used in SAA to reach at least 4 × 107 frozen nucleated cells/kg with no more than 2 of 6 HLA mismatches between the unit and the patient.46 For patients with FA, only 1 CB is recommended with no more than 1 mismatch, because the use of 2 CB units in this situation was associated in our center with high rates of GVHD and consequently unacceptable toxicity).48,49 Chimerism must also be followed up carefully post HSCT (Table 1).8,15
To reduce the risk of GVHD, BM is recommended for MMUDs for whom a FLU, CY, low-dose TBI + ATG or FCC regimen + low-dose TBI seems appropriate outside prospective clinical trials (Table 1).8,15 For haplo, the favored method is unmanipulated BM with high-dose posttransplant CY, introduced by the Baltimore group,15 but very few reports have been published on this latter procedure13 (Table 1).8,15
Regarding infections, particular attention should be paid to the cytomegalovirus (CMV) donor/recipient (D/R) status. CMV-seronegative D/R pairs are far easier to manage in alternative HSCTs. The risk of infections and quality of supportive care are extremely important in this type of graft, which should only be carried out in experienced centers.
Clonal evolution in FA
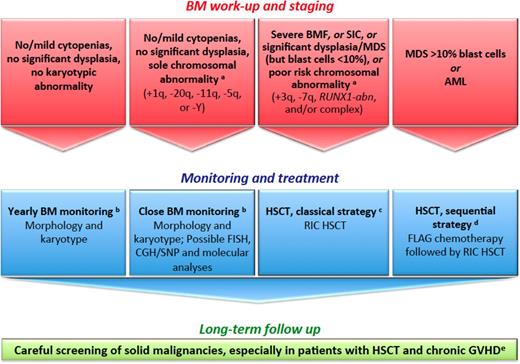
We recently reviewed this issue.24 When clonal evolution is apparent in the BM, patients with FA deserve a personalized therapeutic plan encompassing personal and disease-specific criteria. Although certain situations are indications for prompt transplantation (overt acute myeloid leukemia [AML], significant myelodysplastic syndrome [MDS], cytogenetic/molecular abnormality of chromosome 3q, 7q, RUNX1, and/or complex), it remains unclear whether other patients with a level of isolated clonal evolution, such as a sole +1q clone, should receive immediate treatment (Figure 3).50-53 This situation, along with FA genetic reversion (somatic mosaicism), raises somewhat similar questions to the recently recognized clonal hematopoiesis of indeterminate potential in aging individuals, idiopathic cytopenia of unknown significance, and clonal cytopenia of unknown significance of the general (non-FA) population.52,53 Regarding pre-HSCT cytoreduction, we all agree that toxicity is high in patients with FA. However, specific subsets may benefit from pre-HSCT cytoreduction (MDS with excess blasts, overt AML, or BRCA2/FANCD1 patients).24,54 The risk of prolonged chemotherapy-associated aplasia remains clear, highlighting the importance of securing a donor before offering chemotherapy to these patients. HSCT per se has been addressed before (see Current status of HSCT in aplastic anemia). Alternative donors (MMUD, CB, or haplo-HSCT) may be considered, depending on the experience of each center. Of note, a single-center experience reported on 12 consecutive patients with FA who were given T cell–depleted, CD34+ positively selected cells from a haploidentical related donor after a reduced intensity, fludarabine-based conditioning regimen. Results were good with a 5-year OS of 83% and disease-free survival of 67%.55 In any case, patients with FA should be followed on a very-long-term basis to enable early detection and prompt treatment of secondary cancers (Table 1).8,15
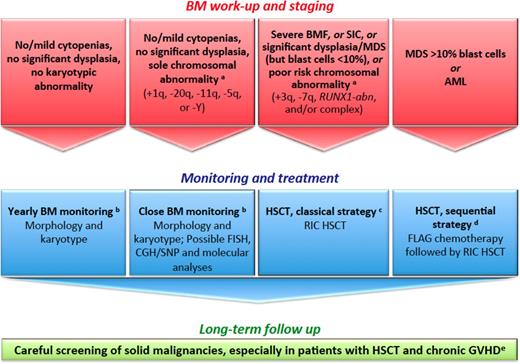
How we diagnose and manage FA patients with MDS and AML. (a) We define provisional cytogenetic/molecular categories by reference to MDS/AML literature in patients with FA50,51 and without FA.52,53 (b) The timing of BM monitoring is discussed, especially because repeated aspiration is poorly tolerated in children, adolescents, and young adults. The overall consensus is that 1 year of BM aspirate is reasonable and should be adapted in response to blood cell count changes, myelodysplastic signs, increased blast proportion, and/or cytogenetic evidence of clonal evolution. (c) Standard RIC regimen combines fludarabine 90 mg/m2 (30 mg/m2 on days −4, −3, and −2) and cyclophosphamide 40 mg/kg (10 mg/kg on days −5, −4, −3, and −2) for MRDs. For MDS/AML, low-dose CY/FLU alone may not suffice as conditioning therapy because of the substantial number of residual host cells early post transplantation with this approach and the risk of relapse; an alternative is to use TBI 2-3 Gy in patients with MDS/AML and an HLA-matched sibling donor.28,54 For MRDs, the conditioning regimen combines fludarabine 120 mg/m2, cyclophosphamide 40 mg/kg, and TBI 2 Gy. GVHD prophylaxis combines mycophenolate acid and cyclosporine. ATG is used in total doses of 5 mg/kg for MUDs only. In CB HSCTs, we do not use ATG in the conditioning regimen. (d) Others do not recommend cytoreduction, except in patients with BRCA2 mutations54 ; the sequential strategy comprises pretransplant chemotherapy with fludarabine 30 mg/m2 per day for 5 days and cytarabine 1 g/m2 × 2 per day for 5 days with granulocyte colony-stimulating factor injections (FLAG), followed 3 weeks later by a RIC conditioning regimen (4 days of cyclophosphamide 10 mg/kg, 4 days of fludarabine 30 mg/m2, and TBI 2 Gy) delivered during chemotherapy-induced aplasia. ATG is used in total doses of 5 mg/kg for MUDs only. In CB HSCTs, we do not use ATG in the conditioning regimen. (e) Screening for malignancies, including oropharyngeal, dental, and gynecological examinations, forms part of long-term patient care. Naturally, long-term multidisciplinary surveillance is also mandatory for all patients post HSCT. Multiple problems in early age, subsequent HSCT requirements, and continuing poor prognosis in survivors as a result of cancer susceptibility is a source of stress for patients with FA and their families. Adequate psychological support and a coordinated multidisciplinary team with dedicated physicians are the cornerstones to successful management. CGH, comparative genomic hybridization; FLAG, fludarabine/cytarabine/granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; RIC, reduced-intensity conditioning regimen; SIC, severe isolated cytopenia; SNP, single nucleotide polymorphism.
How we diagnose and manage FA patients with MDS and AML. (a) We define provisional cytogenetic/molecular categories by reference to MDS/AML literature in patients with FA50,51 and without FA.52,53 (b) The timing of BM monitoring is discussed, especially because repeated aspiration is poorly tolerated in children, adolescents, and young adults. The overall consensus is that 1 year of BM aspirate is reasonable and should be adapted in response to blood cell count changes, myelodysplastic signs, increased blast proportion, and/or cytogenetic evidence of clonal evolution. (c) Standard RIC regimen combines fludarabine 90 mg/m2 (30 mg/m2 on days −4, −3, and −2) and cyclophosphamide 40 mg/kg (10 mg/kg on days −5, −4, −3, and −2) for MRDs. For MDS/AML, low-dose CY/FLU alone may not suffice as conditioning therapy because of the substantial number of residual host cells early post transplantation with this approach and the risk of relapse; an alternative is to use TBI 2-3 Gy in patients with MDS/AML and an HLA-matched sibling donor.28,54 For MRDs, the conditioning regimen combines fludarabine 120 mg/m2, cyclophosphamide 40 mg/kg, and TBI 2 Gy. GVHD prophylaxis combines mycophenolate acid and cyclosporine. ATG is used in total doses of 5 mg/kg for MUDs only. In CB HSCTs, we do not use ATG in the conditioning regimen. (d) Others do not recommend cytoreduction, except in patients with BRCA2 mutations54 ; the sequential strategy comprises pretransplant chemotherapy with fludarabine 30 mg/m2 per day for 5 days and cytarabine 1 g/m2 × 2 per day for 5 days with granulocyte colony-stimulating factor injections (FLAG), followed 3 weeks later by a RIC conditioning regimen (4 days of cyclophosphamide 10 mg/kg, 4 days of fludarabine 30 mg/m2, and TBI 2 Gy) delivered during chemotherapy-induced aplasia. ATG is used in total doses of 5 mg/kg for MUDs only. In CB HSCTs, we do not use ATG in the conditioning regimen. (e) Screening for malignancies, including oropharyngeal, dental, and gynecological examinations, forms part of long-term patient care. Naturally, long-term multidisciplinary surveillance is also mandatory for all patients post HSCT. Multiple problems in early age, subsequent HSCT requirements, and continuing poor prognosis in survivors as a result of cancer susceptibility is a source of stress for patients with FA and their families. Adequate psychological support and a coordinated multidisciplinary team with dedicated physicians are the cornerstones to successful management. CGH, comparative genomic hybridization; FLAG, fludarabine/cytarabine/granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; RIC, reduced-intensity conditioning regimen; SIC, severe isolated cytopenia; SNP, single nucleotide polymorphism.
Conclusions
Very significant progress has been made with HSCT in AA, such that survival is now almost comparable between MRD and MUD HSCT. One concern is recipient age limit. After 40 years for MRD and 30 years for MUD, HSCT should be discussed on an individual patient basis at the respective transplant center. The conditioning regimen is also essential. Using an FCC regimen, up-front MUD HSCT is giving excellent results. This regimen should also be of interest to older patients because of the low rate of chronic GVHD using alemtuzumab. BM is recommended to avoid chronic GVHD. For patients without a MUD, the question of alternative transplantation (MMUD, CB, or haplo) may arise, but should still be considered experimental. In our institution, we do think that candidates for experimental transplantation (ie, mismatched MMUD, CB, and haplo family donors) should be proposed to patients younger than 20 years. However, all stated cutoff ages in this review are recommendations and are thus discussable according to institution and patient specificities. In the coming years, biomarkers (telomere length, mutational status, blood counts) or other pathophysiologic indicators will likely emerge from global assessments and should help in SAA treatment decisions, especially when data on the use of an oral thrombomimetic, eltrombopag, will be available in naïve patients. Regarding inherited AA, matched-donor HSCTs are now routinely accomplished in patients with FA. MRD HSCT is an option in DC, though organ toxicity remains problematic, whereas MUD indication is still being discussed. Prospective international clinical trials are urgently needed to improve GVHD-free survival, the main end point for patients with AA after HSCT.
Acknowledgments
The author thanks Gérard Socié and Jean Hugue Dalle for helpful discussion and critical reading of the manuscript, as well as colleagues from the European Group for Blood and Marrow Transplantation Severe Aplastic Anemia Working Party for helpful discussion.
Correspondence
Régis Peffault de Latour, Service d’Hématologie-Greffe, Centre de Référence Aplasie Médullaire, Assistance Publique Hôpitaux de Paris, Hôpital Saint-Louis, 1 Avenue Claude-Vellefaux, 75010 Paris, France; e-mail: regis.peffaultdelatour@aphp.fr.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from, consulted for, and received honoraria from Alexion, Pfizer, and Novartis, and received research funding from Amgen.
Author notes
Off-label drug use: None disclosed.