Abstract
The clinical manifestations of inherited susceptibility to leukemia encompass a wide phenotypic range, including patients with certain congenital anomalies or early-onset myelodysplastic syndrome (MDS) and some with no obvious medical problems until they develop leukemia. Leukemia susceptibility syndromes occur as a result of autosomal dominant, autosomal recessive, or X-linked recessive inheritance, or de novo occurrence, of germline pathogenic variants in DNA repair, ribosome biogenesis, telomere biology, hematopoietic transcription factors, tumor suppressors, and other critical cellular processes. Children and adults with cytopenias, MDS, dysmorphic features, notable infectious histories, immunodeficiency, certain dermatologic findings, lymphedema, unusual sensitivity to radiation or chemotherapy, or acute leukemia with a family history of early-onset cancer, pulmonary fibrosis, or alveolar proteinosis should be thoroughly evaluated for a leukemia susceptibility syndrome. Genetic testing and other diagnostic modalities have improved our ability to identify these patients and to counsel them and their family members for subsequent disease risk, cancer surveillance, and therapeutic interventions. Herein, the leukemia susceptibility syndromes are divided into 3 groups: (1) those associated with an underlying inherited bone marrow failure syndrome, (2) disorders in which MDS precedes leukemia development, and (3) those with a risk primarily of leukemia. Although children are the focus of this review, it is important for clinicians to recognize that inherited susceptibility to cancer can present at any age, even in older adults; genetic counseling is essential and prompt referral to experts in each syndrome is strongly recommended.
Learning Objectives
Recognize and diagnose the broad spectrum of clinical manifestations associated with inherited susceptibility to leukemia
Understand the importance of early diagnosis and genetic counseling for leukemia susceptibility syndromes
Identify the biology underlying leukemia susceptibility syndromes
Introduction
Although the connection between rare inherited disorders and childhood leukemia has been recognized for decades, the advent of genomics has greatly expanded the number of recognized disorders as well as our understanding of the germline genetic contribution to leukemia etiology. Historically, many childhood disorders associated with leukemia were recognized because of associated dysmorphology, such as in Fanconi anemia (FA). The growing use of multigene panel germline genetic testing and of somatic sequencing to identify potential therapeutic targets has uncovered pathogenic variants (ie, mutations) in patients without classic clinical features of inherited syndromes. Genomics has also greatly facilitated our ability to diagnose patients and at-risk family members and has led to important insights into the underlying biology of leukemia susceptibility.
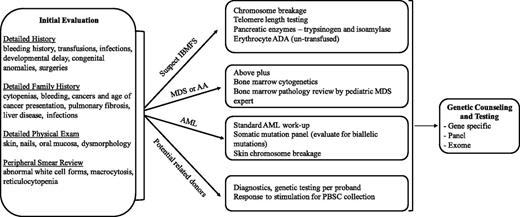
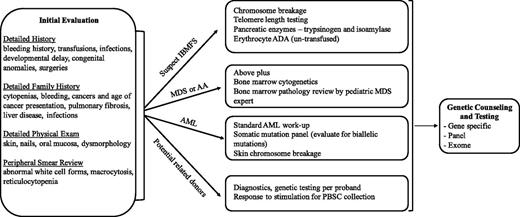
Because a comprehensive review of all leukemia-prone disorders is beyond the scope of this article, we highlight the clinical manifestations and management of some of the more common rare syndromes. A longer list of associated disorders is shown in Table 1. Box 1 and Figure 1 provide guidance on when to suspect and how to diagnose these disorders. We have divided the disorders into those associated with an underlying inherited bone marrow failure syndrome, those typically preceded by myelodysplastic syndrome (MDS), and those disorders with only an increased risk of leukemia.
Box 1. Who do I evaluate for a leukemia susceptibility disorder?
Any pediatric patient with MDS
Any patient aged <40 years with aplastic anemia
Patients aged <40 years with any cytopenia and family member(s) with AML or other cancer(s)
Patients with any cytopenia and family member(s) with pulmonary fibrosis or alveolar proteinosis
Patients with notable infectious history, combined variable immunodeficiency, or persistent warts
Patients with dysmorphology, early gray hair, abnormal nails, deafness, limb anomalies, skin pigmentation abnormalities, or lymphedema
Patients with a prior history of immune thrombocytopenia purpura and new cytopenia or leukemia
Patients with newly diagnosed AML with a CEBPA, WT1, or RUNX1 biallelic mutation reported in leukemia cells or with monosomy 7
Patients with unusual radiation or chemotherapy sensitivity
Patients with hypodiploid ALL
Suggested diagnostic evaluation for leukemia susceptibility syndromes. The order of the evaluation for a suspected leukemia susceptibility syndrome depends, in part, on the way in which the patient first presents. For example, the evaluation of a patient with isolated cytopenias should thoroughly investigate causes of an inherited bone marrow syndrome. Individuals presenting with AML should undergo prompt evaluation and therapeutic intervention for the AML with consideration of familial syndromes. AA, aplastic anemia; ADA, adenosine deaminase; AML, acute myeloid leukemia; IBMFS, inherited bone marrow failure syndromes; MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cell.
Suggested diagnostic evaluation for leukemia susceptibility syndromes. The order of the evaluation for a suspected leukemia susceptibility syndrome depends, in part, on the way in which the patient first presents. For example, the evaluation of a patient with isolated cytopenias should thoroughly investigate causes of an inherited bone marrow syndrome. Individuals presenting with AML should undergo prompt evaluation and therapeutic intervention for the AML with consideration of familial syndromes. AA, aplastic anemia; ADA, adenosine deaminase; AML, acute myeloid leukemia; IBMFS, inherited bone marrow failure syndromes; MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cell.
There are several very important things to remember when considering inherited susceptibility to leukemia. First, the clinical manifestations of these genetic syndromes are myriad, often overlapping, and appropriate diagnosis is essential for management. Second, although children are often the focus of inherited susceptibility to disease, these disorders can present at any age, even in older adults, and proper genetic counseling for patients and family members is essential. Finally, limited evidence-based management guidelines exist; thus, prompt referral to experts in each syndrome is strongly recommended.
Inherited bone marrow failure syndromes associated with leukemia
The inherited bone marrow failure syndromes (IBMFSs) are a set of heterogeneous disorders characterized by the development of ≥1 cytopenias that may progress to pancytopenia associated with an increased risk of MDS and/or acute myeloid leukemia (AML). The specific clinical manifestations vary by syndrome and its underlying biology but may include congenital malformations, mucocutaneous abnormalities, developmental delay, and elevated risk of solid malignancies (Table 1).
Fanconi anemia
FA is caused by pathogenic germline variants in at least 22 different genes of the FA/BRCA DNA repair pathway (FANCA, B, C, D1, D2, E, F, G, I, J, L, N, P, Q, T, U, and V).1 Most FA occurs with autosomal recessive inheritance with 1 X-linked gene (FANCB) and a possible autosomal dominant gene (RAD51) reported. Pathogenic variants in other genes (FANCM, FANCO, FANCR, and FANCS) have been reported in FA but experts disagree as to whether they are true FA genes based on the clinical manifestations of those patients.
Patients with FA are often diagnosed in infancy because of associated congenital anomalies, including aplasia of the thumb, hand, and forearm, kidney and urinary tract malformations, small eyes and characteristic facies, short stature, and numerous café au lait spots.1 A subset of patients, often owing to pathogenic variants in FANCD2/BRCA2 or FANCI, have the VACTERL (vertebral anomalies, anal atresia, cardiac malformations, tracheoesophageal fistula with esophageal atresia, renal and limb abnormalities) or VACTERL-H (with hydrocephalus) constellation. A more specific acronym, PHENOS (pigmentation, small head, small eyes, central nervous system, otology, and short stature), has been proposed to further specify the differences in VACTERL-H and FA-associated anomalies.2 It is important to note that 25% to 30% of patients with FA show no or only subtle physical abnormalities.3 The diagnosis of FA is confirmed by chromosomal fragility testing after exposure of peripheral blood lymphocytes to DNA-crosslinking agents.1
Patients with FA have significantly elevated risks of MDS, AML, head and neck squamous cell carcinomas (HNSCCs), gynecologic cancers, and other solid tumors.4-7 Compared with the general population, the observed/expected (O/E) ratio of any cancer in FA is 50-fold greater than in the general population.4 In addition, the O/E ratios are ∼550-fold for HNSCCs and 3000-fold for vulvar carcinoma.4 Hematopoietic stem/progenitor cell transplantation (HCT) may further increase the risk of solid malignancies in FA, with a reported 4.4-fold increased risk of HNSCCs and occurrence 16 years earlier than in nontransplanted subjects.5
The relative risk of MDS in FA is ∼6600-fold higher than in the general population and has a cumulative incidence of 40% at age 50 years.4 AML risk is ∼700-fold higher in FA than in the general population, with a cumulative incidence of 15% to 20% by age 40 years.4 Notably, AML is even more frequent in the FANCD1/BRCA2 group (cumulative incidence of 80% at age 10 years).4 Most patients with FA develop clinically significant cytopenias before the development of MDS or AML. However, it should also be noted that cancer could be the presenting sign of FA in individuals without congenital anomalies, particularly in those who have excessive toxicity after chemotherapy or radiation therapy for MDS/AML or solid tumors.
Dyskeratosis congenita
Dyskeratosis congenita (DC) became the first known telomere biology disorder (TBD) when pathogenic variants in dyskerin (DKC1) were identified as causative of the X-linked form of DC and is associated with very short telomeres and aberrant telomere biology.8,9 Subsequently, autosomal dominant (ACD, RTEL1, TERT, TERC, TINF2) and autosomal recessive (RTEL1, TERT, ACD, POT1, NHP2, NOP10, CTC1, PARN) forms of DC have been described and are attributable to pathogenic variants in key components of telomere biology, including the telomerase holoenzyme complex (TERT, TERC, DKC1, NOP10, NHP2, NAF1), the shelterin telomere protection complex (TINF2, ACD, POT1), telomere capping proteins (CTC1, STN1), and other components of telomere and DNA replication pathways (RTEL1, WRAP53, PARN).10 The advent of flow cytometry with in situ hybridization as the diagnostic test for DC led to gene discovery and a greater appreciation of the role of telomere biology in DC and in related disorders.11 Hoyeraal-Hreidarsson syndrome and Revesz syndrome are generally considered multisystem early-onset forms of DC. Coats plus was recognized as a TBD with the discovery of CTC1 mutations.8
It is important for the practicing hematologist to understand that although the classic DC mucocutaneous triad of dysplastic nails, reticular skin pigmentation, and oral leukoplakia is diagnostic, it develops at different ages and rates in each patient and may not be present at the time when patients first come to medical attention.8,9 Patients with apparently isolated aplastic anemia or pulmonary fibrosis who have pathogenic variants in a telomere biology gene should be considered as having a TBD and may be at risk of many of the medical problems typically associated with DC, including immunodeficiency, bone marrow failure, pulmonary fibrosis, liver disease, both hematopoietic and solid malignancies, avascular necrosis of the hips and shoulders, and stenosis of the lacrimal ducts, esophagus, and urethra.8,9 Lymphocyte telomere lengths less than the first percentile for age as measured by flow cytometry with in situ hybridization are highly sensitive and specific for DC.11
The first study to quantify cancer incidence in DC was reported in 2009 and reported an O/E ratio of 11 for the occurrence of cancer in patients with DC compared with the general population. The O/E ratios were especially high for tongue squamous cell carcinoma, MDS, and AML (1154, 2362, and 195, respectively).12 An update on malignancies in 197 patients with DC in the National Cancer Institute cohort was presented at the 2016 American Society of Hematology Annual Meeting, and the investigators found a cumulative incidence of 47% for severe bone marrow failure by age 50 years, 2% for leukemia by age 50 years, and 11% for solid cancers by age 50 years.7 Notably, HCT was associated with a 5.7-fold increased risk of cancer in patients with DC, compared with individuals with DC who did not undergo HCT.7
Shwachman Diamond syndrome
Approximately 90% to 95% of Shwachman Diamond syndrome (SDS) is caused by autosomal recessive pathogenic variants in SBDS, a key component of ribosome biogenesis.13-15 The SBDS protein is involved in the joining of the 40S and 60S ribosomal subunits, which is required for proper protein synthesis. Some patients have just 1 SBDS variant and the role of this in SDS etiology is an active area of investigation. Autosomal recessive SDS-like syndrome was recently reported to be attributable to pathogenic variants in EFL1, which interacts with the SBDS protein.16 Biallelic pathogenic variants in DNAJC21 were reported in 3 families with children with SDS.17 DNAJC21 is also reported to have a role in ribosome biogenesis.
Most patients with SDS present with failure to thrive during infancy as a result of pancreatic insufficiency. Clinically significant neutropenia may develop with associated recurrent infections. Intermittent severe neutropenia with absolute counts <0.5 × 109/L has been reported as the initial clinical manifestation. Patients with SDS may have short stature and certain bony anomalies, including delayed appearance of secondary ossification centers, metaphyseal widening and dysotosis, osteopenia, wormian bones of the skull, and thoracic bony abnormalities.13-15 The combination of exocrine pancreatic insufficiency, documented by low levels of serum trypsinogen in children aged <3 years, low serum isoamylase levels in children aged >3 years, and/or elevated fecal fat excretion over 72 hours, with neutropenia is diagnostic of SDS.13-15 However, there is phenotypic variability; a recent study from the SDS registry reported that only 65% of patients with SDS had these clinical features.14
Some patients with SDS develop pancytopenia that evolves to MDS and/or AML. The Severe Chronic Neutropenia International Registry reported an MDS/AML progression rate of ∼1% per year in patients with SDS.18 This registry of 102 patients reported that the cumulative risk of MDS/leukemia was 36% at age 30 years.18
Diamond Blackfan anemia
RPS19 was the first gene identified with pathogenic variants causative of Diamond Blackfan anemia (DBA) and was one of the first links to understanding the role of ribosome biology in human disease.19,20 Since then, autosomal dominant inheritance of pathogenic variants in at least 15 genes encoding ribosomal proteins or key components of this pathway have been reported to cause DBA. Two X-linked recessive genes (GATA1 and TSR2) have been reported in DBA. GATA1 encodes a hematopoietic transcription factor and TSR2 binds to RPS26, suggesting that both direct and indirect aberrations in ribosome biology can cause DBA.21
Most patients with DBA present with severe reticulocytopenic anemia in infancy.19,20 Patients with DBA may have congenital anomalies such as triphalangeal, bifid, or subluxed thumbs or flat thenar eminence with a normal radius, Klippel-Feil anomaly, Sprengel deformity, webbed neck, genitourinary and heart anomalies, and cleft lip and palate. Short stature is also common. These clinical findings are highly variable; some patients may have none of these findings. In fact, because of the variable phenotypic expressivity and incomplete genetic penetrance, heterozygous mutation carriers may not have a clinical phenotype or may not develop anemia until later in childhood or even adulthood.
Patients with DBA have elevated risks of MDS, AML, colon adenocarcinoma, osteosarcoma, and female genital cancers.22 In 2012, the DBA Registry reported on 608 patients with 9458 person-years of follow-up and found O/E ratios of 5.4 for all cancers, 287 for MDS, 28 for AML, 36 for colon carcinoma, 33 for osteosarcoma, and 12 for female genital cancers compared with the general population.22 A presentation at the 2016 American Society of Hematology Annual Meeting provided updated information on 702 patients with 12 376 person-years of follow-up. The new O/E ratio of cancer among patients with DBA was 4.75 for all cancers, with the highest ratios noted for MDS, colon carcinoma, osteosarcoma, and AML (352, 45, 42, and 29, respectively).23
Disorders typically presenting with MDS, at risk of evolution to leukemia
There are several leukemia susceptibility disorders in which MDS often precedes the development of leukemia. Two such disorders are caused by germline mutations in the transcription factors GATA2 and RUNX1, which are discussed here. See Table 1 for further details and references on other MDS/leukemia-associated disorders.
RUNX1 and familial platelet disorder
RUNX1 is a core-binding transcription factor critical for definitive hematopoiesis, particularly megakaryocyte maturation, polyploidization, and proplatelet production.24 It is well known as a binding partner in t(8;21) and t(3;21) AML and in t(12;21) pediatric acute lymphoblastic leukemia (ALL). The RUNX1 gene is commonly somatically mutated in MDS, AML, and ALL. Somatic RUNX1 mutations have been described in MDS arising from FA, severe congenital neutropenia, and therapy-related MDS. Such mutations are associated with a higher risk of transformation from MDS to AML and a shorter time between MDS and AML transformation.24
Familial platelet disorder (FPD) was one of the first autosomal dominant inherited hematologic malignancy syndromes described. Heterozygous pathogenic germline variants in RUNX1 were identified as the cause of FPD in 1999.25 Patients with FPD have thrombocytopenia and bleeding tendency as a result of platelet dysfunction and dense granule storage pool deficiency. Pathogenic variants in RUNX1 tend to cluster in the runt homology or transactivation domains and have a range of effects on protein function.24
The signs and symptoms of RUNX1 germline mutation can be heterogeneous and subtle and they may not be recognized until AML diagnosis. Therefore, it is important to keep this disorder in mind in young patients with newly diagnosed AML. Any patient with a somatic RUNX1 mutation identified by panel screening of the leukemia should be investigated for the presence of germline mutation with Sanger confirmation. This is particularly key when screening potential related HCT donors, given that the thrombocytopenia and bleeding tendency may be mild and MDS or AML may not have developed in a younger donor. In addition, genetic anticipation in families with RUNX1 germline pathogenic variants has been reported; thus, family members should be counseled and closely monitored after the diagnosis of an RUNX1 mutation in a patient.26 Schlegelberger and Heller26 recommend regular clinical examinations including a complete blood count (CBC) with differential and a yearly bone marrow examination, with counseling regarding the signs and symptoms of leukemia.
The overall risk of development of MDS and AML among patients with FPD is 35%, with an average age of MDS or AML onset of 33 years; however, this is highly variable and incomplete penetrance is reported.26 Once a patient with FPD is identified, careful monitoring is critical for both the proband and family to identify disease progression in a timely fashion.
GATA2 deficiency
GATA2 is a zinc finger transcription factor that binds to the consensus sequence W/GATA/R (W = A or T and R = A or G) in the promoter regions of downstream target genes. It has a key role in hematopoiesis, where its expression is critical for maintenance of the stem cell pool.27 In mouse studies, complete loss of Gata2 leads to embryonic lethality because of a lack of definitive hematopoiesis, whereas heterozygous knockout mice have decreased progenitor cell numbers and a reduced capacity to repopulate in serial transplant experiments. Conditional Gata2 mouse models indicate that Gata2 is critical for the formation and survival of hematopoietic stem cells in the early embryo. These models indicate that the level of GATA2 within hematopoietic stem/progenitor cells is critical for normal hematopoiesis.27
In humans, pathogenic germline variants in GATA2 have been reported in exons and in critical regulatory regions of the gene. Several groups have described the constellation of symptoms that compose GATA2 deficiency, each giving it a different name: familial AML, DCML deficiency (dendritic cell, monocyte, B, and natural killer), lymphoid deficiency, MonoMac, or Emberger syndrome.28 In 2011, it was recognized that the underlying genetics of these disorders were autosomal dominant heterozygous germline mutations in GATA2; thus, this constellation of disorders was redefined as GATA2 deficiency. Disease causing germline mutations in GATA2 leads to haploinsufficiency and can be attributable to variants leading to missense, nonsense, or regulatory region alteration. Patients with GATA2 deficiency have a combination of hematologic, immunodeficiency, pulmonary, and lymphatic symptoms as a result of defects in the endothelial, hematopoietic, and immune systems. Patients have severe cytopenias with a profound loss of monocytes as well as B-, natural killer, and dendritic cell lymphopenia. They are immunodeficient and susceptible to the development of nontuberculous mycobacterial infections, Epstein-Barr virus, human papilloma virus (persistent warts), and fungal infections. Defects in alveolar macrophages lead to the development of alveolar proteinosis, and defects in endothelial cells are hypothesized to be the cause of lymphedema that is often present.29 In addition, a subset of patients presenting with aplastic anemia are found to have underlying GATA2 deficiency.30
For patients with GATA2 deficiency, the risk of developing MDS is as high as 84%, whereas the risk of AML is ∼14%.28 With growing recognition of the syndrome, fewer patients are progressing to acute leukemia and the exact risk may thus be underestimated. Patients with GATA2 deficiency may develop various subtypes of AML, but M5 (monocytic/blastic) (a and b) have been described; in addition, a rise in the monocyte count after years of monocytopenia is a concerning sign. Patients with GATA2 deficiency often have monosomy 7 or trisomy 8 MDS.28 In pediatric MDS, patients with monosomy 7 have an ∼40% risk of having an underlying GATA2 mutation, which increases to 70% for adolescents. Thus, screening all pediatric patients with MDS for GATA2 deficiency is critical.31
Patients with GATA2 deficiency should be monitored closely for the development of MDS and new cytopenias, because conversion to acute leukemia can happen quickly with little warning. Routine monitoring is recommended, but there are no evidence-based clinical guidelines at this time. As in RUNX1 mutation, careful screening of family members as potential HCT donors is critical because the signs and symptoms of the disease may be lacking or yet to present.
Disorders associated primarily with increased leukemia risk
There is a growing understanding of inherited disorders predisposing patients to leukemia without associated congenital abnormalities, bone marrow failure, or MDS. Three such leukemia susceptibility disorders are described below and a longer list is provided in Table 1.
CEBPA-associated familial AML
C/EBPα is a basic region-leucine zipper transcription factor that is important for the differentiation of granulocytes encoded by CEBPA. The first described germline CEBPA mutation was in a family in which 2 siblings and their father developed AML at age <30 years.32 Since that time, several other families have been described and heterozygous pathogenic germline CEBPA variants are now well accepted as causative of inherited leukemia predisposition, with nearly 100% penetrance.
Patients with CEBPA mutations develop AML at a younger age (median age of 25 years) than sporadic AML (average age of AML onset of 67 years).33,34 Interestingly, patients with CEBPA AML typically have normal karyotype blasts with the M1 or M2 FAB subtype, Auer rods, and aberrant CD7 expression. A second CEBPA mutation is often present in the blasts as a somatic event; in 1 study, 18 of 18 samples tested had a secondary CEBPA mutation present in addition to the germline variant. Interestingly, patients with familial CEBPA mutation have a similar good prognosis as sporadic CEBPA double mutant AML; however, they are at risk of recurrence of disease with a new leukemic clone even years after initial remission.35 Thus, it is important to test germline tissue for pathogenic CEBPA variants in any patient presenting with biallelic CEBPA mutant AML to identify germline disease.35 The germline mutations are generally located in the N-terminal region with the transactivating domains, whereas the somatic events tend to be toward the C terminus containing the leucine zipper.36 After family genetic counseling is provided, it is also important to test any child or young adult with AML with a first-degree relative who also had AML, particularly at a younger age (<45 years).
PAX5-associated ALL
PAX5 is a hematopoietic transcription factor, with a conserved paired box DNA binding domain that is important for normal B-cell development. PAX5 is expressed in the early, but not late stages, of B-cell development. PAX5 is commonly mutated somatically in pre–B-cell ALL. In 2013, Shah et al37 described 2 families with heterozygous pathogenic germline variants in PAX5 leading to familial ALL. The first family had 5 patients with ALL, 4 of whom were tested and had the familial mutation. The fifth family member had ALL but was not tested. There were 2 yet unaffected carriers identified. In the second family, 4 mutation-positive affected family members and 1 unaffected carrier were identified, with an additional ALL affected family member identified without gene testing. These PAX5 variants were noted to be hypomorphic, leading to a decrease in the transcription of downstream targets. Interestingly, not all family members with the variant developed ALL, which indicates incomplete penetrance and implies that secondary cooperating mutation(s) are necessary for the development of leukemia.38 Although the majority of leukemia susceptibility disorders predispose patients to AML, it is important to remember that germline predisposition to lymphoblastic disease also occurs, as demonstrated by PAX5.
Li-Fraumeni syndrome and hypodiploid ALL
Autosomal dominant inheritance of pathogenic germline variants in the tumor suppressor TP53 lead to Li-Fraumeni syndrome (LFS), a well-described cancer predisposition disorder. Patients with LFS are at very high risk of bone and soft tissue sarcoma, brain tumors, adrenocortical carcinoma, early-onset breast cancer, and other malignancies. The cumulative cancer risk associated with LFS has been estimated to be ∼50% by age 40 years and up to 90% by age 60 years. Individuals with LFS have an increased risk of AML and ALL; however, the risk is lower (1% to 3%) than for solid tumors. The average age of leukemia onset in LFS is 13 years.39
Up to 40% of patients with low hypodiploid ALL have been reported to have pathogenic germline TP53 variants, in addition to somatic TP53 mutations. This higher-than-expected rate has prompted the recommendation that all patients with hypodiploid ALL undergo genetic counseling and testing for germline TP53 mutations to facilitate appropriate cancer screening for patients and their family members.40
Management of patients with a high genetic risk of leukemia
Individuals who are suspected to have or are newly diagnosed with a leukemia-prone syndrome should be referred to providers familiar with the complexities of the disorder to enable coordinated and comprehensive care (Box 1). Genetic education and counseling of individuals and their families is essential to provide the information necessary for the family to make an informed decision about whether to undergo genetic testing. An evaluation by expert clinicians can facilitate a prompt diagnosis by informing the type of diagnostic (eg, chromosome breakage or telomere length) and genetic (eg, single gene, gene panel, or familial genetic testing) testing to perform as well as the best tissue to analyze (eg, blood versus fibroblasts) (Figure 1).41
Evaluations after diagnosis of a leukemia-prone syndrome
Patients should undergo a comprehensive physical examination with a focus on disease-specific manifestations, such as mucocutaneous features in DC or gastrointestinal problems in SDS. Patients and their families should be educated as to the signs and symptoms of leukemia and should undergo a prompt evaluation if any symptoms develop (eg, fatigue, pallor, fever, petechiae, or bruising). Clinical management guidelines exist for some syndromes and provide a good starting point for evaluations.42,43
Cancer surveillance
A CBC with manual differential should be performed at the initial visit to establish a baseline for future comparison. More frequent CBCs should be done if there are any abnormalities on the baseline evaluation or concerning clinical signs. The FA and DC guidelines recommend CBCs for patients every 3 to 4 months, given their high risk of MDS/AML.42,43 Recently, surveillance recommendations for pediatric patients with leukemia predisposing conditions were put forth by a special workshop of the American Association for Cancer Research. These recommendations stress referral to experts, education on the signs and symptoms of leukemia, consultation with transplant specialists, and surveillance.44
Patients with leukemia syndromes associated with pancytopenia and/or MDS should have a bone marrow aspirate with cytogenetics and biopsy at diagnosis. Subsequent bone marrow evaluations should then be tailored based on the underlying syndrome. Many clinicians recommend annual bone marrow aspirate and biopsy in order to follow trends in cellularity and cellular morphology. Patients with worsening dysplasia, leukemic blasts, or progressive bone marrow clonal abnormalities should be followed closely by experts in the specific disorder. Although cytogenetics is informative, it is important to remember that cytogenetics is limited in that only a small number of cells are evaluated and its prognostic value is not fully understood. In addition, other factors that drive the transformation to MDS and AML may be present in the stressed hematopoietic niche in these patients (eg, clonal hematopoiesis, somatic mosaicism, and stem cell exhaustion), and further study is required to understand the role of these factors in leukemogenesis.
Surveillance for solid malignancies should be tailored to the underlying disorder. A comprehensive screening regimen including whole-body and brain magnetic resonance imaging, ultrasound, and blood tests has been shown to be effective in reducing cancer-related mortality in LFS.45 Patients at risk of HNSCCs or other cancers are encouraged to perform self-examinations and to have an annual evaluation performed by otolaryngology, dentistry, gynecology, and other specialists.
Treatment of MDS or leukemia in patients with genetic predisposition
The management of patients with leukemia susceptibility disorders varies widely between the different syndromes and should be based on up-to-date knowledge connecting the underlying biology and latest clinical data. The efficacy of demethylating agents or other MDS treatment modalities in treating associated with an IBMFS or MDS-associated leukemia-prone disorder has not been investigated.
Preemptive HCT before the development of MDS or AML is a viable option for many patients with leukemia-prone syndromes. For patients with IBMFS, the recommendation is to consider HCT when cytopenias become symptomatic.46 For some patients, it may be more beneficial to undergo HCT before the onset of MDS or other organ complications but the risks must be carefully considered. To our knowledge, there are currently no evidence-based guidelines for whether patients with disorders of leukemia predisposition without associated or preceding symptoms should undergo HCT before the development of leukemia.
The HCT-specific regimen for each patient must be tailored based on the patient’s disorder, underlying comorbidities, and consideration of late HCT effects, particularly for those syndromes (eg, DC) in which transplantation will improve hematopoiesis but will not improve other significant symptoms (eg, pulmonary fibrosis) and may increase cancer risk later in life.47 Selection of related HCT donors must take the underlying genetics into account. Given the variable penetrance, delayed presentation and subtle findings in most of these syndromes affected family members can be missed without genetic and/or proper diagnostic evaluations. All related donors must be tested for the presence of the causative pathogenic variant(s) in the affected person.
The specific management of AML in patients with these syndromes is dependent on the underlying condition. Individuals with FA or DC do not tolerate chemotherapy or radiation well because of their underlying DNA repair and telomere biology defects, respectively. Radiation therapy is to be avoided in LFS because of the significantly increased risk of solid malignancies. There are limited data on whether an ablative HCT regimen leads to improved outcomes in patients with MDS and a leukemia-prone syndrome. There are no specific treatment change recommendations for patients with GATA2 deficiency or CEPBA-associated or RUNX1-associated leukemia predisposition who have progressed from MDS to AML.41
Future directions
The wide range of clinical manifestations and highly variable underlying biology in leukemia-prone syndromes makes it essential that these patients and their families are followed closely by a multidisciplinary team in consultation with experts in the specific disorder. Advances in understanding the biology of these disorders have the potential to lead to improved clinical management and possibly leukemia prevention. Ongoing genomic studies of the somatic genetics of MDS and leukemia in these settings may also lead to improvements in clinical care. Patients and their families will benefit as awareness of these disorders by both pediatric and adult hematologists, oncologists, and immunologists grows. These are the first steps in the development of precision clinical care with the ultimate aim of the prevention of disease-associated manifestations.
Correspondence
Sharon A. Savage, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 9609 Medical Center Dr, Room 6E456, MSC 9772, Bethesda, MD 20895-9772; e-mail: savagesh@mail.nih.gov.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.