Abstract
Sickle cell disease (SCD) is an autosomal recessive disease in which homozygosity for a single point mutation in the gene encoding the β-globin chain produces hemoglobin S molecules that polymerize within the erythrocyte during deoxygenation; the result is sustained hemolytic anemia and vaso-occlusive events. As patients live to adulthood, the chronic impact of sustained hemolytic anemia and episodic vaso-occlusive episodes leads to progressive end-organ complications. This scenario culminates in the development of 1 or more major cardiovascular complications of SCD for which there are no approved or consensus therapies. These complications include elevated pulmonary artery systolic pressure, pulmonary hypertension, left ventricular diastolic heart disease, dysrhythmia, sudden death, and chronic kidney disease with associated proteinuria, microalbuminuria, and hemoglobinuria. In patients with advancing age, cardiopulmonary organ dysfunction and chronic kidney injury have significant effects on morbidity and premature mortality. Over the last 15 years, a number of tests have been validated in multiple replicate cohort studies that identify patients with SCD at the highest risk of experiencing pulmonary and systemic vasculopathy and death, providing for screening strategies tied to targeted, more aggressive diagnostic and therapeutic interventions.
Learning Objectives
Appreciate that patients with sickle cell disease can develop different types of pulmonary hypertension and to learn about the definitions of the different types (World Health Organization class 1, pulmonary arterial hypertension; 2, pulmonary hypertension secondary to left heart disease; 4, chronic thromboembolic pulmonary hypertension; and 5, miscellaneous class) and the treatment options available for these types
Learn about combined precapillary and postcapillary pulmonary hypertension that arises in the setting of heart failure with preserved ejection fraction (previously referred to as diastolic left heart disease)
Review the updated information on the established cardiovascular biomarkers that predict an increased risk of death in patients with sickle cell disease, including tricuspid regurgitant jet velocity, hemodynamic measurements from right heart catheterization, N-terminal pro–B-type natriuretic peptide plasma levels, measures of diastolic left heart disease, and urine proteinuria and microalbuminuria
Introduction
Over the last 15 years, a number of cardiovascular complications and related biomarkers have been identified that are strongly and independently related to reduced exercise capacity and to the risk of death among adult patients with sickle cell disease (SCD).1 These include the following: (1) the diagnosis of pulmonary hypertension (PH), both precapillary and postcapillary disease, according to right heart catheterization (defined in the following discussion); (2) an elevated pulmonary artery systolic pressure estimated by using Doppler echocardiography from the tricuspid regurgitant jet velocity (TRV); (3) echocardiographic evidence of left ventricular diastolic dysfunction, using conventional and tissue Doppler echocardiography; (4) an elevated plasma level of N-terminal pro–B-type natriuretic peptide (NT-proBNP); and (5) chronic injurious effects of hemoglobinuria and the development of proteinuria, macroalbuminuria, and chronic kidney disease. Analogous to the composite risk score for coronary disease identified in the classic Framingham cohort studies (eg, smoking, cholesterol, hypertension, age), these “biomarkers” have been strongly validated in multiple independent cohorts of patients with SCD and help identify adult patients at high risk of morbidity and death. This knowledge sets the stage for interventions targeting these patients to improve patient survival. These factors are each discussed in more detail, with particular attention to the clinical classification of PH that affects patients with SCD.
Complications and biomarkers
PH defined by right heart catheterization: definitions and findings in patients with SCD
Pulmonary arterial hypertension (PAH) (World Health Organization [WHO] group 1 classification) is caused by a progressive increase in pulmonary vascular resistance, smooth muscle and intimal proliferation, and in situ thrombosis, ultimately obliterating the pulmonary arterioles and increasing pulmonary vascular resistance.2 This action leads to progressive right heart failure and reduced exercise capacity. PAH is defined according to a mean pulmonary artery pressure ≥25 mm Hg, with a left ventricular end-diastolic pressure ≤15 mm Hg and a pulmonary vascular resistance value ≥3 Wood Units, indicating an increase in the precapillary pulmonary pressures.3 Although these definitions are briefly reviewed herein, the interested reader is referred to more detailed sources elsewhere.2-4
Pulmonary venous hypertension (WHO group 2 classification) is caused by increases in pressures downstream of the pulmonary arterioles and capillaries, typically related to increases in left heart filling pressures caused by diastolic or systolic heart failure.
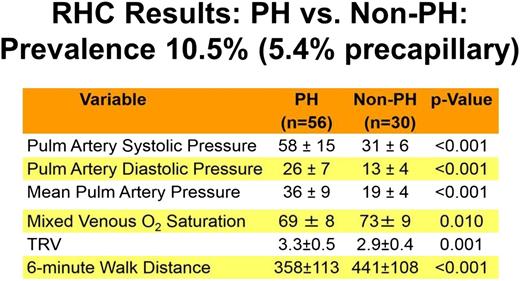
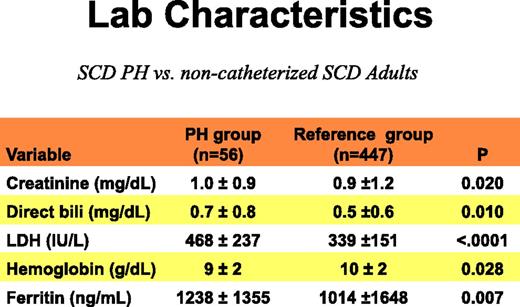
Both hemodynamic forms of PH are among the most common cardiovascular complications of SCD and are independent predictors of death in the adult population. The largest study conducted to date with the longest follow-up is the National Institutes of Health (NIH) PH cohort study, which screened 531 patients followed up for a median of 4.4 years.5-7 As shown in Figures 1 and 2, a total of 86 right heart catheterizations were performed in patients with suspected PH. Fifty-six (10.5%) of the 533 screened patients were diagnosed with PH on the basis of a mean pulmonary artery pressure >25 mm Hg. Of the group with PH, 56.4% had precapillary PAH (group 1 hemodynamic parameters), with a pulmonary artery occlusion pressure ≤15 mm Hg; the remainder had pulmonary venous hypertension (group 2 hemodynamic parameters), with elevated pulmonary artery occlusion pressures >15 mm Hg. Figures 1 and 2 display the clinical and laboratory characteristics of these patients, respectively, and highlight the fact that PH in patients with SCD is associated with a number of risk factors, including the severity of hemolytic anemia, chronic renal dysfunction, and iron overload. They also illustrate that patients with PH develop a reduced cardiac output and can walk less distance in 6 minutes (lower 6-minute walk test).
Prevalence, severity, and clinical characteristics of patients with SCD and PH diagnosed by using definitive right heart catheterization. PH in patients with SCD is associated with a reduced cardiac output (lower mixed venous oxygen saturation) and lower 6-minute walk distance.
Prevalence, severity, and clinical characteristics of patients with SCD and PH diagnosed by using definitive right heart catheterization. PH in patients with SCD is associated with a reduced cardiac output (lower mixed venous oxygen saturation) and lower 6-minute walk distance.
Laboratory characteristics of patients with SCD and PH. PH in patients with SCD is associated with a number of risk factors, including the severity of hemolytic anemia, chronic renal dysfunction, and iron overload. LDH, lactate dehydrogenase.
Laboratory characteristics of patients with SCD and PH. PH in patients with SCD is associated with a number of risk factors, including the severity of hemolytic anemia, chronic renal dysfunction, and iron overload. LDH, lactate dehydrogenase.
PH secondary to heart failure with preserved ejection fraction and combined precapillary and postcapillary PH
Another new name for what was traditionally called diastolic heart disease is heart failure with preserved ejection fraction (HFpEF), distinct from heart failure with reduced ejection fraction. Both the preserved and reduced ejection fraction forms of heart failure occur in patients with SCD; however, HFpEF is clearly more common (and is discussed in more detail in a later section). We now appreciate that there are patients with HFpEF who develop more severe secondary vasoconstriction and proliferation of the pulmonary vasculature.8,9 These patients develop both precapillary and postcapillary PH. The precapillary severity is defined by a high transpulmonary pressure gradient (the mean pulmonary artery pressure minus the left ventricular end-diastolic pressure, often estimated by using the pulmonary capillary occlusion pressure) or by a high pulmonary vascular resistance. This form of PH is included in WHO group 2 and is also called combined precapillary and postcapillary pulmonary hypertension (CpcPH), as recently reviewed by Dixon et al.9 Studies in patients without SCD indicate that patients with CpcPH have a higher mortality than patients with simple postcapillary PH. This scenario appears to be the case for patients with SCD as well (as summarized in the following section): patients with SCD with a high transpulmonary pressure gradient or pulmonary vascular resistance experience higher mortality.5
Chronic thromboembolic PH (WHO group 4) is caused by recurrent pulmonary emboli with secondary fibrotic remodeling of the thrombosed and occluded pulmonary vasculature. Venous and pulmonary thromboembolism is relatively common in patients with SCD,10,11 and Anthi et al12 reported that 6 of 24 patients with PH documented by using right heart catheterization had a high-probability ventilation/perfusion scan. In 3 (12.5%) of these 24 patients, the computed tomography angiogram was suggestive of chronic thromboembolic PH. Evaluation of patients with PH and SCD by using ventilation/perfusion scanning and/or computed tomography angiography is recommended, with referral for consideration of riociguat or surgical therapy.13 Riociguat, a small molecule that binds to and activates the nitric oxide receptor soluble guanylate cyclase, has recently been approved by the US Food and Drug Administration; however, definitive treatment requires surgical thromboendarterectomy, which has been used with success in patients with SCD.14
Miscellaneous cause of PH (WHO group 5)
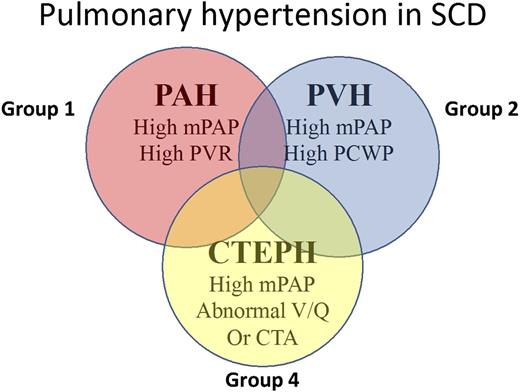
Based on the fact that patients with SCD can have group 1, 2, and 4 forms of PH, the recent classification meetings held in Venice placed SCD in group 5. Figure 3 highlights the presentations of PH in SCD.
Clinical types of PH observed in patients with SCD. CTA, computed tomography angiography; CTEPH, chronic thromboembolic PH; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; V/Q, ventilation/perfusion.
Clinical types of PH observed in patients with SCD. CTA, computed tomography angiography; CTEPH, chronic thromboembolic PH; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; V/Q, ventilation/perfusion.
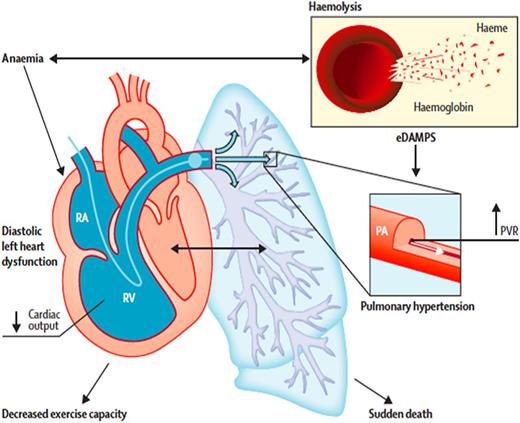
Regardless of the specific hemodynamic parameters, extensive data now clearly define PH as a major cause of early death in patients with SCD. Early studies by Castro’s group using right heart catheterization to define disease severity found that in patients with SCD and PH, an increase of 10 mm Hg in the mean pulmonary artery pressure was associated with a 1.7-fold increase in the hazard ratio of death (95% confidence interval [CI], 1.1-2.7; P = .028).15 Three prospective screening studies have been performed in adult patients with SCD; they evaluated hemodynamic parameters according to right heart catheterization to assess disease prevalence with this gold standard diagnostic test and to evaluate associated prospective mortality risk.5,6,16,17 The NIH PH cohort study (summarized earlier) identified 10.4% of screened patients with PH; 56.4% had precapillary PAH (group 1 hemodynamic parameters), and the remainder had pulmonary venous hypertension with elevated pulmonary artery occlusion pressures >15 mm Hg (group 2 hemodynamic parameters). The median survival time for patients with PH was 6.8 years. Multivariate analysis of hemodynamic variables identified systolic pulmonary artery pressure, pulmonary pulse pressure, transpulmonary gradient, and pulmonary vascular resistance as being significantly associated with high mortality, consistent with pulmonary vascular disease, and not left heart disease, driving the observed relationship between higher pulmonary pressures and death.5 A similar prevalence of PH of 6% to 10%, with one-half having precapillary PH and one-half having postcapillary PH, was reported in 2 other screening studies that used right heart catheterization.16,17 In both studies, PH was strongly related to increased risk of death during follow-up. Figure 4 highlights how chronic intravascular hemolysis leads to precapillary PAH and pulmonary arteriole vasoconstriction and smooth muscle hypertrophy, and how chronic anemia leads to diastolic left heart disease (now also called HFpEF).1,18 These 2 disease manifestations are diagnosed by using Doppler echocardiography and right heart catheterization.
How chronic intravascular hemolysis leads to precapillary pulmonary arterial hypertension (PAH) and pulmonary arteriole vaso-constriction and smooth muscle hypertrophy, and how chronic anemia leads to diastolic left heart disease (now also called HFpEF). These 2 disease manifestations are diagnosed by using Doppler echocardiography and right heart catheterization. PVR, pulmonary vascular resistance; RA, right atrium; RV, right ventricle. Reprinted from The Lancet, 387, Gladwin MT, Cardiovascular complications and risk of death in sickle cell disease, 2565-2574, 2016, with permission from Elsevier.
How chronic intravascular hemolysis leads to precapillary pulmonary arterial hypertension (PAH) and pulmonary arteriole vaso-constriction and smooth muscle hypertrophy, and how chronic anemia leads to diastolic left heart disease (now also called HFpEF). These 2 disease manifestations are diagnosed by using Doppler echocardiography and right heart catheterization. PVR, pulmonary vascular resistance; RA, right atrium; RV, right ventricle. Reprinted from The Lancet, 387, Gladwin MT, Cardiovascular complications and risk of death in sickle cell disease, 2565-2574, 2016, with permission from Elsevier.
Tricuspid regurgitant jet velocity
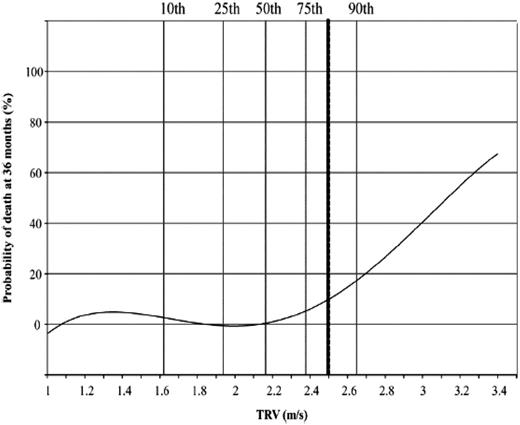
The measured TRV is obtained by using Doppler echocardiography. This technique is a direct measure of the velocity of blood that moves backward from the right ventricle during systole into the right atrium, and it can be used to estimate the right ventricular systolic pressure, which equals the pulmonary artery systolic pressure. This parameter has been evaluated in numerous studies, and even mild increases ≥2.5 to 2.7 m/s are associated with an increased risk of death.4,19-23 A total of 45 screening studies have now been subjected to meta-analysis and include >6000 patients.24 Patients with elevated TRV walked an estimated 30.4 m (95% CI, 6.9-53.9 m) less than those without elevated TRV and had a mortality hazard ratio of 4.9 (95% CI, 2.4-9.7). In a recently published study of patients from Créteil (Paris, France), the probability of death was low in patients with TRV <2.5 m/s, whereas TRV ≥2.5 m/s was associated with a hazard ratio of 6.81 in multivariate analysis (P < .001).2 5 As shown in Figure 5, the risk of death rose linearly above a value of 3 m/s, with a 50% probability of death at TRV 3.2 m/s.
In a recently published study of patients from Créteil, the probability of death was low in patients with TRV <2.5 m/s, whereas TRV ≥2.5 m/s was associated with a hazard ratio of 6.81 in multivariate analysis (P < .001). Remarkably, the risk of death rises linearly above a value of 3 m/s with a 50% probability of death at TRV 3.2 m/s. Reprinted from Damy T, Bodez D, Habibi A, et al., Haematological determinants of cardiac involvement in adults with sickle cell disease, Eur Heart J, 2016, 37, 14, 1158-1167, by permission of Oxford University Press.
In a recently published study of patients from Créteil, the probability of death was low in patients with TRV <2.5 m/s, whereas TRV ≥2.5 m/s was associated with a hazard ratio of 6.81 in multivariate analysis (P < .001). Remarkably, the risk of death rises linearly above a value of 3 m/s with a 50% probability of death at TRV 3.2 m/s. Reprinted from Damy T, Bodez D, Habibi A, et al., Haematological determinants of cardiac involvement in adults with sickle cell disease, Eur Heart J, 2016, 37, 14, 1158-1167, by permission of Oxford University Press.
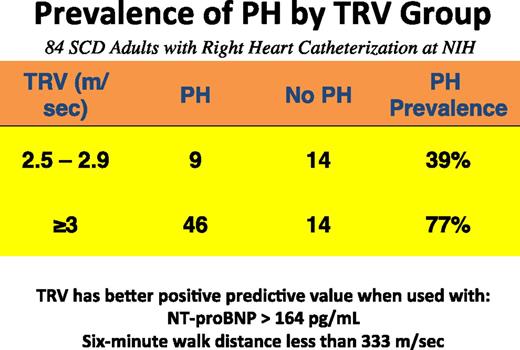
There are controversies surrounding TRV as a measure of PH. TRV can be used to estimate pulmonary artery systolic pressure and clearly identifies adult patients at high risk of death, but it should not be considered a gold standard for the diagnosis of PH. A value between 2.5 and 3 m/s identifies only 25% to 39% of patients with a mean pulmonary pressure ≥25 mm Hg, whereas a value ≥3 m/s identifies ∼66% to 77% of patients with PH.5,6,16 Using combinations of a high TRV and a high NT-proBNP level >164 pg/mL or a low 6-minute walk distance <333 m can increase the positive predictive value; this method has been proposed as a diagnostic strategy for patients with TRV between 2.5 and 3.0 m/s.4,16 Figure 6 highlights the utility of TRV for PH screening.
Utility of TRV combined with a low walk distance or a high NT-proBNP level to diagnose patients with PH. Data from the NIH cohort screening study were used (data are unpublished analyses, courtesy of Xin Tian and Gregory Kato, and include data from Parent et al16 ).
Utility of TRV combined with a low walk distance or a high NT-proBNP level to diagnose patients with PH. Data from the NIH cohort screening study were used (data are unpublished analyses, courtesy of Xin Tian and Gregory Kato, and include data from Parent et al16 ).
Diastolic left heart disease or HFpEF
Although systolic heart dysfunction is relatively rare in patients with SCD, measures of diastolic heart disease are more common and define patients at high risk, independent of increases in pulmonary pressures.1,22,26 For example, in the Walk-PHaSST (Treatment of Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy) screening study, both the left ventricular lateral E/e′ ratio (P = .014) and TRV (P = .019) were independent predictors of a shorter 6-minute walk distance.22 In the NIH PH screening study, diastolic dysfunction as reflected by a low E/A ratio was associated with excess mortality, even after adjustment for TRV, with a risk ratio of 3.5 (95% CI, 1.5-8.4; P < .001).26 The E/A ratio represents the ratio of peak velocity flow in early diastole (the E wave) to peak velocity flow in late diastole caused by atrial contraction (the A wave). When considered together, diastolic dysfunction and an elevated TRV conferred a risk ratio for death of 12.0 (95% CI, 3.8-38.1; P < .001). Proportional hazards regression analysis found that most diastolic parameters are associated with increased mortality, including E/A (P < .001), peak E velocity (P = .002), deceleration time (P = .002), and tissue Doppler septal Em/Am ratio (P = .026). The use of left atrial size as a measure of diastolic dysfunction should be used with care because all chambers increase with anemia. This measure will have low specificity for HFpEF in patients with SCD.
The importance of diastolic dysfunction and its relationship to myocardial microvascular disease and cardiomyopathy in SCD has recently been highlighted in clinical studies, with corroborating data from mouse models.27,28 A recent study evaluated the relationship between diastolic dysfunction and objective measures of myocardial fibrosis by using magnetic resonance imaging methods that calculate the extracellular volume; the findings suggest that chronic effects of anemia and ischemia and reperfusion injury may result in diffuse myocardial fibrosis.29 In these studies, a high extracellular volume correlated with echocardiographic measures of diastolic dysfunction, a low hemoglobin level (r = –0.46; P = .03), and a high NT-proBNP level (r = 0.62; P = .001).
Elevation in the plasma levels of NT-proBNP
The brain natriuretic peptide is a pre-pro hormone released from cardiomyocytes of the left and right ventricles subjected to pressure overload and wall stress. It also identifies patients at higher risk of having PH, with lower exercise capacity and increased mortality risk. This role has been shown in archived samples from the historic MSH (Multicenter Trial of Hydroxyurea) and the CSSCD (Cooperative Study of SCD) cohorts,30 as well as the more recent NIH PH29 and Walk-PHaSST cohorts.31 It is worth nothing that because renal failure will increase the BNP level, high levels may not indicate right or left ventricular dysfunction in this setting.
Chronic kidney disease
Stage 3 chronic kidney disease (CKD) is defined by an estimated glomerular filtration rate <60 mL/min/1.73 m2, and macroalbuminuria is defined as urine albumin >300 mg/g creatinine. The kidneys are among the most commonly affected organs in patients with SCD,32,33 and the presence of CKD is an independent predictor of developing PH as well as early mortality in adults with SCD.20,34,35 Proposed mechanisms for sickle cell nephropathy include ischemia-reperfusion injury, hyperfiltration, and hypertension.36 Gordeuk’s group recently found that urine dipstick and microscopy-defined hemoglobinuria is present in >20% of adults with SCD, is associated with markers of hemolysis, and is a predictor of CKD stage and progression.32
Combined risk factor analysis identifies a group of adults with SCD at high risk for hospitalization and death
In an unpublished data analysis performed by Mehdi Nouraie (analysis in May 2017) at the University of Pittsburgh, the prevalence of TRV ≥2.5 m/s and an NT-proBNP level ≥160 pg/mL was evaluated in patients from the Walk-PHaSST and NIH patient cohorts. An elevation of both biomarkers was observed in 19.2% of adults screened in the Walk-PHaSST trial (n = 527 patients with TRV and BNP data) and 19.4% of 407 adult patients in the NIH PH screening cohort. Elevation of both of these parameters identified a group at high risk of death. In the Walk-PHaSST study, the 12-month mortality rate was 7.9% in patients with both risk factors compared with 0.5% in patients with normal TRV or BNP values. In the NIH PH cohort, these rates were 8.6% and 0.7%, respectively. This group also had a higher risk of hospitalization (risk ratio, 1.6). In the Walk-PHaSST cohort, the 12-month hospitalization risk was 78% for patients with abnormal TRV and BNP values compared with 45% for patients with normal TRV or BNP values.
How do we screen and treat adult patients for risk of PH and death?
Recent consensus guidelines for PH in SCD from the American Thoracic Society (ATS), the American College of Chest Physicians, and the Pulmonary Hypertension Association have been published.4,37 Experts in pediatric and adult SCD, cardiology, and pulmonary medicine developed a recommended approach to screening and therapy for adult patients with SCD. The consensus statement argued for screening by using Doppler echocardiography, plasma NT-proBNP testing, and (if indicated) right heart catheterization to identify adult patients at the highest risk of death for additional diagnostic testing and potentially more aggressive therapies as indicated by the clinical scenario (Figure 74 ). It is recommended that patients with borderline TRV values of 2.5 to 3.0 m/s undergo additional risk stratification with NT-proBNP level and results of 6-minute walk testing. For this group with an NT-proBNP level >164 pg/mL and a 6-minute walk distance <333 m, or if the TRV is ≥3.0 m/s, right heart catheterization is recommended to define group 1 vs group 2 hemodynamic parameters. For all patients with high TRV or NT-proBNP levels, the intensification of SCD-specific care is recommended with hydroxyurea, supplemental oxygen therapy, iron chelation, or other interventions, as indicated. Aggressive hydroxyurea therapy to maximally increase fetal hemoglobin levels reduces vaso-occlusive events and the acute chest syndrome that are known to increase risk of death in patients with SCD who have preexisting PH or high TRV values.38 Chronic transfusions would be expected to control hemolytic anemia, reduce vaso-occlusive episodes, and improve cardiopulmonary reserve, with small cohort studies suggesting benefit.39,40 Finally, patients identified with chronic thromboembolic PH (WHO group 4 PH) would be amenable to receive riociguat,13 surgical thromboendarterectomy,14 and required anticoagulation therapy.
Recent consensus guidelines for PH in SCD. Guidelines from the ATS, the American College of Chest Physicians, and the Pulmonary Hypertension Association have been published. Experts in pediatric and adult SCD, cardiology, and pulmonary medicine developed a recommended approach to screening and therapy for adult patients with SCD. The consensus statement argued for screening using Doppler echocardiography, plasma NNT-proBNP testing, and (if indicated) right heart catheterization to identify adult patients at the highest risk of death for additional diagnostic testing and potentially more aggressive therapies as indicated by the clinical scenario. For all patients with high TRV or NT-proBNP levels, the intensification of SCD-specific care is recommended with hydroxyurea, oxygen, iron chelation, or other interventions, as indicated. It is recommended that patients with borderline TRV values of 2.5 to 3.0 m/s undergo additional risk stratification with NT-proBNP and 6-minute walk testing. For this group with an NT-proBNP level >164 pg/mL and a 6-minute walk distance (6MWD) <333 m, or if TRV is ≥3.0 m/s, right heart catheterization is recommended to define group 1 vs group 2 hemodynamic parameters. The treatment of patients with PAH (group 1 hemodynamics) was limited to Level C evidence based on a lack of randomized placebo-controlled trials. However, until such evidence exists to change management, patients with PAH hemodynamic variables (pulmonary artery mean pressure ≥25 mm Hg and a pulmonary capillary wedge pressure ≤15 mm Hg, with pulmonary vascular resistance [PVR] >160 dyn·s/cm5) should be considered for PAH-specific therapy after fully maximizing underlying SCD-specific care. A number of case series have been published suggesting that phosphodiesterase-5 inhibitors, prostanoids, and endothelin receptor blockers may be effective in these patients.41-43 Reprinted with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society. Cite: Klings ES, Machado RF, Barst RJ, et al; American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease/2014/An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease./Am J Respir Crit Care Med./189/727-740. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Recent consensus guidelines for PH in SCD. Guidelines from the ATS, the American College of Chest Physicians, and the Pulmonary Hypertension Association have been published. Experts in pediatric and adult SCD, cardiology, and pulmonary medicine developed a recommended approach to screening and therapy for adult patients with SCD. The consensus statement argued for screening using Doppler echocardiography, plasma NNT-proBNP testing, and (if indicated) right heart catheterization to identify adult patients at the highest risk of death for additional diagnostic testing and potentially more aggressive therapies as indicated by the clinical scenario. For all patients with high TRV or NT-proBNP levels, the intensification of SCD-specific care is recommended with hydroxyurea, oxygen, iron chelation, or other interventions, as indicated. It is recommended that patients with borderline TRV values of 2.5 to 3.0 m/s undergo additional risk stratification with NT-proBNP and 6-minute walk testing. For this group with an NT-proBNP level >164 pg/mL and a 6-minute walk distance (6MWD) <333 m, or if TRV is ≥3.0 m/s, right heart catheterization is recommended to define group 1 vs group 2 hemodynamic parameters. The treatment of patients with PAH (group 1 hemodynamics) was limited to Level C evidence based on a lack of randomized placebo-controlled trials. However, until such evidence exists to change management, patients with PAH hemodynamic variables (pulmonary artery mean pressure ≥25 mm Hg and a pulmonary capillary wedge pressure ≤15 mm Hg, with pulmonary vascular resistance [PVR] >160 dyn·s/cm5) should be considered for PAH-specific therapy after fully maximizing underlying SCD-specific care. A number of case series have been published suggesting that phosphodiesterase-5 inhibitors, prostanoids, and endothelin receptor blockers may be effective in these patients.41-43 Reprinted with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society. Cite: Klings ES, Machado RF, Barst RJ, et al; American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease/2014/An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease./Am J Respir Crit Care Med./189/727-740. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
The treatment of patients with PAH (group 1 hemodynamic parameters) was limited to Level C evidence based on a lack of randomized placebo-controlled trials. However, until such evidence exists to change management, patients with PAH hemodynamic variables (pulmonary artery mean pressure ≥25 mm Hg and a pulmonary capillary wedge pressure ≤15 mm Hg, with pulmonary vascular resistance >160 dyn·s/cm5) should be considered for PAH-specific therapy after fully maximizing underlying SCD-specific care. A number of case series have been published suggesting that phosphodiesterase-5 inhibitors, prostanoids, and endothelin receptor blockers may be effective in these patients.41-43 Although phosphodiesterase-5 inhibition may be helpful in aggressively transfused or hydroxyurea-treated patients, the Walk-PHaSST clinical trial was stopped early due to increases in hospitalization for pain in the sildenafil treatment arm.44 It is notable that a number of recently published studies suggest that aggressive chronic transfusions39,40 or stem cell transplantation45 can improve PH in patients with SCD, suggesting the need for further study in this at-risk group.
How do we screen and treat pediatric patients for risk of PH and cardiovascular morbidity?
Gordeuk et al46 conducted a prospective longitudinal screening study of 310 sickle cell patients aged 3 to 20 years. The investigators showed that TRV values are higher in children, and a value of 2 standard deviations above the population mean was 2.6 m/s (compared with the adult cutoff of 2.5 m/s), suggesting that this threshold should be used for screening in children.46,47 Elevated jet velocity (defined as ≥2.60 m/s) occurred in 11.0% of 310 children.47 The high TRV was associated with a higher rate of steady-state hemolysis, lower oxygen saturation, and history of more episodes of transfusion, acute chest syndrome, and stroke. In a longitudinal follow-up of 160 children with hemoglobin SS disease for a median time of 22 months, 14% of this group had an elevated TRV ≥2.60 m/s. Importantly, patients with both a high TRV and more severe hemolytic anemia (measured by using principal component analysis31,48 ) had an estimated 4.4-fold increase in the odds of a ≥10% decline in age-standardized 6-minute walk distance during follow-up (P = .015). This finding suggests that combination risk factor analysis may be needed in children to identify higher risk of future functional decline.
These studies suggest that a higher cutoff value for TRV with associated clinical symptoms or further laboratory abnormalities may be required to justify more intensive evaluations in the pediatric population. The risk of death is low in children with SCD, and no studies have followed up patients long enough to show a mortality risk related to high TRV at an early age. For these reasons, the recommendations for screening are not yet established. The ATS guidelines suggest, based solely on expert opinion, that screening be reserved for children presenting with additional risk factors which suggest the development of early vasculopathy, such as dyspnea, hypoxemia, symptoms of right heart failure, or laboratory measures of high levels of hemolysis or urine proteinuria.4
Should we consider exchange transfusion therapy for patients with more severe PH or diastolic heart disease?
Erythrocytapheresis is expected to rapidly improve cardiopulmonary function and reduce risk in adult patients with SCD at the highest risk of death. From a physiological standpoint, red blood cell transfusion will increase hemoglobin levels, improve oxygen delivery, reduce cardiac wall stress by decreasing stroke volume and work, improve exercise capacity, and improve arterial hemoglobin oxygen saturation. Chronic exchange transfusion therapy would be expected to control hemolytic anemia, reduce vaso-occlusive sickling episodes, and improve cardiopulmonary reserve, with small cohort studies suggesting benefit.39,40 From a clinical standpoint, exchange transfusion also reduces vaso-occlusive events and the acute chest syndrome that are known to increase the risk of death in patients with SCD.38 Prophylactic simple transfusion of antigen-matched blood has been successful in ameliorating all of the complications of SCD tested to date, including postsurgical acute chest syndrome, vaso-occlusive crisis, and stroke, but is complicated by iron overload. We thus hypothesize that exchange transfusion will limit disease progression, improve exercise capacity, and prevent interval episodes of vaso-occlusive crisis and acute chest syndrome that often acutely increase pulmonary pressures and cause right heart failure in patients with baseline increased pulmonary pressures.38
Despite the safety and wide utilization of erythrocytapheresis in adult patients with SCD, there is no consensus or quality efficacy data on its use to improve outcomes in aging, high-risk SCD patients with progressive end-organ dysfunction. Based on a recent survey of transfusion practices, as many as 10% of adult patients with SCD undergoing chronic exchange transfusion are being treated for PH, heart failure, or high TRV,49 and the recent ATS guidelines of PH in SCD recommended transfusion in patients with PH who did not tolerate or had suboptimal responses to hydroxyurea.4 However, recent National Heart, Lung, and Blood Institute guidelines did not support this recommendation and equivocated on the need for screening for cardiovascular risk factors such as TRV and NT-proBNP based on the fact that there are no proven effective interventions.37,50 These controversies establish clear equipoise and highlight the urgent need for an interventional trial of exchange transfusion in adults with SCD with cardiovascular risk factors for morbidity and mortality. This equipoise is acutely illustrated by conflicting recommendations in letters from 2 guideline committees,37,50 with the National Heart, Lung, and Blood Institute guidelines committee calling for “direct evidence needed before universal adoption.”
Conclusions
Over the last 15 years, investigators have identified a series of clinical tests that identify patients at high risk of pulmonary and systemic vasculopathy and death. The recommended approach is to screen patients by using these tests to identify potentially treatable end-organ complications and to enroll patients in more aggressive interventional research studies.
Acknowledgments
The author thanks Victor Gordeuk, Seyed Mehdi Nouraie, Maria Brooks, Sally Campbell-Lee, and Darrell Triulzi for reading and editing parts of this review.
Correspondence
Mark Gladwin, 1218 Scaife Hall, 3550 Terrace St, Pittsburgh, PA 15261; e-mail: Gladwinmt@upmc.edu.
References
Author notes
Conflict-of-interest disclosure: M.T.G. is on the Board of Directors or an advisory committee for Bayer; has received research funding from Bayer; holds patents with or receives royalties from the University of Pittsburgh, the NIH, and Globin Solutions, Inc; and has equity ownership in Globin Solutions, Inc. He is listed as a coinventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases and on provisional patents for the use of recombinant neuroglobin and heme-based molecules as antidotes for carbon monoxide poisoning; the former has been licensed by United Therapeutics. M.T.G. is a co-investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguat as a treatment for patients with SCD.
Off-label drug use: None disclosed.

![Figure 7. Recent consensus guidelines for PH in SCD. Guidelines from the ATS, the American College of Chest Physicians, and the Pulmonary Hypertension Association have been published. Experts in pediatric and adult SCD, cardiology, and pulmonary medicine developed a recommended approach to screening and therapy for adult patients with SCD. The consensus statement argued for screening using Doppler echocardiography, plasma NNT-proBNP testing, and (if indicated) right heart catheterization to identify adult patients at the highest risk of death for additional diagnostic testing and potentially more aggressive therapies as indicated by the clinical scenario. For all patients with high TRV or NT-proBNP levels, the intensification of SCD-specific care is recommended with hydroxyurea, oxygen, iron chelation, or other interventions, as indicated. It is recommended that patients with borderline TRV values of 2.5 to 3.0 m/s undergo additional risk stratification with NT-proBNP and 6-minute walk testing. For this group with an NT-proBNP level >164 pg/mL and a 6-minute walk distance (6MWD) <333 m, or if TRV is ≥3.0 m/s, right heart catheterization is recommended to define group 1 vs group 2 hemodynamic parameters. The treatment of patients with PAH (group 1 hemodynamics) was limited to Level C evidence based on a lack of randomized placebo-controlled trials. However, until such evidence exists to change management, patients with PAH hemodynamic variables (pulmonary artery mean pressure ≥25 mm Hg and a pulmonary capillary wedge pressure ≤15 mm Hg, with pulmonary vascular resistance [PVR] >160 dyn·s/cm5) should be considered for PAH-specific therapy after fully maximizing underlying SCD-specific care. A number of case series have been published suggesting that phosphodiesterase-5 inhibitors, prostanoids, and endothelin receptor blockers may be effective in these patients.41-43 Reprinted with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society. Cite: Klings ES, Machado RF, Barst RJ, et al; American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease/2014/An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease./Am J Respir Crit Care Med./189/727-740. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2017/1/10.1182_asheducation-2017.1.423/4/m_hem00059f7.jpeg?Expires=1768117054&Signature=zfiTQk6hN8AA6aTHYKATYHGG9JEJ7GiA~1ZkNSgKHgsUgHuMg5RYP0jMe3tpO-xcAMM~EcWUr4zRY3Dii0qAIvcVaDNycCWfJFFHnMrllkL5fE-O8dhFJ9Pk6KEQqM6lFhCukC0rfIlg90tjK5v3K4xRhlyLzgJADeAtgrzx8SedWavLwmZeH3~Pdr31zUrXerzI1iNF9kQZY3~b8FNYSw63AQVKvhQ7~ALGTVSrjizluO4Wd9xmGX6rmMybRyPWfvib6fMf9tRy5DR1iEOfyzFmrGfyNr1kwaxephzJ~vZIqMPSiDXI5acy4vbE3rparYMNteoBl4kSmGXC~BR7dg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)