Abstract
Vitamin K antagonists (VKAs) have been the only oral anticoagulants for decades. The management of anticoagulant therapy with VKA is challenging because of the intricate pharmacological properties of these agents. The success of VKA therapy depends on the quality of treatment that is ensured through continuing comprehensive communication and education. The educational program should address important issues of the VKA therapy such as beginning of treatment, pharmacological, dietary, and drug–drug interactions, as well as treatment temporary suspension during surgical interventions or invasive maneuvers. In addition, the initial and continuing patient education is of imperative importance. A major role in the educational process may be addressed by patient associations. The quality of treatment is better reached if patients are followed in anticoagulation clinics. Moreover, a federation of anticoagulation clinics may improve patient care through regular meetings to update knowledge on VKA treatment. Learning objectives of this paper is to allow readers to correctly approach patients starting VKA treatment, recognize possible pitfalls of treatment, and provide adequate solutions.
Learning Objectives
Initiate warfarin treatment and monitor patients in the follow-up period by modulating warfarin daily dose and performing bridging therapy in the occasion of surgery or invasive maneuvers
Summarize the activities of an anticoagulation clinic in which patient education, clinical and laboratory monitoring, and drug–drug interaction are essential for a well-managed warfarin treatment
Introduction
Vitamin K antagonists (VKAs) have been the only oral anticoagulants for decades. Among them, warfarin is the most widely used agent, although others such as acenocoumarol, phenprocoumon, and indandione derivatives are also prescribed in some countries.1
The indications of VKA therapy range from the prevention of stroke in patients with atrial fibrillation, prevention of recurrent thromboembolism in patients with deep vein thrombosis and pulmonary embolism, to the prevention of thrombosis in patients with valvular heart disease or prosthetic cardiac valves.
The management of anticoagulant therapy with VKA is challenging because of the intricate properties of these agents. VKA therapy is among the top drugs associated with serious adverse events and emergency department admissions. Therapeutic errors, such as inadequate prescription or administration, inappropriate monitoring, absence of treatment resulting from poor adherence, sector change (admission to or discharge from hospital), or undergoing surgery are the most common.2-5 These issues may have medico-legal implications ranging from the failure to prescribe anticoagulants when clinically indicated to inadequate monitoring of the therapy afterward. Despite the introduction of direct oral anticoagulants (DOACs), VKA therapy is still broadly used for other indications or in economical situations where DOACs cannot be afforded. Despite DOACs’ efficacy and safety profiles, their advantage comes less at sites with good international normalized ratio (INR) control, underlining the importance of local standards of care to affect the benefits of anticoagulation therapy.6 DOAC should not be seen as an absolute substitution of VKA, but as an extension of the antithrombotic armamentarium and the knowledge and education on VKA should not come less.
It is important to ensure continuing comprehensive communication and education concerning the beginning of treatment, pharmacological and dietary interactions, as well as suspension during surgical interventions or invasive maneuvers. In addition, the continuous patient education is of fundamental importance for a successful long-term therapy and requires permanent attention.
For these reasons, specialized structures have emerged in many countries (thrombosis centers or anticoagulation clinics), dedicated to monitoring VKA therapy. In this review, we will focus on the various issues of VKA therapy and provide adequate solutions.
Well-managed warfarin treatment
This high-quality anticoagulation management is poorly defined and difficult to assess.7 However, as with other chronic therapies, the ultimate goal is finding a safe balance between efficacy and safety. The achievement of a high-quality VKA therapy goes through a number of topics:
Initial patient education
Optimal laboratory control
Rapid identification of maintenance dose
Establishment of rules in the management of excess anticoagulation
Management of surgical or interventional procedures (bridging therapy)
Use of a computer-aided prescription and provision of written dosage instructions
Close clinical and laboratory monitoring
Management of intake or suspension of interfering drugs
Consensus (hospital- or regional-based) treatment decisional pathways
Initial patient education
The pivotal importance of patient education has been reaffirmed for more than a decade by a report from the World Health Organization: Therapeutic Patient Education, Continuing Education Programs for Health Care Providers in the Field of Prevention of Chronic Diseases.8 This program is well suited to the condition of the chronic patient undergoing long-term oral anticoagulant therapy. It emphasizes the way education should be structured, organized, and offered systematically to each patient using different communication methods and must provide an assessment of the learning process and its effects. It is a continuous process, integrated into patient care and administered by properly trained personnel. The role of dedicated nurses in this process is unique.
The basic elements of the educational process (Table 1) should start with broad explanation of the condition and the purpose and the characteristics of the therapy. Particular attention should be given to therapy duration and the risks of self-interruption. Other elements should include practical issues in dealing with diet, comedications, and the necessity of continuous INR measurement, and different dosing regimens. VKA therapy is perceived by patients as cumbersome, difficult to deal with and potentially risky; patient education should focus on “demystifying” VKA therapy and gaining confidence at dealing with it. Several predictors of nonadherence or noncompliance to warfarin therapy have been reported. Nonadherent patients are more likely to be younger, male, and those with poor comprehension of oral anticoagulation. In 1 study,9 social isolation, interventional fragility, and information discomfort were found to be predictors of nonadherence. Noncompliance is more likely in low-income populations and related to perceived barriers, marital status, and living arrangements.10 All these factors of poor VKA therapy management call for patient educational programs, that when applied, have been shown to improve the overall outcomes.11-13
Laboratory control
The favorable risk-benefit profile of VKA therapy is directly related to the achievement and maintenance of a therapeutic INR value. Prothrombin time varies according to the type of reagent used. The INR is a mathematically transformed value converting the prothrombin time in seconds to a standard ratio value using the International Sensitivity Index of reagent/instrument combination. The INR eliminates the differences in sensitivity of various prothrombin time reagents. An optimal laboratory control essentially means consistency and standardization in INR results. Laboratory accreditation and proficiency testing are essential for the quality of laboratory results.14
Point of care testing (POCT) for the self-determination of INR and self-management of anticoagulation is widely spread in some countries. In self-management, the patient takes an active part on determining the INR and deciding the dosage of VKAs. One review of 28 randomized trials found that self-monitoring and self-management halved thromboembolic events, but had a neutral effect on major bleeding.15 Despite the potentials of self-managed VKA therapy, it is still underused,16 especially in a setting of continuing VKA use in indications other than atrial fibrillation. It is still not well defined which subset of patients (in terms of indication for oral anticoagulation, age, comorbidity, social status, and training) might benefit more from self-management,17 but there have been attempts to identify the predicts of poor outcome18 useful to identify patients that might benefit from additional training, tight follow‐up, or even management in anticoagulation clinics. Self-management has been used to different extent in different health realities depending on the legislation. In some countries, such as Italy, because of health policy constrains and medicolegal issues, the POCT is used mainly by health care personnel such as visiting nurses in confined to bed patients. In this way. the INR obtained are centralized to the thrombosis center where an experienced physician compiles the dosage calendar for the patient. External quality control for the POCTs19 as well as training are essential for a successful monitoring.
Initiation of anticoagulation (how we start treatment with warfarin)
The initiation of warfarin therapy is one of the most delicate aspects of the long-term management of VKA therapy. It requires an appropriate starting dose, frequent INR monitoring, and proper overlap with subcutaneous low-molecular-weight heparin (LMWH) if an immediate anticoagulant effect is required (such as in venous thromboembolism). The antithrombotic effect of VKA requires up to 5 or more days depending on the time required for complete reduction of the vitamin K–dependent coagulation factors (II, VII, IX, X). To achieve a rapid antithrombotic effect, bridging with LMWH (administered concurrently) for at least 5 days until the INR reaches therapeutic levels is necessary. Reaching therapeutic INR on the first 2 to 3 days is not indicative of an effective antithrombotic treatment. Different schemes for therapy initiation have been proposed when considering age and gender,20 indication for anticoagulation,21,22 and comorbidities. Initiating therapy with 5 mg warfarin, at least in hospitalized elderly patients, resulted in better outcomes in terms of excessive anticoagulation by day 4 or 5, than a 10-mg protocol.23,24 A 10-mg dose of warfarin resulted more effective in achieving more rapidly therapeutic range without excessive anticoagulation in patients with venous thromboembolism; however, there were few elderly patients included in the study.22 Lower initial starting doses have been shown as appropriate in patients with prosthetic heart valves.21 We adopt a 5-mg protocol for the initiation of warfarin therapy. On a previous observation, we found that a 10-mg protocol for 2 consecutive days resulted in excessive anticoagulation by day 3, especially in elderly patients.25 We therefore switched to a 5-mg protocol for 4 days that resulted in a more gradual increase of the INR values. Interestingly, we found a correlation between the INR achieved on day 5 and the expected maintenance dose.26 Using the best-fit nonlinear regression curve, it was possible to calculate the weekly maintenance dose of warfarin based on INR on day 5 after 4 consecutive days on warfarin (5 mg/day). For example, if INR at day 5 is 1.5, then the weekly maintenance dose is 35 mg/week (5 mg/day).
The different response in terms of INR to a fixed dose of warfarin depends on several factors including genetics. Specifically, the involvement of CYP2C9 and VKORC1 in the warfarin pharmacology is known to influence individual warfarin response. Under these premises, several trials attempted to address the effect of genotype-guided warfarin dosing in improving warfarin dosing algorithms. Two randomized controlled trials generated different results. The Clarification of Optimal Anticoagulation Through Genetics trial27 compared an algorithm with clinical variables vs an algorithm with clinical variables and genotyping. The genetic algorithm did not significantly outperform the clinical variables algorithm. The European Pharmacogenetics of Anticoagulation Therapy–Warfarin trial,28 compared a standard fixed dose loading algorithm with a genetic-guided algorithm. The genetic-guided algorithm outperformed the fixed dose algorithm in terms of achieving a stable warfarin dose more quickly. Several factors might explain these contrasting results, including the comparator to genetic dosing.29 A fixed dose algorithm might perform worse than an algorithm, including clinical characteristics of the patients. We performed a study aimed to determine whether pharmacogenetics algorithm (based on CYP2C9- VKORC1- CYP4F2 polymorphism determination)30 was superior to our treatment scheme with standard fixed dosing.26 No significant differences were found in the number of out-of-range INR (INR < 2.0 or >3.0) (P = .79) and in the mean percentage of time spent in the therapeutic range (TTR) after 19 days in the pharmacogenetic (51.9%) and in the control arm (53.2%, P = .71).31 Genotype-guided warfarin dosing was not superior in overall anticoagulation control when compared with our standard pharmacodynamic method. With data in our hands, feasibility and cost-effectiveness32 does not justify an extensive use of genotype-based treatment algorithms. Their use might be confined in few specific situations.29,31
Maintaining therapeutic anticoagulation and computer-aided prescription
After identification of best possible maintenance dose for optimal anticoagulation, it should be maintained within therapeutic range as much as possible. The percentage of time a patient’s INR is within the desired treatment range is measured by the TTR. TTR is the best recognized indicator of VKA anticoagulation quality. TTR has been shown to correlate with thrombosis recurrence and bleeding with VKA,33 and thus is indirectly a measure of the quality of anticoagulation. Once achieved, maintenance of optimal anticoagulation may not be straightforward given the fluctuation of INR even within the same individual. Predicting future dosage in a patient requires adequate training as well as a deep knowledge of VKA pharmacology. Dedicated software can aid in determining the correct warfarin dosage based on the present and past INR. This can make dosing of warfarin easier and more efficient especially in unexperienced hands.34 Computer-based dosing is reliable in most cases, although operator intervention should not be overlooked.35 The computer decision-aided support improves the laboratory quality of anticoagulant treatment, both during long-term maintenance and in the early, highly unstable phase of treatment, and significantly reduces the number of scheduled laboratory controls.36 Importantly, the computer-aided prescription makes easily available a calendar with the patient’s daily dose, thus improving compliance and reducing dosing errors and adverse events. It also stores INR history for the patient in a repository allowing for a readily available TTR calculation.
Supratherapeutic or subtherapeutic anticoagulation (high or low INR)
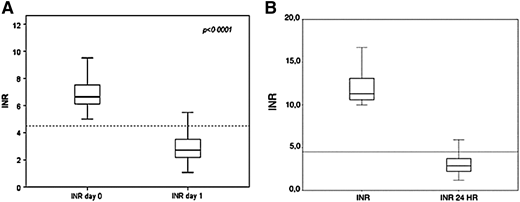
The problem of an excess anticoagulation during warfarin treatment is common in clinical practice. The main reasons for high or low INR are fluctuations in vitamin K dietary intake, interfering drugs (eg, amiodarone), incurring comorbidities, or poor compliance. Reversal of overanticoagulation can be achieved in several escalating ways, from up- or downregulation of the warfarin weekly dosage (for INR up to 4), to withholding anticoagulation and administration of vitamin K (for INR >5). No standardized regimen exists, and present regimens have not been individually compared. When vitamin K is used in nonemergency situations, small oral doses are recommended to avoid overcorrection and inducing warfarin resistance. In 2 registries, we found that oral doses of vitamin K of 2 to 3 mg are effective in correcting overanticoagulation.37,38 For patients with an INR between 5 and 10, in addition to omitting the day’s dose of warfarin, 2 mg of oral vitamin K are administered and INR values tested the following day.38 As shown in Figure 1A, the median (interquartile range) INR at presentation (day 0) fell to a safe median (interquartile range) the day after (day 1). We apply a different protocol to patients presenting with INR >10. In this case, in addition to omitting the day’s dose of warfarin, 3 mg of oral vitamin K are administered and INR tested the day after (Figure 1B). Results are reassuring because most patients had a safe INR on day 1.37
Effect of administering (A) 2 or (B) 3 mg of vitamin K to reverse overanticoagulation in asymptomatic patients or those presenting with minimal bleeding. Reprinted by permission from (A) Springer Customer Service Center GmbH: Springer Nature (Denas G, et al, J Thromb Thrombolysis, 2009) and (B) Schattauer GmbH, Thieme Group (Denas G, et al, Thrombosis Haemostasis, 2009).
Effect of administering (A) 2 or (B) 3 mg of vitamin K to reverse overanticoagulation in asymptomatic patients or those presenting with minimal bleeding. Reprinted by permission from (A) Springer Customer Service Center GmbH: Springer Nature (Denas G, et al, J Thromb Thrombolysis, 2009) and (B) Schattauer GmbH, Thieme Group (Denas G, et al, Thrombosis Haemostasis, 2009).
Moreover, the use of oral vitamin K in high-risk thromboembolic patients (such as those with mechanical heart valves (MHV) or recent cerebral ischemic events) warrants caution.
In case of low INR (0.5-1 below the lower limit), it is urgent to restore the correct anticoagulant regimen, particularly in high-risk patients (eg, those with mechanical prosthetic heart valves).39 Although the use of LMWH treatment is not evidence-based,40 in patients with mechanical prosthesis or mitral stenosis, we recommend its use at therapeutic doses for a limited amount of days (3-5). In the low-moderate risk patients, upregulation of warfarin dosage is enough to bring the INR back to therapeutic range in a short period. Thus, doubling the day’s dosage and modulating the following weekly dosage under a strict INR control might be sufficient to safely bring the INR back to therapeutic level. Because the most common cause of subtherapeutic INR is poor adherence to treatment, a conversation face-to-face to remind the importance of correct intake of warfarin is also advisable.12
Bridging anticoagulation
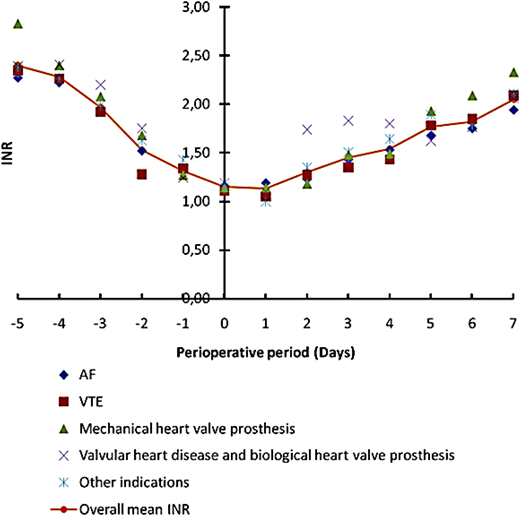
This term defines the introduction of a short-acting anticoagulant (LMWH) during warfarin interruption for a 10- to 12-day period when the INR is not within the therapeutic range to minimize the risk of thromboembolism. Several prospective cohort studies were performed looking at thromboembolic and bleeding events during bridging therapy either at therapeutic or subtherapeutic regimens. In a multicenter prospective inception cohort management study, we assessed the efficacy and safety of an individualized bridging protocol applied to outpatients.41 An oral anticoagulant, warfarin or acenocoumarol, was stopped 5 days before the procedure and LMWH was started 3 to 4 days (in case of warfarin or acenocoumarol, respectively) before surgery. Anticoagulants were not administered at the day of surgery. LMWH was started the day after surgery with a dosage of 70 anti-factor Xa U/kg twice daily in high-thromboembolic-risk patients and prophylactic once-daily doses in moderate- to low-risk patients. Oral anticoagulation was also resumed the day after the procedure with a 50% increased dosage for 2 days and maintenance doses afterward. As shown in Figure 2, INR levels declined to intervention-safe levels on day 0 and recovered correctly after warfarin reintroduction. The bridging protocol appeared feasible, effective, and safe for most patients. It showed that tailoring the bridging regimen to thromboembolic risk and using subtherapeutic doses (70%) of LMWH was effective and safe. To conclusively establish its safety in patients with mechanical prosthetic valves, we performed a further cohort study.42 This study included bearers of MHV in the mitral position, or in the aortic position associated with risk factors. We found that bridging with subtherapeutic doses of LMWH in high-risk MHV patients is effective, although complicated by nonfatal major bleeding. Whenever major bleeding occurred, it was related to the surgical setting or to surgery’s bleeding risk.42 A recent clinical trial compared bridging therapy with heparin with a strategy of continued warfarin treatment at the time of pacemaker or implantable cardioverter–defibrillator surgery (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) and showed that warfarin continuation markedly reduced the incidence of clinically significant device-pocket hematoma.43 The Effectiveness of Bridging Anticoagulation for Surgery study randomized patients with atrial fibrillation undergoing elective operation or invasive maneuvers to bridging therapy (dalteparin 100 IU per kg twice daily) or placebo.44 Forgoing bridging anticoagulation was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding; however, most patients were at low thromboembolic risk. The latest guidelines of American College of Chest Physicians suggest (grade 2C) the use of bridging with LMWH only in patients with a mechanical heart valve, atrial fibrillation, or venous thrombosis at high risk for thromboembolism.45 High-level evidence on bridging has started to build for low thromboembolic risk patients and low bleeding risk procedures. Oral anticoagulation should not be interrupted for low risk procedures. Patients at high thromboembolic risk should undergo bridging, especially if at low bleeding risk. In this setting, subtherapeutic doses of LMWH were shown to be safe and effective. A tailored approach should be reserved for each patient balancing thromboembolic and bleeding risk.
Declining of INR values during the 5 days of warfarin suspension before surgery/intervention. Different colors denote different warfarin indications. The orange line show the mean INR values for all indications. Warfarin was resumed at a 50% increased maintenance dose for 2 days starting the first or second day after surgery/intervention. Therapeutic INR values were usually reached after 1 week. AF, atrial fibrillation; VTE, venous thrombosis.
Declining of INR values during the 5 days of warfarin suspension before surgery/intervention. Different colors denote different warfarin indications. The orange line show the mean INR values for all indications. Warfarin was resumed at a 50% increased maintenance dose for 2 days starting the first or second day after surgery/intervention. Therapeutic INR values were usually reached after 1 week. AF, atrial fibrillation; VTE, venous thrombosis.
Management of intake or suspension of interfering drugs
Drug–drug interaction using warfarin are frequently reported in the literature.46,47 Although clinically relevant drug interactions (Table 2) are few,48 amiodarone, a drug widely used in patients with atrial fibrillation, determines an excess of anticoagulation that requires a reduction of the amount of warfarin of 25% to 50%. The opposite happens at the suspension that should be carefully monitored given the long half-life of this drug (90 days). Other commonly used medications that interfere with warfarin therapy are azole antibiotics, macrolides, quinolones, and nonsteroidal anti-inflammatory drugs, including selective cyclooxygenase-2 inhibitors, selective serotonin reuptake inhibitors, omeprazole, fluorouracil,46 and rifampicin. Whenever possible, coadministration with these agents should be avoided or INRs should be closely monitored.
Improvement of quality of anticoagulation across anticoagulation clinics
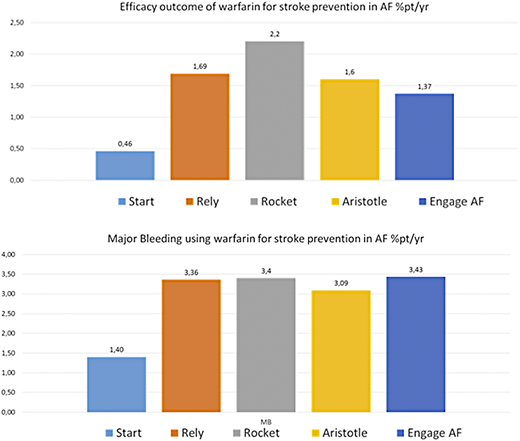
VKA have been used for more than 60 years. Because of the complexity of the therapy, efforts have been made worldwide to improve management. The introduction of anticoagulation clinics has had a positive impact on oral anticoagulation management, and represent the best management model.49 Anticoagulation clinics have a central role in determining appropriate indications for anticoagulation, initiating and monitoring treatment through laboratory tests (INR), determinations of time intervals for testing, and dosage modifications, providing indications for therapy interruptions and bridging therapy. They also have a central role on education of patients and health care personnel. In Italy, the Italian Federation of Centers for surveillance of antithrombotic treatments annually publishes updated recommendations on oral anticoagulant treatment, including the management of specific clinical situations, and invites members to participate in an educational program aimed at providing accreditation for the anticoagulation center. This educational effort has been translated to overall satisfactory levels of TTR control across the country (Figure 3).14 The satisfactory levels of quality of anticoagulation with VKA gives very good results50,51 in terms of stroke prevention in atrial fibrillation even when compared with the DOAC registration studies (Figure 4). However, even under the premises of good TTR, DOAC maintain the superior safety profile halving the incidence of intracranial bleeding.52
Boxplot of center TTR by study year (reproduced from Tosetto et al.14 doi: 10.1371/journal.pone.0145318 under Creative Commons Attribution [CC BY] license).
Boxplot of center TTR by study year (reproduced from Tosetto et al.14 doi: 10.1371/journal.pone.0145318 under Creative Commons Attribution [CC BY] license).
Efficacy and safety of warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Results from the Survey on Anticoagulated Patients Register compared with those using direct oral anticoagulants in registration studies.
Efficacy and safety of warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Results from the Survey on Anticoagulated Patients Register compared with those using direct oral anticoagulants in registration studies.
In the Internet era, efforts have been made worldwide to provide patients and health care personnel with updated knowledge and tools on oral anticoagulation treatment and self-management.53-55
Consensus (hospital- or regional-based) treatment decisional pathways
The quality of treatment improves through homogenous management of the therapy. A robust common pathway for outpatient care offers clear, evidence-based guidance for the management of patients receiving VKA. It includes the correct identification, diagnosis, and treatment of patients followed in an outpatient setting providing references to health care personnel not directly involved in this setting. A common pathway avoids misunderstanding among all specialists involved in the patient care. Consensus on VKA treatment modalities should be reached at hospital and ideally at regional level.
Conclusions
Oral anticoagulation therapy with VKA remains the mainstay therapy for the prevention and treatment of thromboembolism in certain indications and socioeconomic settings. Because the management of VKA treatment is complex, its success heavily relies on the quality of treatment. An optimal quality of treatment with VKA involves initiating appropriate therapy, maintaining therapeutic anticoagulation measured through TTR, monitoring anticoagulation at the appropriate frequency, managing perioperative dosing, and managing nontherapeutic INR, which is best performed in anticoagulation centers. Optimal quality of treatment also involves continuous patient education and extensive communication.
Correspondence
Vittorio Pengo, Cardiology Clinic, Department of Cardiac, Thoracic, and Vascular Sciences, Padua University Hospital, Via Giustiniani 2, 35121 Padua, Italy; e-mail: vittorio.pengo@unipd.it.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.

![Figure 3. Boxplot of center TTR by study year (reproduced from Tosetto et al.14 doi: 10.1371/journal.pone.0145318 under Creative Commons Attribution [CC BY] license).](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2018/1/10.1182_asheducation-2018.1.332/2/m_hem01845f3.png?Expires=1767846944&Signature=cVDMHk3bEa3G-wVGHA2Us2b5zgErZpev3m4l21e~zY~U~QvtWvj~JIlA2RxsqMfYKzbgq9BtNLDYLHcKa3kmpZBiCWo8exBWbIvXXHZWbgFDN6k9fW0HlVGW-0F9NaWY9-MMVEmdrX4GZi-DpW0GTQHHo8JIr2CS60CnwLih~~l-Z-0pu-KKUyNKWpVpVuTgIorWIsV5xNsmVCfko-wGjCaNHbNcLlhm2DatRnd-fVWkys51KAUfJs9C~wCxh514~GvIKMe~ajYDASWia0dp-UKKQ9aRxVYmU2AzGNBoW7ZLM5LLSGrGfegMjGZo-MlcKKZm4n7q-k~s8upkSJt2SQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)