Abstract
Myeloma patients not eligible for clinical trials have many treatment options. Choosing the next best therapy starts with careful assessment of the biology and dynamics of the disease at relapse, as well as the condition and situation of the patient. Fit patients should be considered for triplet regimens, whereas intermediate and frail patients warrant dose-reduced triplets or doublets. An indolent serologic relapse may be treated with dose intensification, especially in a maintenance situation, whereas a rapid relapse requires a more aggressive approach with drug class change or a second-generation immunomodulatory drug (IMID) or proteasome inhibitor (PI). Monoclonal antibodies, in combination with PIs and IMIDs, have proven highly efficacious in early and late relapse. Key elements of supportive care include infection prevention, bone health, thromboprophylaxis, and management of active symptoms, such as pain and distress.
Learning Objectives
Understand treatment approaches in frail and high-risk relapsed multiple myeloma patients
Choose the optimal and evidence-based treatment for relapsed and/or refractory multiple myeloma patients
Introduction
The treatment of relapsed and refractory multiple myeloma is challenging due to the heterogeneity of the disease and patients and the plethora of effective agents and the ways in which they can be used and combined. Monoclonal antibodies have now become firmly incorporated among the other cornerstones of therapy: proteasome inhibitors (PIs), immunomodulatory drugs (IMIDs), alkylators, and steroids. Here, we review recently reported combinations and provide our approach to refractory disease. Our focus is on patients not eligible for a clinical trial; thus, we primarily discuss US Food and Drug Administration (FDA)-approved agents.
Clinical case
A 65-year-old man with essential hypertension presented with anemia, hypercalcemia, and back pain. He was diagnosed with immunoglobulin G (IgG) κ multiple myeloma, Revised International Staging System stage II, and without high-risk chromosomal abnormalities on CD138-selected fluorescent in situ hybridization (FISH). He was treated with bortezomib (btz), lenalidomide (len), and dexamethasone (dex), along with zoledronic acid for bone disease and aspirin for thromboprophylaxis. He received 4 cycles of induction therapy, and tolerated it well, attaining a very good partial response (VGPR). He then underwent autologous stem cell transplantation (ASCT), followed by initiation of len for maintenance treatment. He subsequently attained a complete response (CR). Minimal residual disease (MRD) testing was not performed. Eighteen months following the start of maintenance, he presented to the emergency department with chest and back pain and was diagnosed with a myocardial infarction, requiring placement of 3 stents. During hospitalization, he was noted to have anemia, and additional workup found recurrence of his IgG κ M-protein, at 1.6 mg/dL, and new lytic bone lesions in his thoracic spine and pelvis. Repeat bone marrow sampling demonstrated 30% clonal plasma cells, and FISH and karyotype did not demonstrate high-risk features.
Is this patient really not eligible for trial?
An estimated 2% to 5% of adult cancer patients participate in clinical trials. A meta-analysis examining clinical trial enrollment across oncology found that the biggest barrier to enrollment was trial availability; 56% of cancer patients did not enroll because no trial was available at their centers. When a trial was available, an additional 22% could not enroll because they were not eligible, and another 15% did not enroll because of patient or physician decision making.1 Arguments have been made for broadening eligibility criteria (eg, to include HIV-infected patients who are healthy and at low risk for AIDS-related outcomes) to make trials more inclusive and generalizable.2 Although the patient in our vignette would not be considered a trial candidate because of unstable cardiovascular function, he may become eligible for trials once his cardiac condition stabilizes. We strongly encourage consideration of clinical trials upon detecting relapse and referrals to local centers that have trials when practical.
Assessments at relapse
Evaluations at relapse include serum and urine analyses, as well as total body imaging (Table 1). Recent data from the Spanish Myeloma Group suggest that patients who do not have light chain–only disease (ie, those with myeloma that make intact Ig) can be assessed and followed with serum immunofixation without urine immunofixation (thus avoiding 24-hour urine collection).3 Bone marrow biopsy should be considered but may not always provide information that impacts treatment. Our approach to assessing the patient and disease biology is summarized in Table 2 and discussed further below.
Patient-related considerations
Frailty.
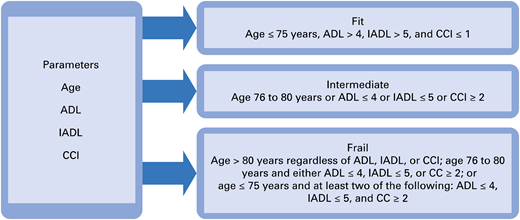
We believe that it is important to assess the patient’s frailty through a careful history and physical examination. Age or performance status scores do not fully capture the comorbidities and functional limitations of patients.4 The International Myeloma Working Group (IMWG) has developed a model of frailty based on age, comorbidities (Charlson Comorbidity Index), ability to conduct basic self-care (activity of daily living [ADL] instrument), and ability to conduct more complex activities required for independent living (instrumental ADL instrument) (Figure 1). This frailty score predicted for mortality (hazard ratio [HR], 1.61 in intermediate; HR, 3.57 in frail), incidence of grade ≥ 3 nonhematologic toxicity (HR, 1.74 in frail), and treatment discontinuation (HR, 2.21 in frail) in elderly myeloma patients (median age 74 years in the study cohort).5 A number of other frailty models can be used to predict survival and/or likelihood of toxicity.4 As a general principle, fit patients are suitable for full-dose therapy with 3-drug combinations, and one should be careful to not undertreat them. Intermediate and frail patients should receive dose reductions (Table 3) or be given 2-drug regimens (doublets), and an approach of starting low and increasing as tolerated may reduce cumulative toxicity.
Framework for assessing frailty. CCI, Charlson Comorbidity Index; IADL, instrumental ADL. Reprinted with permission from Larocca and Palumbo.80
Framework for assessing frailty. CCI, Charlson Comorbidity Index; IADL, instrumental ADL. Reprinted with permission from Larocca and Palumbo.80
Comorbidities and prior toxicities.
Peripheral neuropathy is most commonly associated with btz (5%-8% grade 3/4 in pivotal trials6,7 ) and thalidomide (thal; 10%-13% grade 3/4 in pivotal trials8,9 ). To reduce the incidence and severity, btz can be given once weekly and in subcutaneous form, although it is important to note that once-weekly dosing has not been compared with twice-weekly dosing in a prospective randomized trial. Thal can be dose reduced. If neuropathy is severe, it may be best to avoid these agents. Heart failure and hypertension have been linked to carfilzomib (cfz) and should be avoided in patients with significant heart failure or uncontrolled hypertension. Cytopenias occur with many therapies but can be prominent with IMIDs and limit dosing. Rashes are frequently seen upon introduction of IMIDs and can be managed with topical corticosteroids and/or oral antihistamines. More severe cases may require systemic steroids and holding the IMID, and desensitization can be considered for patients at high risk for recurrence.10 Given their efficacy at myeloma control, we urge practitioners not to abandon IMIDs prematurely because of rash. Steroid-induced hyperglycemia can be managed by reducing the dose of steroid, oral hypoglycemic agents, or insulin.
Practical limitations.
Difficulties with travel make frequent visits for infusional therapy less practical, although travel and lodging resources are often available and should be explored. Patients without insurance or with Medicaid are more likely to present with advanced disease and are less likely to undergo treatment.11 High out-of-pocket costs can limit access to oral drugs, such as len,12 and although copayment assistance programs help to fill part of the gap, those who use them can have delayed initiation.13 Cognitive impairment may make it more difficult for the patient to adhere to a complex treatment regimen or to take pills consistently. Many patients rely on caregivers, who are at risk for burnout.
Disease-related considerations
High-risk disease assessment.
The IMWG defines high-risk myeloma as disease that contains ≥1 of the following findings: t(4;14), t(14;16), t(14;20), del(17/17p), or gain(1q) on FISH, nonhyperdiploid or del(13) on karyotype, and a high-risk signature on gene-expression profiling.14 A more recent analysis that performed whole-exome sequencing on 1273 newly diagnosed myeloma patients identified 2 subsets of patients at particularly high risk (median progression-free survival [PFS], 15.4 months; overall survival [OS], 20.7 months): biallelic TP53 inactivation or amplification of CKS1B (1q21), which was worse than 1q gain.15 Additional features that suggest high-risk disease include plasma cell leukemia, extramedullary disease, and poor response (limited depth or brief duration) to prior therapy. High-risk disease is itself a heterogeneous entity but, generally speaking, patients with the above features are less likely to achieve durable responses. We try to consider these patients for clinical trials; if trials are not an option, we favor treating them with combinations containing a PI and IMID and/or an antibody. Many of the recent 3-drug (triplet) regimens that we review below demonstrate efficacy in high-risk patients (Tables 4 and 5).
Disease biology.
An estimated 2% to 4% of myeloma patients develop plasma cell leukemia during their disease trajectory,16 and up to 30% of myeloma patients develop extramedullary disease.17 Both of these entities are associated with poor prognosis; in fit patients, we would consider changing therapy significantly to one of the following options: a new triplet with agents that the patient has not been exposed to, possibly with daratumumab (dara) in addition,18 or dex, cyclophosphamide, etoposide, cisplatin (DCEP), or VDT-PACE, or stem cell transplantation. Another important entity to monitor for during the course of myeloma treatment is amyloid light-chain (AL) amyloidosis. A Mayo Clinic study of 4319 myeloma patients between 1990 and 2008 who had ≥6 months of follow-up identified 47 patients who were subsequently diagnosed with amyloid,19 although the population incidence remains unclear.20 Diagnosing amyloid can shift treatment in important ways: change the target for hematologic response to be at least VGPR (defined by a difference between the involved and uninvolved free light chain < 4 mg/dL), greater caution in using IMIDs,21 consideration of novel agents (eg, anti-amyloid antibody CAEL-101, which is in clinical testing,22 and doxycycline, which has retrospective evidence23 and is under prospective testing), and greater attention to monitoring end-organ function.
Dynamics of relapse.
The dynamics of relapse should help to guide therapy, and 3 patterns are commonly seen. One is an indolent biochemical relapse that typically presents late after last therapy (>2 years) and manifests as a slow increase in the monoclonal protein. The doubling time of disease is relatively long, and there is a significant time gap between serologic relapse and end-organ dysfunction. These relapses may be observed for a few months or, if the patient is on treatment, managed by an increase in dose, possibly with addition of dex. A second pattern is early relapse without high-risk features. In these cases, the reappearance of monoclonal protein is typically earlier (<2 years from previous therapy), and the rate of progression is more rapid, although aggressive features, such as rapid end-organ damage or extramedullary disease, are absent. Here, we start therapy earlier and use triplet combinations. A third pattern is early relapse with high-risk features. These are typically seen later in the course of disease in most patients, with the exception of those with high-risk features at the time of diagnosis. Patients frequently have high-risk FISH or karyotype findings (described above). For these patients, we rapidly start multiagent regimens (triplet or quadruplet), with drugs to which the patient has not been exposed in the past. For patients with resistance to most novel agents, we consider regimens such as DCEP or VDT-PACE (btz, dex, thal, cisplatin, doxorubicin, cyclophosphamide, etoposide) (discussed in Additional therapies section), followed by second autologous transplant.
Refractoriness.
Refractory myeloma can be subdivided into 2 entities: relapsed and refractory and primary refractory. Relapsed and refractory myeloma is a disease that has not attained at least minor response while on salvage therapy or that has progressed within 60 days of last therapy. Primary refractory myeloma is disease that has not attained at least a minor response with any therapy. Separately, relapsed myeloma is disease that has progressed and requires initiation of salvage therapy but does not meet the criteria for relapsed and refractory or primary refractory myeloma.24 It is essential to review the patient’s treatment history carefully to identify which agents his or her disease has become refractory to. Patients who have become refractory to 1 PI or IMID may respond to other agents within the same class, albeit typically with lower frequency and depth of response than someone who is naive to that class.
Clinical case continued
Our patient is recovering well from his myocardial infarction and is not limited in his ADLs or instrumental ADLs. By the IMWG model for frailty, he was considered fit. He tolerated his prior therapies well and did not develop peripheral neuropathy with btz. He had good family support, was insured, and had no travel limitations. Although his remission was notably shorter than observed in the IFM 2009 study (median PFS was 50 months in the btz/len/dex and ASCT arm25 ), our repeat bone marrow sampling did not find features of high-risk disease, and he did not have symptoms to raise concern for extramedullary relapse or AL amyloidosis. He relapsed with myeloma-related symptoms, worsening anemia, and bone disease, which warranted a change in therapy. Because he relapsed while on len, he was considered refractory to len, and we discussed treatment options outside of clinical trials with him.
Treatment options
There are multiple ways to conceptualize and organize studies of relapsed and refractory myeloma. Here, we organize studies based on the refractoriness of their patient populations (ie, refractory to IMID, refractory to PI, dual refractory, and later) to help clinicians select evidence-based options pertinent to the circumstances of their patient. Summaries of the studies we review are provided in Tables 4 and 5. We also identify our preferred regimens in refractory populations and in some special scenarios in Table 6.
Many of the phase 3 studies discussed below compare triplet regimens with doublets. In these comparisons, triplets have invariably shown superior response rates (RRs) and PFS, with, importantly, acceptable toxicity. In 2 studies (ASPIRE and ELOQUENT-2), triplets have provided an OS advantage. Given these favorable results, we recommend triplet therapy for patients who are fit and have limited comorbidity, as well as for disease that is high risk, rapidly progressive, or heavily refractory. That said, in other circumstances (eg, frail patients, indolent relapse), doublets retain their place and can often be used to good effect.
Early relapse, refractory to IMID
This is the most common initial relapse seen in the United States, given that a majority of patients are on len maintenance therapy. Approaches to consider include adding a PI, changing to a PI, changing to a PI and antibody, changing to a PI and alkylator, or switching to a second-generation IMID, such as pomalidomide (pom). If the relapse is indolent and biochemical only, then increasing len dose, with or without the addition of dex, is also an option.
Ixazomib-based regimens.
Although btz has been in use the longest and is a good choice, ixazomib (ixa), an oral inhibitor of the 20S proteasome, is convenient to dose and associated with less neuropathy. Ixa can be added to IMID regimens. The TOURMALINE-MM1 study compared ixa/len/dex with placebo/len/dex (n = 722). Median prior therapies was 1; 55% of the study cohort was IMID exposed, and 12% were thal refractory; 69% had btz exposure, but PI-refractory patients were excluded. The ixa arm had a higher overall response rate (ORR; defined as partial response [PR] or better) (78% vs 72%) and CR rate (12% vs 7%). Median PFS was superior in the ixa arm (20.6 vs 14.7 months), but not in the subsets of patients who were IMID exposed or thal refractory. The ixa arm had more thrombocytopenia, rash, and gastrointestinal toxicity.26,27 In frail patients or those who are not tolerating an IMID, ixa can substitute for the IMID. The combination of ixa/dex was examined in a phase 2 study (n = 35 at the 4-mg dose) in a population with a median of 4 prior regimens and in which 46% of the cohort was len refractory (none were PI refractory). ORR was 31%, CR rate was 3%, and RR in len-refractory patients was 31%.28 Given the modest benefits seen with ixa in these studies, we favor its use in patients with indolent relapse or in those who may have difficulty tolerating btz or cfz.
Carfilzomib.
Cfz is an irreversible inhibitor of the proteasome that produces more sustained inhibition than btz. As with btz and ixa, cfz can be used by itself (with or without steroid) or with an IMID. ENDEAVOR compared cfz 56 mg/m2 and dex with btz biweekly and dex (n = 929). Median prior regimens was 2; 73% of the study cohort was IMID exposed, and 25% was len refractory. The cfz arm had higher ORR (76% vs 63%), CR rate (13% vs 6%), median PFS (18.7 vs 9.4 months),29 and OS (47.6 vs 40.0 months).30 In len-refractory patients, PFS was not improved.29 We more commonly give cfz in conjunction with an IMID, as in the ASPIRE study, which compared cfz/len/dex with len/dex (n = 792). Median prior therapies was 2, and 22% of the study cohort was len refractory. The cfz arm had a higher ORR (87% vs 67%), CR rate (32% vs 9%), median PFS (26.3 vs 17.6 months),31 and OS (48.3 vs 40.4 months).32 In the len-refractory patients, cfz use was associated with improved PFS (HR, 0.64).31 In both studies, the cfz arm had higher rates of hypertension, heart failure, and respiratory symptoms.
Daratumumab.
This CD38-targeted IgG κ monoclonal antibody exerts direct tumor cytotoxicity and also has immunomodulatory effects. The CASTOR trial demonstrated the utility of dara in early IMID-exposed/refractory relapse. Here, dara/btz/dex was compared with btz/dex (n = 498). Median prior lines was 2 (range, 1-10); 76% of the study cohort was IMID exposed, and 21% was len refractory at their last line of therapy. Of note, there was treatment imbalance between the arms: the btz/dex arm stopped treatment after 8 cycles, whereas the dara arm continued on single-agent dara until progression. Dara use yielded higher ORR (83.8% vs 63.2%), CR rate (28.8% vs 9.8%), and median PFS (16.7 vs 7.1 months), including in the subsets of IMID-exposed and len-refractory (9.3 vs 4.4 months) patients. The dara arm had more grade 3/4 neutropenia (14% vs 5%) and thrombocytopenia (46% vs 33%) and more all-grade upper respiratory infections (31% vs 18%).33
Elotuzumab.
This IgG1 antibody targets signaling lymphocytic activation molecule F7 and induces myeloma cell death by activating natural killer cells or mediating antibody-dependent cellular cytotoxicity. A comparison of elotuzumab (elo)/btz/dex with btz/dex (n = 152; median prior lines, 1; 74% IMID exposed; refractoriness not described) found comparable ORR (66% vs 63%), CR rate (4% vs 3%), and median PFS (9.7 vs 6.9 months, P = .09). In the subset of IMID-exposed patients, no difference in PFS was noted.34 Combining elo with IMIDs (discussed below) appears more compelling.
Pomalidomide.
Patients refractory to len can switch to pom. Studies of pom/dex and pom/cfz/dex are reviewed in the dual-refractory section, because a majority of patients in those studies were refractory to btz and len. Pom can be combined with btz and dex, and this combination was evaluated in a phase 1/2 study (n = 50). Median prior regimens was 2; all patients were len refractory, and 17% was btz refractory. ORR was 86%, CR or better rate was 22%, and median PFS was 13.7 months.35 A phase 3 trial is comparing this regimen with btz/dex in relapsed and refractory patients (NCT01734928).
Bendamustine.
Alkylating agents, such as cyclophosphamide (with btz as in the CyBorD regimen36 or with cfz [NCT03336073]) and bendamustine, have activity in IMID-refractory disease. A phase 2 study examined bendamustine (70 mg/m2 on days 1 and 8), btz (biweekly), and dex in 75 patients. Median prior therapies was 1, 32% was refractory to IMIDs, and 47% had prior exposure to btz (but none were refractory). ORR was 77%, CR rate was 20%, and median PFS was 15.5 months. Refractoriness to IMIDs did not affect response, whereas prior btz use was associated with lower time to progression (9 vs 17 months).37 Another phase 1/2 study examined the combination of bendamustine (75 mg/m2 on days 1 and 2), len (10 mg, days 1-21), and dex (n = 29). Median prior therapies was 3, 97% of the study cohort had IMID exposure, and 52% was refractory to len and/or thal. ORR was 52%, best response was VGPR in 24%, and median PFS was 6.1 months. In the subset of IMID-refractory patients, RR was 69%.38 Grade 3/4 neutropenia and thrombocytopenia were the most prominent toxicities in both studies, particularly with the bendamustine/len combination. In our practice, we typically use bendamustine in dual-refractory or later relapse.
Early relapse, refractory to PI
Patients in this category have been exposed to PIs and may have developed refractoriness. Common approaches in this setting include changing to an IMID, adding an IMID, changing to an IMID and antibody, and switching to a different PI.
Lenalidomide.
Len/dex can be used in patients who are frail or have indolent relapse. Len can sensitize myeloma cells to btz, and the combination of len/btz/dex was examined in a phase 2 multicenter United States study of 64 patients (53% were btz exposed, 42% with refractory myeloma). Of note, btz was given IV (1.0 mg/m2 biweekly), and len was used at a reduced dose (15 mg). ORR was 64%, with VGPR or better rate of 28%, and mean PFS was 9.5 months.39 Len can also be combined with cfz and dex, as in the ASPIRE study above. In a phase 2 study of cfz/len/dex (n = 52), in which 25% of the study cohort was btz refractory, ORR was 77% (VGPR, 37%), RR was 69% in btz-refractory patients, and median PFS was 15.4 months.40
Daratumumab.
Dara appears to synergize particularly well with IMIDs. POLLUX compared dara/len/dex with len/dex (n = 569). Median prior lines of therapy was 1; 84% was btz exposed, and 21% was btz refractory (len-refractory patients were excluded). The dara arm had higher ORR (93% vs 76%), CR rate (51% vs 21%), and, notably, MRD-negative CR (26% vs 6.4%). At a median follow-up of 25.4 months, median PFS was not reached in the dara arm vs 17.5 months. In the subset of btz-refractory patients, RR was 88% in the dara arm vs 68%, and median PFS was 26.1 in the dara arm vs 11.3 months. There were more cases of neutropenia and pneumonia in the dara arm.41
Elotuzumab.
ELOQUENT-2 compared elo/len/dex with len/dex (n = 646). Patients had a median of 2 prior therapies, and refractoriness was not described, although 70% were btz exposed, 48% were thal exposed, and 6% were len exposed. The elo arm had higher ORR (79% vs 66%), although with fewer CRs (4% vs 7%). It also had superior median PFS (19.4 vs 14.9 months)42 and OS (48 vs 40 months). In btz-exposed patients, use of elo improved PFS (HR, 0.66). The elo arm had more grade 3/4 lymphopenia (79% vs 49%).43
Carfilzomib.
Clinical case continued
Our patient had multiple treatment options: change to a PI (btz or ixa), add a PI and increase len dose, change to a dara-based regimen, change to a pom-based regimen, or change to a PI and alkylator. Given his recent myocardial infarction, we avoided cfz. Among the many options, we favored dara/btz/dex on the basis of the high efficacy rates seen in the CASTOR study, which had a substantial number of CRs or better. Our patient is relatively young and remained fit; thus, our goal was to maximize the depth and duration of his next remission. We would ensure the patient receives zoster prophylaxis (recommended for patients on dara) and a bone-modifying agent (BMA), the latter because of his new lytic lesions. If the patient strongly preferred an oral regimen, we would consider ixa/pom/dex (discussed in Dual-refractory and later relapse section) because response rates will likely be higher than with ixa/len/dex.
The patient started on dara/btz/dex. He initially tolerated therapy well, but then developed a progressive painful neuropathy in his feet and hands that began to affect his walking. By this time, he had completed 7 cycles, and his btz was stopped. Repeat serum, urine, and bone marrow sampling indicated that he had attained a CR (determined with the help of the dara interference reflex assay46 ), flow MRD positive. His cardiovascular status had remained stable, without development of heart failure. He was maintained on monthly dara and did well until 12 months later (19 months since the start of current therapy, 44 months since diagnosis), when his M-protein returned and he was noted to have a mild anemia. Positron emission tomography–computed tomography at this time revealed mild uptake throughout the axial skeleton, without new lytic bone disease.
Dual-refractory and later relapse
Dual-refractory patients have become refractory to a PI (typically btz) and IMID (typically len in the United States). Outcomes in this population have historically been poor; an IMWG international retrospective study of 543 dual-refractory patients diagnosed between 2006 and 2014 found a median OS of 13 months and PFS to the next regimen of 5 months.47 Approaches to consider include antibody with a second-generation PI or IMID, changing the PI and/or IMID to ones previously not used, an alkylator-based regimen, or a histone deacetylase inhibitor–based regimen.
Daratumumab.
If not used previously or if patients are not dara refractory, a dara-based combination is a preferred option in dual-refractory or later disease, because remissions tend to be deeper than with other regimens, and tolerability is good. EQUUELEUS was a phase 1b study that evaluated dara/pom/dex in 103 patients. Median prior therapies was 4; 80% of the study cohort was refractory to a PI (including 30% to cfz), 89% was refractory to len, and 71% was refractory to both PI and IMID. ORR was 60%, CR or better rate was 17%, and RR was 58% in the subset of dual-refractory patients. Median PFS was 8.8 months, and OS was 17.5 months. Grade 3/4 neutropenia was a prominent side effect of this regimen (77% of patients).48 MMY1001 was a phase 1b study examining dara/cfz/dex in patients with previous btz and IMID use (n = 85; 60% len refractory, 29% dual refractory). Cfz was given at 70 mg/m2 once weekly. ORR was 84% with CR rate of 33%; for the dual-refractory patients, 12-month PFS was 58%.49
Beyond dual-refractory disease, dara has demonstrated activity as a single agent in late relapse, although it is not typically used as a single agent in the United States. As a single agent in a very refractory population (n = 42 for 16-mg/kg group, 64% dual refractory, 48% triple refractory, 19% quad refractory), ORR was 36%, and RR was 33% in dual-refractory patients, 30% in triple-refractory patients, and 38% in quad-refractory patients.50 In combination with dex in the phase 2 SIRIUS study (n = 106 for 16-mg/kg group, 82% dual refractory, 66% triple refractory, 31% quad refractory), ORR was 29%, and RR was 26% in dual-refractory patients, 29% in triple-refractory patients, and 21% in quad-refractory patients. Median PFS was 3.7 months, and OS was 17.5 months.18
Elotuzumab.
Elo was examined with pom/dex in a predominantly dual-refractory population and demonstrated good activity. ELOQUENT-3 compared elo/pom/dex with pom/dex (n = 117) in patients with a median of 3 prior lines of therapy, among whom 70% were refractory to len and PI. The elo arm had higher ORR (53% vs 26%) and PFS (10.3 vs 4.7 months) and was well tolerated (safety comparable to the pom/dex arm). In the subset of dual-refractory patients, elo/pom/dex improved PFS relative to pom/dex (HR, 0.56).51
Carfilzomib.
Cfz and its combinations have demonstrated activity in dual-refractory disease. ARROW compared cfz given at 70 mg/m2 once weekly with the more conventional cfz 27 mg/m2 twice weekly, and both arms received dex (n = 478). The number of dual-refractory patients was not provided, but 42% of the study cohort was refractory to btz, 74% was refractory to len, and 18% was refractory to thal. The once-weekly arm had higher ORR (63% vs 41%) and PFS (11.2 vs 7.6 months) and did not appear to be associated with more toxicity.52 The combination of cfz/pom/dex has also shown promise. A phase 1 study of this regimen (n = 32; cfz given at 27 mg/m2 biweekly) in a cohort in which 91% of patients were dual refractory found an ORR of 50%, although most were PRs. Median PFS was 7.2 months.53 Another phase 1/2 study also examined cfz/pom/dex but gave cfz once weekly at 27 mg/m2. Among 47 patients treated at the maximum tolerated dose, 100% were refractory to len, and 45% were dual refractory (len and btz). ORR was 62%, and RR was 71% in dual-refractory patients. Median PFS was 10.3 months.54
Ixazomib.
Ixa/pom/dex appears to have some activity in dual-refractory disease. A phase 1/2 study in 25 patients (all refractory to len, 56% dual refractory) found an ORR of 48%, with best response being VGPR in 20%. Median PFS was 8.6 months. RR in the dual-refractory patients was 29%.55
Pomalidomide.
Several studies have demonstrated the efficacy of pom/dex (without a PI) in dual-refractory patients. MM-003 compared pom and low-dose dex (40 mg once weekly) with high-dose dex alone (40 mg daily on days 1-4, 9-12, and 17-20). Of 455 enrolled patients, 94% were refractory to len, 79% were refractory to btz, and 74% were refractory to both. The pom arm had higher ORR (31% vs 10%), median PFS (4 vs 1.9 months), and OS (12.7 vs 8.1 months). In the subset of dual-refractory patients, use of pom improved RR (28% vs 12%) and PFS (3.7 vs 2 months) but not OS.56 MM-010 was a single-arm phase 3b study examining pom/dex in 682 patients, 80% of whom were dual refractory. Results were similar to that of MM-003: ORR was 33%, median PFS was 4.6 months, OS was 11.9 months, and RR in dual-refractory patients was 32%.57 In fit patients, we prefer to use pom in a triplet because of the higher response rates, as seen with the cfz/pom/dex studies discussed above.
Cyclophosphamide.
Cyclophosphamide has direct antimyeloma activity but also immune-stimulatory activity. It can be given IV or orally and at weekly or daily dosing intervals. A phase 1/2 study (REPEAT) examined len, cyclophosphamide, and prednisone in 82 patients, all of whom were refractory to len, and 66% were also refractory to btz (dual refractory); 57% had prior cyclophosphamide exposure. Cyclophosphamide was given at a metronomic dosing of 50 mg daily. ORR was 67%, median PFS was 12.1 months, and OS was 29 months. In dual-refractory patients, RR was 60%. Infection was a notable toxicity of this regimen, with grade 3-5 infection occurring in 21%.58 Cyclophosphamide was used in combination with pom/dex in a phase 2 study (n = 34, arm C) in which all patients were dual refractory to len and a PI. ORR was 65%, and median PFS was 9.5 months.59
Panobinostat.
Panobinostat (pano), a pan-deacetylase inhibitor that affects the aggresome protein–degradation pathway, showed synergistic cytotoxicity with btz in preclinical models. PANORAMA 1 compared pan/btz/dex with placebo/btz/dex in patients with early relapse (n = 768, median prior lines 1); it found a 3.9-month PFS benefit but no OS benefit.60 PANORAMA 2 evaluated pano/btz/dex in a more refractory population (n = 55, median prior therapies 4, all refractory to btz and had prior IMID exposure). ORR was 34.5% (all were PRs except 1), and median PFS was 5.4 months. Toxicities included all-grade diarrhea (71%), nausea (60%), and grade 3/4 thrombocytopenia (64%).61 Combining pano with cfz62 or len63 may be more promising: the former because of greater response (ORR, 67%) and the latter because of much lower gastrointestinal toxicity. Nonetheless, the limited clinical efficacy in PANORAMA 1 and toxicity concerns have limited the use of pano at this time.
Clinical case continued
We reassessed our patient’s relapse. Because the patient relapsed while on dara, we would stop it at this time. Using the IMWG model for frailty, he remained fit. Repeat bone marrow sampling should be discussed, but it may not be strictly necessary outside of a clinical trial. Given the stabilization of his cardiovascular status, he may now be eligible for clinical trials, including chimeric antigen receptor T-cell and/or bispecific antibody studies. He can also now receive cfz. Nontrial treatments to consider include an elo-based regimen, a cfz and IMID regimen, an alkylator-containing regimen, and repeat ASCT. Among these options, we favor elo/pom/dex: the patient has not been exposed to these agents previously, and the response rate in ELOQUENT-3 was favorable. Another choice in this situation would be cfz/pom/dex. Using the clonal tides model for myeloma evolution,64 it may make sense to reintroduce dara (and other prior therapies) with a later line of therapy65 (being examined in NCT03871829).
Additional therapies
Stem cell transplantation.
Per a 2015 international consensus statement, transplant-eligible patients should be considered for ASCT at relapse if they have not received it previously or if they had ASCT and remained in remission for >18 months.66 Numerous mostly single-center studies have demonstrated that salvage ASCT produces substantial responses and prolongs PFS. Transplant-related mortality remains low in the salvage setting, and the cost of a second ASCT may compare favorably with that of intensive combination regimens.67 There are few data to guide sequencing (ie, whether salvage ASCT should be performed in early or later relapse); however, as observed with salvage chemotherapy, we expect ASCT that is performed later in relapse to be less effective. Allogeneic stem cell transplantation is only recommended in the context of a clinical trial and should be considered for younger fit patients who relapsed early (within 24 months) and have high-risk disease (adverse cytogenetics, extramedullary disease, plasma cell leukemia) or who relapsed early after primary therapy that included ASCT.66
DCEP, VDT-PACE.
Multiple variations of these intensive infusional regimens consisting of traditional chemotherapies exist: DCEP, VDT-PACE, and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dex. DCEP and VDT-PACE are more commonly used, and modifications to add or subtract agents or reduce doses are often made with these regimens. These therapies were all established before the current treatment era, in nonrandomized studies, and in refractory patient populations different from those of today. They require hospitalization for continuous infusion and are associated with significant rates of febrile neutropenia and transfusions.68 We tend to use these therapies briefly (ie, as a bridge to transplant, adoptive cellular therapy, or rapid tumor debulking before switching to another regimen) in patients who are not frail.
Supportive care
Bone disease
There is little evidence to guide the use of BMAs at relapse. The Spanish Myeloma Group demonstrated that use of zoledronic acid at the time of biochemical relapse reduces the development of skeletal-related events.69 In accordance with IMWG guidelines, we favor resuming BMAs (zoledronic acid, pamidronic acid, denosumab) at relapse, particularly in patients with bone pain, skeletal-related events, or increased bone disease on imaging. If zoledronic acid is used, we would consider administration every 3 months instead of monthly based on CALGB 70604.70 We consider denosumab for patients with persistent hypercalcemia or creatinine clearance < 30 mL/min, realizing that it is associated with more hypocalcemia but less renal toxicity than zoledronic acid.71 We give calcium and vitamin D supplementation to patients who are on BMAs.
Infection
Patients on PIs, dara, or elo should be given zoster prophylaxis. We recommend pneumococcal and annual influenza vaccination for all patients. We consider antibiotic prophylaxis (fluoroquinolone72 or trimethoprim/sulfamethoxazole) and/or IVIG in patients who have recurrent life-threatening infections. We also consider IVIG for patients with severe hypogammaglobulinemia (IgG level < 400 mg/dL).73 We maintain a low threshold to give granulocyte colony-stimulating factor to attenuate the duration and severity of neutropenia in susceptible patients. Our approach to thromboprophylaxis, anemia, and prevention of renal dysfunction is consistent with the National Comprehensive Cancer Network guidelines.74
Future directions
There are many questions and debates surrounding the assessment and interpretation of MRD in myeloma. The current evidence suggests that MRD-negative status predicts improved PFS in relapsed myeloma41,75 and that dara combinations (btz/dex or len/dex) are capable of attaining MRD negativity in a fraction of relapsed patients.76 Currently, MRD testing is not recommended outside of clinical trials. Much more data are needed, and will be forthcoming, in this area.
We expect the therapeutic space for relapsed and refractory myeloma to continue changing rapidly. Selinexor, an oral inhibitor of the nuclear export protein exportin 1, has demonstrated promise in refractory disease. STOMP (selinexor/btz/dex) found activity (ORR, 63%) in single-refractory patients (RR, 43% in PI refractory) and dual-refractory patients (RR, 42%).77 STORM (selinexor/dex) found activity in heavily refractory patients (ORR, 21%; RR, 21% in quad refractory and 20% in penta refractory).78 However, toxicities (hematologic, gastrointestinal, weight loss) have also been prominent. In July 2019, the FDA granted accelerated approval for selinexor in combination with dexamethasone for patients refractory to 2 PIs, 2 IMIDs, and an anti-CD38 antibody.
Venetoclax is an oral inhibitor of BCL-2 that helps to restore apoptosis in malignant cells. Preclinical models suggested synergy with btz, which inhibits MCL-1. A phase 1 study of venetoclax/btz/dex found an ORR of 67%, with an RR of 31% in btz-refractory patients vs 97% in btz-nonrefractory patients. Patients with t(11;14) translocation have higher ratios of BCL-2/MCL-1 expression and appear to have higher responses than other patients.79 Earlier in 2019, the FDA put a partial hold on venetoclax myeloma trials because there were more deaths in the venetoclax arm of the phase 3 BELLINI trial, many due to severe infection; however, this hold has been lifted for patients with t(11;14).
There is ongoing interest in molecular profiling (eg, next-generation sequencing) and identification of druggable targets (eg, TAPUR study [NCT02693535]), such as BRAF, KRAS/NRAS, and BCL-2. Interest in chimeric antigen receptor T-cell therapy has surged, and promising results have also been seen with bispecific antibodies and antibody-drug conjugates. Breakthrough results in these areas may drastically alter our approach to treatment sequencing. At the same time, the delivery of cancer care continues to change at breakneck pace, and we foresee more intense discussions about affordability, access to care, and quality care in the future.
Correspondence
Suzanne Lentzsch, Columbia University Irving Medical Center, 161 Fort Washington Ave, New York, NY 10032; e-mail: sl3440@cumc.columbia.edu.
References
Competing Interests
Conflict-of-interest disclosure: S. Lentzsch has acted as a consultant for Janssen, Bristol-Myers Squibb, Takeda, AbbVie, Bayer, Sanofi, Proclara Biosciences, and Caelum Biosciences; has equity ownership in Caelum Biosciences; has received research funding from Karyopharm and Sanofi; has served on the Board of Directors for Caelum Biosciences; and has received honoraria from Multiple Myeloma Research Foundation, Clinical Care Options, and Physician Education Resources. S. Leng and D.B. declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.