Abstract
The low prevalence of pulmonary embolism (PE) among pregnant patients presenting with suspected PE implies that most of these patients will be found not have the disease. Given this low prevalence, excluding PE in this population has necessitated the use of sensitive and specific diagnostic imaging, such as computed tomography pulmonary angiography or ventilation-perfusion scanning. Recent studies suggest that a clinical prediction rule with D-dimer testing can also be used to exclude a subset of pregnant patients with suspected PE without the need for diagnostic imaging. The YEARS criteria, which consist of clinical signs and symptoms of deep venous thrombosis, hemoptysis, and PE as the most likely diagnosis (a subjective variable), combined with selective D-dimer levels, seem to safely exclude up to one-third of these patients without imaging. The revised Geneva rule using objective variables, combined with nonpregnancy cutoffs for D-dimer levels, offers some promise, although fewer patients avoided imaging (14%). These recent studies provide evidence in support of radiation avoidance for some patients; however, for most, imaging remains the only option. Future studies should focus on improving the safety and techniques of imaging modalities, in addition to improving the specificity of D-dimer testing and objective prediction rules. Studies assessing patients’ and physicians’ values, preferences, and risk perceptions are also required to assist clinicians in shared decision making when counseling pregnant patients with suspected PE.
Learning Objectives
Learn to apply clinical decision rules and D-dimer results in pregnant women presenting with suspected pulmonary embolism, and avoid diagnostic imaging in some of these patients
Recognize that improvements in safety and quality of imaging techniques are still required
Explore patients' and physicians' values and risk tolerance to optimize decisions on diagnostic imaging use in these patients
Case
A 35-year-old woman pregnant for the first time presents to the emergency department at gestational age of 28 weeks complaining of shortness of breath lasting several days. She has no hemoptysis or clinical signs or symptoms of deep venous thrombosis (DVT). The emergency physician does not want to miss possible pulmonary embolism (PE) but is concerned about ordering computed tomography pulmonary angiography (CTPA) or a ventilation-perfusion (VQ) scan. You are asked about the safety of the tests.
Diagnostic imaging
Until recently, excluding PE in pregnant patients presenting with suspicious symptoms required diagnostic imaging.1,2 Unlike in nonpregnant patients, specific prediction rules in pregnant patients have not been developed, and D-dimer tests with manufacturers’ suggested cutoffs might not be specific enough to be used in pregnant patients.3 In the absence of prospective studies defining the use of these ancillary tests to exclude PE, imaging diagnostic tests (ie, VQ scanning and CTPA) are the sole tests used to exclude PE. In addition to their performance, the major concerns surrounding these tests are radiation exposure of both maternal chest and developing fetus.
Several systematic reviews examining the performance of CTPA vs VQ scanning in pregnant women4,5 have been published. In a recent review4 that compared the diagnostic efficiency of both techniques, a total of 1270 patients who had a VQ scan were compared with 837 women who underwent CTPA. The negative predictive value of negative CTPA or VQ scan was 100% in the setting of low disease prevalence of 1% to 7%.4 The sensitivity rates of CTPA and VQ scanning in pregnancy, using clinical follow-up (3-24 months) as a surrogate for the reference standard, were 83% (95% confidence interval [CI], 0% to 100%) and 100% (95% CI, 0% to 100%), respectively.5 The specificity of CTPA and VQ scanning for pregnant women with suspected PE will likely remain unknown. Although the specificity of these tests is assumed to be 100%, this is not accurate.6,7
These imaging studies do not always offer a result of certainty. The pooled rates of nondiagnostic tests of VQ scanning vs CTPA were similar in 1 review,4 at 14% (95% CI, 10% to 18%) and 12% (95% CI, 6% to 17%), respectively. However, the authors acknowledged that the rates associated with nondiagnostic VQ scanning may have been overestimated. Moreover, the management of patients with a nondiagnostic VQ scan is vastly different from the management of those undergoing nondiagnostic CTPA. Patients with a nondiagnostic VQ scan might not require further imaging in the setting of low prevalence of disease.8 In contrast, results from a nondiagnostic CTPA study,9 in which the study protocol was inadequately modified to accommodate for the physiological changes of pregnancy, are problematic, and further imaging is recommended for definitive diagnosis.1,2
Above all, the major concern surrounding the use of any diagnostic imaging technique is the risk of maternal and fetal radiation exposure. In these studies, the risk of fetal radiation exposure with VQ scanning ranged from 0.32 to 0.73 mGy, whereas the risk with CTPA ranged from 0.03 to 0.66 mGy.10 With exposure <50 mGy, the fetal risk of death, malformation, impaired neurodevelopment, or malignancy is likely minimal.11 Beyond fetal radiation exposure, exposure of maternal chest to radiation with CTPA is substantial. The estimated mean effective radiation doses for CTPA and VQ scanning are 7 to 21 and 0.9 to 1.29 mSv, respectively, with breast-absorbed doses of 10 to 44 and 0.11 to 0.95 mGy, respectively.10,11 Although the risk of early-onset breast cancer was not observed to increase in a large population cohort study12 with median follow-up of 5.9 years in the computed tomography group and 7.3 years in the VQ scanning group, these findings are not yet reassuring because the length of follow-up is inadequate and the contribution of many confounders is unknown. More importantly, the cumulative effect of multiple chest diagnostic studies over a woman’s lifetime was not explored.
As timely access to VQ scanning becomes less available in many centers, CTPA will likely become the diagnostic modality of choice for most pregnant women with suspected PE. Fortunately, the radiation dose to the maternal breast associated with modern CTPA techniques has also steadily declined over the last decade, with median exposure reported to be as low as 3 to 4 mGy.13 Unfortunately, the number of studies performed to diagnose PE in pregnancy using CTPA has increased fourfold, without an increase in the rate of positive tests.14 A prospective study is currently under way to evaluate the use of a low-dose CTPA protocol for pregnant women15 to ensure that such an approach remains safe in excluding PE.
After your reassurance that imaging tests are safe, the patient wants to know if she really needs the tests?
In the last few years, 2 prospective management studies using D-dimer testing and the clinical prediction rule to exclude PE in pregnant women were published.16,17 Guidelines incorporating the results of these 2 studies were also published.18 The results from both these studies are summarized in Table 1.
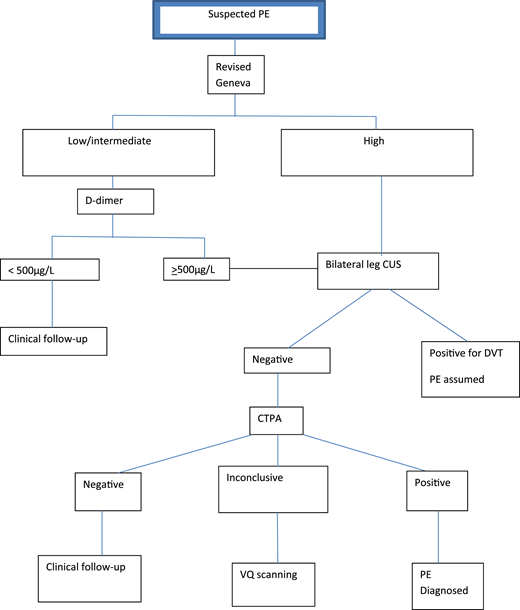
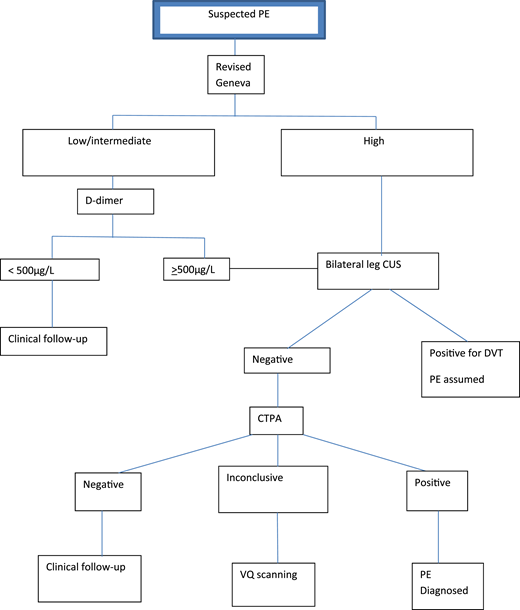
In the first study, involving 395 patients, Righini et al16 applied the revised Geneva score18 and D-dimer testing to stratify pregnant women with suspected PE (defined as acute onset of new or worsening shortness of breath or chest pain without another obvious cause) into 2 groups: those who did and those who did not require further imaging (Figure 1). Pregnant women with high PTP for PE underwent CUS and CTPA (after negative CUS). Those with nonhigh PTP were further stratified using D-dimer levels into those who required CTPA (≥500 μg/L) and those who did not (<500 μg/L).
Diagnostic algorithm for the revised Geneva/D-dimer protocol for pregnant women with suspected PE. CUS, compression ultrasound.
Diagnostic algorithm for the revised Geneva/D-dimer protocol for pregnant women with suspected PE. CUS, compression ultrasound.
The revised Geneva prediction rule was adopted with no modification for pregnant women; most patients were classified as low or intermediate PTP (99.2%). Applying a D-dimer level cutoff of <500 μg/L among patients with low or intermediate PTP, 46 women (11.7%) did not undergo imaging. No VTE was diagnosed during follow-up in these patients, but 2 women received ongoing anticoagulation. Five patients with symptoms of DVT at presentation underwent bilateral CUS to identify DVT. Overall, CTPA was avoided in 14.2% of patients presenting with suspected PE in the study (ie, 85.8% required imaging). Of the 308 CTPAs performed, 5.2% were nondiagnostic; therefore, further imaging with subsequent VQ scanning was indicated.
The incidence of PE in the study by Righini et al16 was 7.1% at study entry; the calculated incidence of PE among patients requiring CTPA was 6.1% (Table 1). The reported rate of symptomatic VTE in women with low or intermediate probability and a negative D-dimer test or a positive D-dimer test and negative or nondiagnostic imaging after completion of clinical follow-up was 0% (95% CI, 0% to 1.0%). The calculated risk of VTE on follow-up managed solely by PTP and D-dimer level alone was 0% (95% CI, 0% to 8%).
The revised Geneva/D-dimer study had several limitations: it was underpowered to evaluate the safety of excluding PE on the basis of ancillary tests alone; although the revised Geneva rule relied on objective variables, these were not specific to pregnancy; and a single unmodified D-dimer cutoff was used, limiting its utility beyond the earlier trimesters of pregnancy.
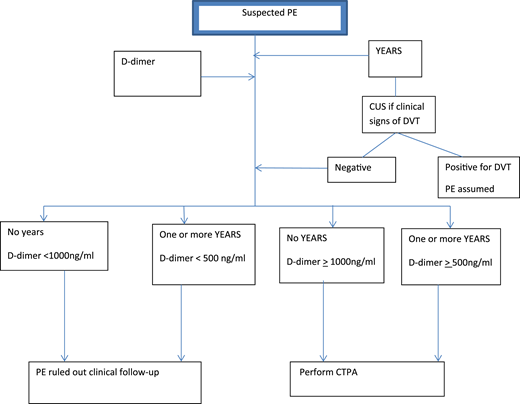
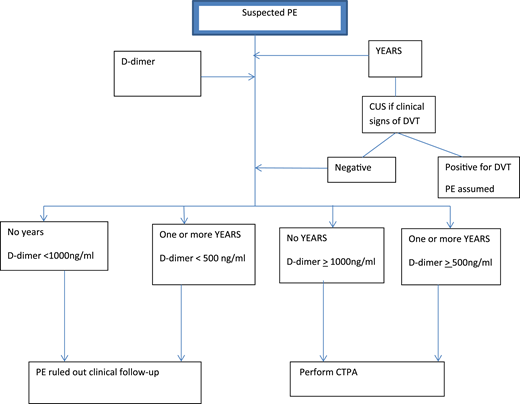
In the other prospective study of 498 patients, van der Pol et al17 explored the use of selective D-dimer criteria and the YEARS prediction rule in the management of pregnant women with suspected PE. These women were assessed using the following variables19 : clinical signs of DVT, hemoptysis, and PE as the most likely diagnosis. Management of these women with suspected PE is shown in Figure 2. At presentation, PTP was assessed using the YEARS criteria. Patients with clinical signs and symptoms of DVT underwent CUS. Selective D-dimer levels were used to stratify patients into those who required further imaging and those who did not. Patients not meeting YEARS criteria and with D-dimer <1000 μg/L and those with meeting YEARS criteria and with D-dimer <500 μg/L did not undergo CTPA and did not receive anticoagulation. All women with symptoms for DVT, and some without symptoms (not defined), underwent CUS testing.
Diagnostic algorithm for the YEARS/D-dimer protocol for pregnant women with suspected PE.
Diagnostic algorithm for the YEARS/D-dimer protocol for pregnant women with suspected PE.
With this approach, the authors avoided CTPA in 39% (95% CI, 35% to 44%) of all pregnant women presenting with suspected PE: 65% in the first and 32% in the third trimesters. The overall incidence of VTE on follow-up with the YEARS/D-dimer management strategy was 0.21% (95% CI, 0.04% to 1.2%). For the 195 patients who did not undergo CTPA, the incidence of VTE was 0.51% (95% CI, 0.9% to 2.9%).
Notably, of the 299 CTPA tests performed, there were no CTPA tests deemed inconclusive or nondiagnostic (verified with the author), following the standardization of CTPA with modification for pregnant women in all participating centers.17
Some limitations of the study included: the assessment of whether PE was the most likely diagnosis was the most decisive variable in the YEARS criteria, but how this subjective variable was determined and by whom are uncertain; PTP was not always assessed without knowledge of D-dimer level and might have influenced a clinician’s determination of the likelihood of PE; and the possible use of thromboprophylaxis in patients with a history of VTE (6%) and those with known thrombophilia (2.8%) was not detailed (this is especially relevant for VTE detection after initial assessment).
External validation of these 2 approaches was conducted in a UK cohort of 219 patients with PE diagnosed primarily via imaging.20 The authors of the study raised some concerns that the strategies may not be safe; using the revised Geneva/D-dimer strategy would avoid imaging in 21% of women, including 3 of 12 patients with PE, whereas 44% would avoid imaging with the YEARS/D-dimer strategy, including 5 of 12 patients with PE. Sensitivity rates were calculated at 75% (95% CI, 42.8% to 93.3%) and 58.3% (95% CI, 28.6% to 83.5%) for the Geneva/D-dimer and YEARS/D-dimer strategies, respectively.
The failure of the 2 strategies in that specific UK cohort might have been secondary to the influence of the anticoagulation effect in lowering D-dimer levels. In addition, the UK study employed a different design (patients were identified with PE both retrospectively and prospectively), and a fixed diagnostic algorithm was not prescribed. Other factors affecting the performance of these strategies may include the test characteristics of the D-dimer assays used.20
Nevertheless, when the YEARS/D-dimer approach was applied to the revised Geneva/D-dimer cohort,21 it was found that 21% (77) of the patients would not have required imaging, and no patient (0 of 77) would have been diagnosed with VTE on follow-up (95% CI, 0% to 3.9%).
Is CUS of lower extremities necessary as part of PE workup?
Many guidelines1,2 recommend the use of bilateral CUS in the diagnostic management of pregnant women with suspected PE to possibly reduce the need for ionizing radiation; this was part of the diagnostic algorithm in both studies.16,17 In the revised Geneva/D-dimer study,16 CUS was performed in 75% of patients with suspected PE. DVT was diagnosed in 1.8% (7 of 328) of asymptomatic patients, compared with 8.8% (5 of 57) of patients with symptoms. In the YEARS/D-dimer study,17 CUS was performed in 88% of the cohort; 7% (3 of 43) of patients with symptoms of DVT were positive, and 1.3% (1 of 79) of asymptomatic patients were diagnosed with DVT.
The reported rates of DVT in pregnant patients from these studies investigating pregnant women with suspected PE16,17 were similar (7% to 8.8%) to those reported from prospective studies of pregnant women with suspected DVT (7.2% to 8.3%).22,23 Therefore, CUS is clearly indicated if patients presenting with suspected PE have concurrent signs and symptoms of DVT. Even in the absence of DVT symptoms, DVT is diagnosed in 1% to 2% of patients; universal screening of these pregnant patients to avoid radiation exposure could still be considered. However, based on personal experience, obtaining CUS testing before timely diagnostic imaging is sometimes challenging.
Applying the diagnostic algorithm to this patient case and counseling the patient
The revised Geneva score for this specific patient would place her as low or intermediate PTP; her PTP based on YEARS criteria could be 1 or 0 based on the level of experience or risk aversion of the clinician who assessed her. Her D-dimer test was subsequently reported as 995 ng/mL.
Based on the study reported by Righini et al,16 CTPA would be indicated after negative CUS. If the patient were assessed with no YEARS criteria, no further imaging would be required17 ; if she were assessed with 1 criterion, imaging would be indicated.
The subjectivity of this variable would likely be based on the experience and risk tolerance of the clinician. A notable observation from the 2 prospective studies in pregnancy16,17 was the fact that the proportion of physicians who felt that PE was the most likely diagnosis at presentation was 71% in the revised Geneva/D-dimer study16 vs 44% in the YEARS/D-dimer study,17 even though the participants in both studies were recruited from similar settings, and the proportion of patients diagnosed with PE was similar.
From the patient’s perspective, the likelihood of diagnosed PE is low. At presentation, the incidence of PE is 4%17 ; if she were assessed as meeting 1 YEARS criterion and requiring imaging, the incidence of PE would marginally increase to 6%.17 Given these low rates, the patient might opt not to comply with medical recommendations.
Indeed, protocol violations reported from the 2 prospective studies of PE diagnosis in pregnancy were fairly common.16,17 Comparing the YEARS/D-dimer study in pregnant patients17 with the corresponding study in nonpregnant patients,19 protocol violations were observed more often among pregnant patients.17 When CTPA was not indicated as per the protocol, 6% (12 of 199) of women had imaging, compared with 1.5% (24 of 1651) in the study of nonpregnant patients; when CTPA was indicated, 4.7% (14 of 299) of patients in the pregnancy study17 versus 0% (0 of 1814) in the nonpregnancy study19 did not undergo the test. Similarly, when CTPA was indicated in the revised Geneva/D-dimer study, 2.9% (10 of 342) of tests were not completed; in addition, 42% (14/33) of patients refused VQ scanning when indicated.
The reasons for protocol violations are unclear; whether they are driven by physicians who are risk adverse or by patients who fear radiation exposure, or a combination of both, is not known. Not infrequently, a patient might also insist on imaging even if the risk of PE is low. Studies addressing patients’ values, preferences, and risk perceptions would be helpful to aid clinicians in counseling these patients and arriving at an agreeable management decision. Further exploration of clinicians’ assessments of the likelihood of PE vs common pregnancy symptoms of benign conditions could aid in more selective use of diagnostic imaging.
Conclusion
The prevalence of PE among pregnant women presenting with nonspecific symptoms of chest pain and dyspnea is low. Both D-dimer testing and clinical prediction rules can be used to assist in the management of pregnant women presenting with symptoms suspicious of PE, especially for those in the first 2 trimesters of pregnancy. The revised Geneva criteria are expectedly less specific for pregnant women, when compared with the YEARS criteria. Although the YEARS criteria performed better in negating the need for diagnostic imaging when combined with variable D-dimer levels, as compared with the revised GENEVA/D-dimer approach, the third variable used in the YEARS criteria (ie, that PE is the most likely diagnosis) is highly subjective. In addition to developing more specific PTP criteria for pregnant patients with suspected PE, future studies should explore how patients’ values and risk tolerance and physicians’ risk tolerance influence decision making.
Correspondence
Wee-Shian Chan, University of British Columbia, 4500 Oak St, Vancouver, BC V6H 3N1, Canada; e-mail: weeshian.chan@cw.bc.ca.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.