Learning Objectives
Management of high-risk pulmonary embolism when systemic thrombolysis is contraindicated due to high risk of bleeding
Indications for the use of extracorporeal membrane oxygenation in the management of pulmonary embolism
Clinical case
A 67-year-old man presented to the emergency room with progressively worsening shortness of breath and chest pain of 2 days duration. He has a history of hypertension, obesity and an ischemic stroke in the right middle cerebral artery, which was treated with systemic thrombolytics 4 weeks ago, and the patient was discharged on aspirin 81 mg daily. On arrival to the emergency room, the patient was tachycardic (heart rate 122 beats per minute) and hypotensive with a blood pressure of 88/60 mmHg. His respiratory rate was 24/minute, oxygen saturation was 88% on room air and improved to 92% on 2 liters of oxygen via nasal canula. Laboratory results were notable for a normal complete blood count and comprehensive metabolic panel, high-sensitivity troponin of 57 ng/L (normal 3-15 ng/L) and N-terminal pro-brain natriuretic peptide of 685 pg/ml (normal <100 pg/ml). CT angiogram of the chest showed large bilateral pulmonary emboli. Both bed-side echocardiogram and CT angiogram of the chest showed evidence of right heart strain. The patient was started on intravenous fluids, heparin infusion and the hospital’s pulmonary embolism response team (PERT) was consulted for further management. Due to worsening respiratory status and persistent hypotension, he was intubated and started on inotropic support. Blood pressure remains low despite using maximal doses of norepinephrine and dobutamine. How should this patient be treated?
Introduction
The outcomes in patients with pulmonary embolism (PE) vary widely based on their clinical, imaging and laboratory parameters, and several prognostic scores have been developed to assess early PE-related mortality.1 High-risk PE is characterized by the presence of shock or persistent hypotension (systolic blood pressure <90 mmHg, need for vasopressors, or a decrease in the systolic blood pressure by ≥40 mmHg from baseline for 15 minutes or longer despite resuscitation), and is associated with poor outcomes.2,3 While low risk patients can be treated as out-patient, current guidelines recommend systemic thrombolysis with anticoagulation for patients with high-risk PE and hemodynamic compromise.2,3 Thrombolytic therapy is associated with an increased risk of major bleeding, including intracranial hemorrhage, especially in the elderly, obese patients and those with comorbidities.4 Advanced treatments or adjunctive treatments, including catheter based therapies and extracorporeal membrane oxygenation (ECMO), respectively, are being increasingly used for management of high-risk PE, though their optimal use remains uncertain. In this review, we discuss the management of high-risk PE when systemic thrombolysis is contraindicated due to a high risk of major bleeding, with an emphasis on the role for ECMO.
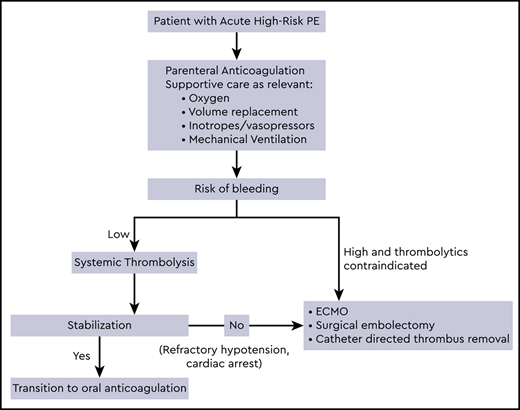
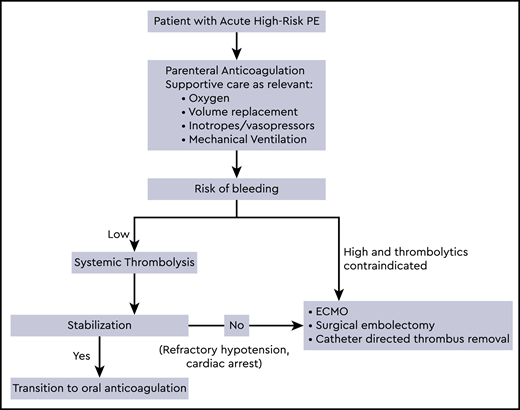
Initial treatment of high-risk PE
Supportive therapy including oxygen, mechanical ventilation, volume optimization, and use of vasopressors and inotropic support is often required in conjunction with anticoagulation as initial management of high-risk PE patients. Parenteral anticoagulation is recommended in patients who are hemodynamically compromised or at high risk of decompensation, and oral anticoagulants should be avoided in the acute phase.3 Low molecular weight heparin and fondaparinux carry a lower risk of major bleeding and heparin induced thrombocytopenia, and unless contraindicated, are the preferred anticoagulant agents for the initial treatment of PE.2,3 However, unfractionated heparin may be considered in patients with hemodynamic decompensation, due to the anticipated necessity for reperfusion treatment.3 Patients in whom initial therapy fails to improve hemodynamic parameters and have persistent cardiogenic shock or develop cardiac arrest may require advanced therapies as described below. Anticoagulation should be continued to prevent new thrombosis while these therapies are administered. In massive PE, a significant reduction in recurrent PE or mortality was noted from 19% with heparin alone to 9.4% with full dose fibrinolysis.5 However, systemic thrombolysis with anticoagulation is associated with a 3 fold increased risk of major bleeding compared to anticoagulation alone, with a 9.9% incidence of major bleeding and 1.7% incidence of intracerebral or fatal bleeding.6-8
Treatment of PE when systemic thrombolytics are contraindicated
In patients receiving anticoagulation, a history of stroke is known to increase the risk of major bleeding,9,10 and ischemic stroke within 3 months is a major contraindication for systemic or locally administered thrombolysis.2 Absolute and relative contraindications to fibrinolysis are provided by several society guidelines,2,3,11 and other major contraindications include structural intracranial disease, hemorrhage, recent brain or spinal surgery, brain injury, bleeding diathesis and active bleeding.2 A risk stratification score to assess the risk of intracranial hemorrhage in PE patients receiving thrombolytics, the PE-CH score, was developed including 4 independent prognostic factors: preexisting peripheral vascular disease, age >65 years, prior stroke with residual deficit, and prior myocardial infarction.12 In patients with high-risk PE and perceived high risk of bleeding, surgical embolectomy, mechanical catheter based treatments and ECMO could be considered.
Surgical pulmonary embolectomy
Surgical embolectomy with cardiopulmonary bypass is an effective strategy when thrombus removal is indicated but there is an absolute contraindication for thrombolytic therapy, or to rescue patients that are refractory to thrombolysis. 13 In patients with massive PE who do not respond to thrombolysis within the first 36 hours as evident by persistent clinical instability and residual echocardiographic right ventricular dysfunction, rescue surgical embolectomy led to better in-hospital course when compared with repeat thrombolysis, and was associated with lower recurrent PE and mortality.14 A recent meta-analysis involving 1,579 patients who underwent surgical embolectomy showed an in-hospital all-cause mortality of 26.3% and a long-term all-cause mortality rate of 6.5 deaths per 100 person-year. Among these patients, 36% had preoperative contraindications to systemic thrombolysis, 33.9% suffered preoperative cardiac arrest, and 27% required the use of ECMO.15
Catheter-directed therapies
Interventional catheter-based treatments for acute PE include catheter directed therapies (CDT) with or without thrombolytics, and should be offered to patients who have a moderate to high risk of bleeding with systemic thrombolysis, and have access to the expertise and resources required to perform these procedures.2,3 CDT achieves a high local concentration of thrombolytic drug by infusing drug directly into the PE, and can be combined with thrombus fragmentation resulting from placement of the infusion catheter and additional maneuvers, or ultrasound delivered via the catheter. The SEATTLE II study evaluated the safety and efficacy of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis in massive and sub-massive PE, and showed moderate bleeding in 10% of participants though none had intracranial hemorrhage.16 In pooled data from 348 patients, clinical success with percutaneous therapy alone for patients with acute massive PE was 81% (aspiration thrombectomy 81%; fragmentation 82%; rheolytic thrombectomy 75%) and 95% when combined with local infusion of thrombolytic agents (aspiration thrombectomy 100%; fragmentation 90%; rheolytic thrombectomy 91%).17
Although bleeding rates have not been directly compared in the contemporary era with advanced CDT, the incidence of bleeding may be lower than that reported in studies of systemic agents (approximately 0 to 4% vs 10 to 20% for major bleeding and <1% vs 2 to 5% for intracranial hemorrhage respectively).7,16,18,19 However, since the evidence comes from prospective cohorts and registries that included small number of patients with massive PE, the efficacy and safety of these techniques in high-risk PE patients is uncertain.16,20-22
Extracorporeal membrane oxygenation
For patients with life-threatening PE at high risk of bleeding, catheter-directed thrombolytic therapy is relatively contraindicated, especially since the associated risk of major bleeding is unknown. Mechanical cardiopulmonary support, mostly with veno-arterial ECMO, is helpful in high-risk PE to maintain the circulation and oxygenation of organs during acute right ventricular failure and cardiogenic shock. Multiple case series and retrospective studies have shown good outcomes with ECMO for massive PE, but there are no randomized controlled trials comparing ECMO to other treatments.23 ECMO has been used in different clinical scenarios, to rescue patients when thrombolytic treatment fails or as a temporary hemodynamic support prior to surgical24 or catheter-based embolectomy,25 and in patients with refractory cardiogenic shock or cardiac arrest. Surgical embolectomy requires sternotomy and cardiopulmonary bypass and carries a significant morbidity and mortality in patients with advanced shock and multiorgan failure. In these patients, heparin therapy with ECMO may offer a rapid and effective alternative treatment option until heparin-induced and endogenous thrombolysis permits weaning-off support, often within few days.26-28 Pasrija et.al., reported that implementation of a protocolized strategy of triaging patients with massive PE based on an algorithmic approach rather than aggressive early surgical approach reduces morbidity and mortality.29
Consensus regarding the optimal management of PE patients with persistent shock and when thrombolysis is contraindicated is lacking. It is unclear if these patients benefit from ECMO first or embolectomy first approach, and ECMO is often used following failure of other therapeutic options.30,31 Mortality rates are high when ECMO is used as a salvage therapy in patients who have failed other advanced therapies, and ECMO with therapeutic anticoagulation is emerging as a promising initial support strategy and as a bridge to recovery or surgical pulmonary embolectomy at several centers.29,32-34 In a protocol using ECMO support for 3 to 5 days followed by reevaluation of right ventricular function, 73% of the patients responded to anticoagulation alone and 27% required subsequent surgical embolectomy. Prolonged shortness of breath, elevated N-terminal pro-brain natriuretic peptide, enlarged pulmonary artery diameter, and previous history of venous thromboembolism were associated with lack of right ventricular recovery, and early surgical intervention may be considered in these patients. The use of ECMO requires full dose anticoagulation to maintain the functionality of the system; hence, is associated with complications such as bleeding and infection, and judicious patient selection and center experience play a role. In a multicenter study assessing outcomes of 52 patients treated with ECMO, patients who failed fibrinolysis and those who did not receive reperfusion therapy had unfavorable prognosis compared with ECMO performed in addition to surgical embolectomy.35 Among patients with high-risk PE who require anticoagulation and treatment with ECMO, several patients also received thrombolysis in the published literature (Table 1). The bleeding rate was higher in patients who had thrombolysis or surgery combined with ECMO, than those with ECMO alone.35 Considering that heparin-induced clot dissolution and spontaneous fibrinolysis allows ECMO weaning after only a few days on support, the benefit of additional mechanical clot-removal therapies, catheter-based or surgical thrombectomy on ECMO, warrant further investigation. The appropriateness of these recommendations for a specific patient may vary depending on several factors and should be best judged by the clinician. Optimal medical decisions must incorporate factors such as age, comorbidities, life expectancy, patient wishes, and quality of life. Establishment of multidisciplinary PE response teams (PERTs) is encouraged, as they address the needs of modern systems-based healthcare (Class IIa recommendation, Level C).36
Recent guidelines from the European Society of Cardiology recommend surgical embolectomy (Class I recommendation, Level C) or percutaneous catheter directed treatment (Class IIa recommendation, Level C) in patients with massive PE at high risk of bleeding, if they have access to the expertise and resources. ECMO may be considered in conjunction with these treatments in patients with refractory cardiogenic shock or cardiac arrest (Class IIb recommendation, Level C).3 Level of evidence for embolectomy is probably at the same level as for CDT and ECMO in the current era, as more studies show benefit of ECMO with heparin as a stand-alone therapy.37 Following hemodynamic stabilization, patients recovering from high-risk PE can be switched from parenteral to oral anticoagulation. As these patients were excluded from the direct acting oral anticoagulant clinical trials, the optimal time point for this transition and their efficacy has not been determined by existing evidence.
In summary, the use of ECMO, whether utilized as a bridge to embolectomy or as a therapeutic intervention, seems promising in high-risk PE patients who have contraindication for systemic thrombolysis. Further randomized studies to compare ECMO to other interventions to determine their efficacy and safety are warranted.
Resolution to the Patient Case
In our patient who is elderly with multiple co-morbidities and has high-risk PE due to persistent hypotension, history of recent stroke is an absolute contraindication for systemic thrombolysis. Due to refractory cardiogenic shock, PERT team recommended treatment with heparin anticoagulation and ECMO, and cardiovascular parameters improved after 4 days. He was transferred to the floor after a week and discharged home on apixaban 5 mg twice daily. At a 1 month follow up visit, he reported no complications from anticoagulation and was recovering well.
Conclusion
The use of ECMO with anticoagulation seems promising in high-risk PE when systemic thrombolysis is contraindicated, though quality of evidence is low and future studies are urgently needed to better define its optimal use.
Correspondence
Radhika Gangaraju, University of Alabama at Birmingham, 1600 7th Ave, Birmingham, AL 35233; email: rgangaraju@uabmc.edu.
References
Competing Interests
Conflict-of-interest disclosure R.G. serves as a consultant for Sanofi Genzyme for cold agglutinin disease. This is not relevant to the current article. F.A.K. reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, MSD and Actelion, the Dutch Heart Foundation, the Netherlands Organization for Health Research and Development, and the Dutch Thrombosis Association, all outside the submitted work.
Author notes
Off-label drug use None disclosed.