Abstract
The development and approval of novel substances have resulted in substantial improvements in the treatment of acute myeloid leukemia (AML). In the current era of novel treatment options, genetic and molecular testing at the time of diagnosis and relapse becomes increasingly relevant. Midostaurin in combination with intensive chemotherapy is the standard of care as upfront therapy in younger AML patients with mutated fms-related tyrosine kinase 3 (FLT3). Gilteritinib, a second- generation FLT3 inhibitor, represents a key drug for relapsed/refractory (R/R) FLT3-mutated AML patients. Targeted therapy has also been developed for patients with mutated isocitrate dehydrogenase 1 (IDH1) and IDH2. The US Food and Drug Administration (FDA) approved ivosidenib as a monotherapy for newly diagnosed older adult IDH1-mutated patients and enasidenib for R/R IDH2-mutated AML patients. CPX-351, a liposomal formulation of daunorubicin and cytarabine, has become an important upfront treatment strategy for fit patients with therapy-related AML or AML with myelodysplasia-related changes that are generally challenging to treat. The antibody drug conjugate gemtuzumab ozogamicin was approved in combination with intensive therapy for patients with newly diagnosed (FDA/European Medicines Agency [EMA]) as well as R/R CD33+ AML. The combination of venetoclax, an oral selective B-cell leukemia/lymphoma-2 inhibitor, with hypomethylating agents or low-dose AraC (LDAC) has changed the treatment landscape and prognosis for older adult patients very favorably. The addition of glasdegib, a small-molecule hedgehog inhibitor, to LDAC is another example of novel options in older patients. Further substances have shown promising results in early clinical trials.
Learning Objectives
Learn about the indications and efficacy of newly approved drugs in AML
Become familiar with a treatment algorithm for newly diagnosed and relapsed AML patients
CLINICAL CASE
A 66-year-old man with no significant underlying health conditions developed leukocytosis, anemia, and thrombocytopenia. Bone marrow biopsy revealed an infiltration of 60% myeloblasts. Immunophenotypically, the blasts were CD34+, CD13+, and CD33+. He was diagnosed with acute myeloid leukemia (AML). Mutational screening showed an FLT3-ITD (allelic ratio 0.6), isocitrate dehydrogenase 2 (IDH2), and TP53 mutation. The cytogenetic analysis revealed a normal karyotype.
What are the treatment options for this patient in the era of novel therapies?
Intensive therapy with novel agents
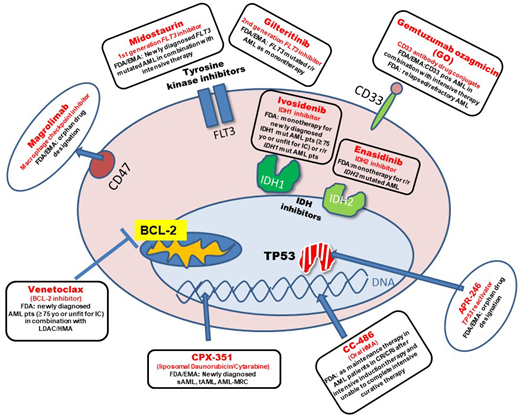
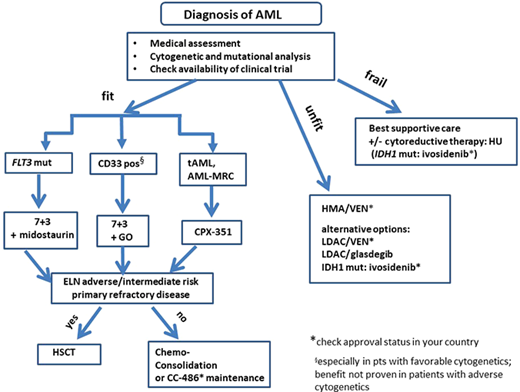
In recent years, treatment strategies for AML have evolved beyond “7 + 3” for younger patients and past monotherapy with hypomethylating agents (HMAs) for older adult AML patients (Figure 1). Novel mechanisms of action underlie these recently approved drugs. Some of the new treatment strategies were designed for or have shown the most benefit in a distinct genetic group of patients. Therefore, molecular and cytogenetic analysis becomes increasingly relevant for the application of novel drugs. In addition, genetic risk stratification at the time of diagnosis is an essential element for guiding the decision regarding whether allogeneic hematopoietic stem cell transplantation (HSCT) is recommended in the first complete remission (CR).1 In 2017 the European LeukemiaNet (ELN) recommended mutational screening for FLT3-ITD (including allelic ratio), NPM1, CEBPA (biallelic status), TP53, ASXL1, and RUNX1 in addition to cytogenetic analysis for the allocation to one of the three prognostic categories.1 Based on the risk-benefit ratio for allogeneic HSCT, patients in the adverse and pos-sibly in the intermediate group are candidates for allo-geneic HSCT in first CR. In our patient three important mutations were identified (FLT3-ITD, IDH2, TP53). The detection of FLT3-ITD in this patient allows the application of frontline-targeted therapy with midostaurin. Midostaurin is an oral first-generation tyrosine kinase inhibitor (TKI) and is now the standard of care in FLT3-mutated AML patients who can receive intensive therapy. Its approval by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) was based on the RATIFY trial, a large international phase 3 trial that randomized FLT3-mutated patients to treatment with midostaurin vs placebo in combination with intensive therapy.2 Both overall survival (OS) and event-free survival (EFS) were significantly longer in the midostaurin compared to the placebo arm without differences in severe adverse events. The 4-year OS was 51.4% in the midostaurin arm vs 44.3% in the placebo arm. Of note, the survival benefit in the midostaurin arm was observed when looking at the whole cohort. In a separate analysis of molecular subgroups (FLT3-ITD high or low, FLT3-TKD), the survival advantage in the midostaurin arm did not reach statistical significance, which is likely due to the subgroups being underpowered.2 The survival advantage observed in the midostaurin arm for the whole cohort was due to a significantly lower cumulative incidence of relapse (CIR) if transplantation was not taken into account. After the completion of consolidation therapy, midostaurin or placebo was given for up to 12 cycles as maintenance therapy. A landmark analysis at the end of consolidation therapy with high-dose cytarabine showed no significant differences in OS and EFS between both treatment arms. Due to these inconclusive results with respect to the quantitative effect of maintenance therapy, the efficacy of the continued treatment after consolidation therapy is currently unclear.3 The favorable safety profile of midostaurin was confirmed in the RADIUS-X expanded access program.4
Frontline treatment algorithm in AML patients considering recently approved substances. mut, mutated.
Frontline treatment algorithm in AML patients considering recently approved substances. mut, mutated.
Another novel treatment option for newly diagnosed AML patients with CD33 expression on leukemic blasts is gemtuzumab ozogamicin (GO) in combination with 7 + 3.5 GO combines a CD33-directed antibody with the chemotherapy agent calicheamicin. CD33 is a suitable target in AML because it is highly expressed in most AML cells and much less on normal hematopoietic cells. While prior data have been conflicting, especially with regard to toxicity, the French ALFA-0701 trial was designed to look directly at the effect of GO on the survival of older adults undergoing intensive therapy.6 In this randomized, open- label phase 3 study, 280 older patients (aged 50-70 years) were either treated with GO (n = 140) on day 1, 4, and 7 or without GO (n = 140) during the 7 + 3 induction therapy.6 Patients achieving CR were treated with 2 cycles of consolidation therapy with or without GO according to their initial randomization. The EFS at 2 years was 17.1% in the control arm vs 40.8% in the GO arm. Two-year OS was also significantly better in the GO (53.2%) vs standard arm (41.9%; P = 0.037).6 A meta-analysis by Hills et al. looked at the efficacy of GO in 5 randomized trials involving 3325 patients.7 Interestingly, the absolute survival benefit was most apparent in the patient group with favorable cytogenetics (20.7%), followed by patients with intermediate cytogenetics (5.7%).7 GO had no benefit in patients with adverse cytogenetics.7 In the AMLSG 09-09 trial, NPM1-mutated AML patients were randomized to receive intensive chemotherapy with or without GO. In this trial the EFS was not significantly different between treatment arms.8 However, the early death rate during inductions was higher, while the CIR was lower in the GO arm. This is in line with the finding that the addition of GO leads to a better reduction in NPM1 mutant transcript levels across all treatment cycles.9 Subgroup analysis revealed that the addition of GO showed a significant benefit for younger, FLT3-ITD-negative, and female patients.8 The FDA and the EMA approved GO in combination with intensive chemotherapy for patients with newly diagnosed CD33+ AML. Considering the data outlined above, GO might be best suited for patients with favorable cytogenetics and without an increased risk for early mortality due to underlying infection.
For patients with newly diagnosed secondary AML (s-AML), therapy-related AML (tAML), or AML with myelodysplasia-related changes (AML-MRC), treatment with CPX-351, a liposomal formulation of daunorubicin and cytarabine in a fixed combination, is now available.10 In the initial phase 2 trial comparing CPX-351 with standard intensive treatment in older AML patients, only the subgroup of patients with sAML and adverse cytogenetics showed a significantly higher response rate (57.6% in the CPX-351 arm vs 31.6% in the standard arm).11 Based on these results, a large phase 3 trial was initiated in older (aged 60-75 years) patients with high-risk AML or sAML.10 Here the median OS was 9.56 in the CPX-351 arm and 5.95 months in the 7 + 3 arm. The overall remission rate was also significantly better with CPX-351 (47.7%) vs the standard arm (33.3%).10 Of note, 34% of patients in the CPX-351 arm underwent allogeneic HSCT, compared to 25% in the 7 + 3 arm. In an exploratory landmark survival analysis from the time of transplant, outcomes were more favorable in the CPX-351 arm. This is in line with results from an Italian compassionate-use program for CPX-351. Here, CIR was reduced in 71 older adult AML patients when allogeneic HSCT was performed in the first CR, with a very favorable overall outcome following transplantation.12 The encouraging results of the phase 3 trial resulted in FDA and EMA approval of CPX-351 for this distinct subgroup of AML patients. In order to identify these patients with AML with MRC, cytogenetic analysis is indispensable.13 Real-life experience from a French retrospective study looked at 103 sAML, tAML, or AML-MRC patients treated with CPX-351.14 The overall response rate (ORR) was 59% after induction with an OS of 16.1 months at a median follow-up time of 8.6 months. Importantly, patients with ASXL1 and RUNX1 mutations, who are in the ELN unfavorable prognostic group, showed similar response rates as wild-type patients in contrast to patients with mutated TP53.14 The safety profile was very favorable for CPX-351, with a low rate of alopecia (11%) and gastrointestinal toxicity (50%). Interestingly, a retrospective study involving 25 AML patients who received CPX-351 as outpatients suggests that the administration of CPX-351 without planned admission is safe and might lead to a decreased utilization of health care resources.15
Now we return to our patient. Based on the data above, he was treated with 7 + 3 and midostaurin. He achieved a CR after induction therapy. Importantly, as he was allocated to the adverse prognostic group (FLT3-ITD high without NPM1 mutation as well as the presence of TP53 mutation), it was recommended that he undergo allogeneic HSCT in first CR. However, for those patients in CR who cannot complete intensive therapy after induction therapy, maintenance therapy with CC-486 has become another novel option. CC-486 is an oral HMA that is not bioequivalent to injectable azacitidine and has shown efficacy in patients who have developed resistance to injectable HMAs in prior studies.16,17 In the phase 3 QUAZAR AML-001 trial, 472 AML patients (aged 55 to 86 years) were randomized to receive CC-486 (238 patients) or placebo (234 patients).18 Median OS as well as EFS was significantly longer in the CC-486 arm (24.7 months, 10.2 months, respectively) compared to the placebo arm (14.8 months, 4.8 months). Hematological toxicity with neutropenia was more common in the CC-486 arm (41% in the CC-486 arm vs 24% in the placebo arm). Based on the QUAZAR AML-001 results, the FDA and EMA granted approval for CC-486 as a maintenance therapy for adult AML patients in CR/CR with incomplete hematologic recovery (CRi) after intensive induction therapy who are unable to complete intensive curative therapy (eg, allogeneic HSCT). CC-486 should not be substituted for injectable azacitidine because there are differences in pharmacokinetic properties between the oral and the injectable version.
We still lack sufficient data about the efficacy of combining the above novel agents with each other.
Nonintensive therapy with novel agents
Treatment has remained challenging for older adult patients who cannot receive intensive therapy.19 Hypomethylating agents such as azacitidine and decitabine have become important treatment strategies for older patients and have shown survival benefits when compared to low-dose AraC (LDAC).20 However, the median OS for patients treated with azacitidine in a phase 3 trial was 10.4 months and only 7.1 months in a large population-based study.21 In light of the fact that HMA monotherapy does not result in long-term remissions, clinical trials combining HMAs (and LDAC) with venetoclax have demonstrated impressive survival data. Venetoclax is an oral, highly selective small-molecule B-cell leukemia/lymphoma-2 inhibitor with poor efficacy as a single agent in AML.22 However, in vitro and in vivo studies have shown synergism between chemotherapy and venetoclax, supporting the use of venetoclax combinations in AML. One mechanism of synergism between azacitidine and venetoclax is through transcriptional induction of the proapoptotic BH3-only protein NOXA by azacitidine.23,24 The VIALE-C study, an international phase 3 randomized double-blind trial, compared venetoclax vs placebo in combination with LDAC in older (≥75 years) or younger patients unfit for intensive therapy.25 At a median follow-up of 17.5 months, patients treated with venetoclax and LDAC (VEN-LDAC) achieved a significantly longer median OS compared to patients receiving placebo and LDAC (PBO-LDAC) (8.4 vs 4.1 months). Similarly, the CR rates as well as EFS were better in the VEN-LDAC arm compared to the PBO-LDAC arm (EFS, 4.9 months vs 2.1 months). The combination of azacitidine and venetoclax (VEN-AZA) yielded even more encouraging results (VIALE-A trial).26 Here, older, previously untreated AML patients who were ineligible for standard induction received VEN-AZA or placebo and azacitidine (PBO-AZA).26 The median OS was 14.7 months in the VEN-AZA arm vs 9.6 months in the PBO-AZA arm at a median follow-up of 20.5 months. Thus, the median OS in the VEN-AZA arm was longer than reported in any other prior trial for frontline, older adult AML patients. In addition, the CR rate, at 36.7% vs 17.9%, was also more favorable with the venetoclax combination. Grade ≥3 thrombocytopenia and neutropenia were more common in patients treated with venetoclax. Likewise, febrile neutropenia occurred in 42% of patients in the VEN-AZA arm vs 19% of patients in the PBO-AZA arm. Thus, it is essential to observe the patient closely for hematological toxicity in the form of cytopenias and follow recommendations for dose adjustments when treating patients with venetoclax combinations.27 Dose adjustments also become necessary when comedications have strong CYP3A4 inhibitory activity (eg, antifungals, fluoroquinolones).27 Recent data suggest that IDH1- and IDH2-mutated AML patients achieve impressive response rates, suggesting that this molecular subgroup, especially, benefits from adding venetoclax to azacitidine treatment.28 The FDA has approved venetoclax in combination with HMAs or LDAC for newly diagnosed AML patients ≥75 years of age or patients with comorbidities precluding intensive induction therapy (the EMA has approved venetoclax only in combination with HMAs). As HMA/VEN has evolved as a standard of care (in countries with approval), this combination also represents the backbone of trials with novel substances. Unfortunately, a retrospective analysis suggests that once patients become unresponsive to HMA/VEN the prognosis is very poor.29 This might be related to the acquisition of high-risk cytogenetic and molecular features (eg, mutations in TP53, N/KRAS, and/or KIT).29 For patients who are older but lack comorbidities, the question arises as to whether intensive therapy or HMA/VEN treatments are the wiser treatment choice. To date, there is no randomized trial comparing intensive chemotherapy with HMA-VEN head-to-head. However, a retrospective study compared outcomes of patients with intensive chemotherapy vs decitabine over 10 days in combination with venetoclax (DEC10-VEN). The CR/CRi rates and relapse rates were more favorable in the DEC10-VEN arm. In addition, OS was significantly longer in DEC10-VEN-treated patients.30 In summary, the introduction of venetoclax has markedly changed the survival outlook for older AML patients.
Glasdegib, a small-molecule hedgehog inhibitor, is another drug that has received approval by the FDA and the EMA for older, newly diagnosed AML patients (≥75 years) or patients with comorbidities that preclude intensive therapy. The approval was based on a phase 2, randomized, open-label, multicenter study that compared LDAC vs LDAC/glasdegib. Median OS was 8.8 months with the combination compared to 4.9 months with LDAC alone.31 Of note, a head-to-head comparison between VEN/LDAC or VEN/HMA and LDAC/glasdegib is currently missing.
For one molecular subgroup of patients, targeted therapy is now available: older AML patients (≥75 years) or AML patients with significant comorbidities who carry a mutation in IDH1. Ivosidenib is an oral, targeted agent that inhibits mutant IDH1 and consequently blocks the production of the oncometabolite 2-hydroxyglutarate. Ivosidenib has been studied as monotherapy in IDH1-mutated AML patients in a phase 1b trial.32 Of the 258 patients in the trial, the majority had R/R AML (179 patients). In the primary efficacy cohort, 21.6% of patients achieved CR and 41.6% an overall response, with a median duration of response of 9.3 and 6.5 months, respectively. The IDH differentiation syndrome is a distinct side effect of IDH inhibitors and requires a physician's awareness. It was observed in 3.9% of patients in this trial. The FDA (but not the EMA) approved ivosidenib for older (≥75 years) patients or patients with significant comorbidities as a monotherapy for newly diagnosed, IDH1-mutant as well as R/R AML. In a phase 1b trial, the combination of azacitidine with ivosidenib was well tolerated, and patients showed durable remissions.33 Thus, we eagerly await the results of the placebo-controlled phase 3 trial with this combination.
Treatment of AML in relapse
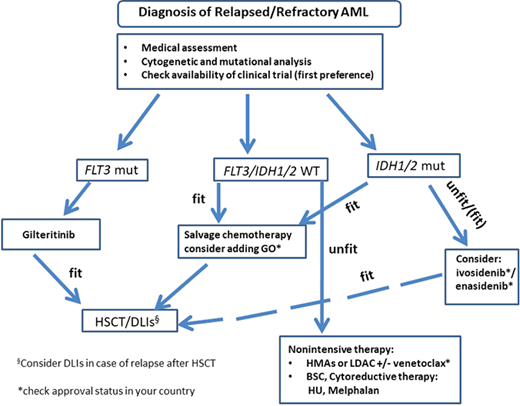
Due to clonal evolution, it is advisable to repeat mutational profiling at the time of relapse.34 While some mutations are stable during disease progression, others are lost or gained at the time of relapse.35 Interestingly, the follow-up data of the RATIFY trial showed that 46% of patients became FLT3-ITD-negative at the time of disease resistance or progression.36 For patients who show an FLT3 mutation in relapse, gilteritinib, an oral, second-generation TKI, is now available. Gilteritinib was compared to salvage therapy in a large randomized phase 3 trial (ADMIRAL).37,38 Here, patients were randomized to receive gilteritinib vs salvage chemotherapy according to local investigators' choice. Gilteritinib was associated with higher CR/CRi rates (34% vs 15.4%). This positive effect translated into a longer OS in the gilteritinib arm (9.3 vs 5.6 months).38 Importantly, severe adverse events were less frequent in the gilteritinib arm. While only 5.7% of patients in the ADMIRAL trial received midostaurin during frontline treatment, a retrospective study of 13 medical centers analyzed the efficacy of gilteritinib in R/R AML patients previously treated with a TKI. In this patient population, the CR rates were 58% with an OS of 7.8 months, suggesting that gilteritinib is no less effective after previous TKI treatment. This is relevant, as in the RATIFY trial 11% of patients developed ITD clones resistant to midostaurin during disease progression.36
For IDH2-mutated AML patients, the FDA (not the EMA) approved enasidenib, an oral inhibitor of mutated IDH2, as a monotherapy for R/R AML patients.39,40 The approval was based on a phase 1/2 trial.29 Here, enasidenib monotherapy achieved an ORR of 40.3%, with a median duration of response of 5.8 months and a median OS of 9.3 months in R/R IDH2-mutated AML patients.40
GO also received approval by the FDA (not the EMA) for R/R patients with CD33+ AML and can be added to intensive therapy. So if our patient relapses after HSCT with the same molecular profile (FLT3-ITD pos, IDH2 mut), targeted therapy is available with gilteritinib or enasidinib (Figure 2).
Treatment of relapsed/refractory AML patients considering recently novel substances. dDLI, donor lymphocyte infusion; BSC, best supportive care; HU, hydroxyurea; mut, mutated.
Treatment of relapsed/refractory AML patients considering recently novel substances. dDLI, donor lymphocyte infusion; BSC, best supportive care; HU, hydroxyurea; mut, mutated.
Further directions
Various agents currently undergo evaluation in early clinical AML trials. Magrolimab is a monoclonal anti-CD47 antibody. CD47 is known to be a macrophage immune checkpoint that functions as a “don't eat me” signal to cancer so that magrolimab can promote phagocytosis of leukemic cells. Magrolimab was combined with azacitidine in a phase 1b trial enrolling 52 AML patients.41 In this trial, 65% of patients achieved an objective response, with 44% of patients achieving a CR. Magrolimab received orphan drug designation by the FDA for myelodysplastic syndrome (MDS) and AML and by the EMA for AML in 2020. APR-246 is a promising agent developed for patients with mutated TP53, as it restores its function as a tumor suppressor gene.42 Early trials have combined APR-246 with azacitidine in MDS and AML. In a phase 1/2 trial, the ORR was 64% with a 36% CR rate in TP53-mutated AML patients.43 These encouraging results have been confirmed in a second study showing a CR rate of 56% at 6 cycles. APR-246 has received orphan drug and fast-track designations from the FDA for MDS and orphan drug designation from the EMA for AML and MDS. Menin is a promising target for AML patients with mixed-lineage leukemia translocations, and menin inhibitors are currently being studied in clinical trials.44 Many more substances are in early development for AML patients. Importantly, clinical trials studying novel agents with new combinations are also ongoing (eg, IDH inhibitors with chemotherapy, FLT3 inhibitors with HMAs). Finally, yet importantly, advancement in measurable residual disease monitoring is also likely to influence our treatment decisions and have a positive impact on outcome.45 In summary, the treatment landscape has evolved remarkably over the last 5 years with the approval of a number of new drugs (Table 1). This has direct implications for our 66-year-old patient.
Conflict-of-interest disclosure
Felicitas Thol: Advisory Board: Celgene, Novartis, Jazz, Abbvie, Astellas, Pfizer.
Off-label drug use
Felicitas Thol: nothing to disclose.