Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare complication in pulmonary embolism (PE) survivors, characterized by chronic vascular occlusion and pulmonary hypertension. The identification and diagnosis of CTEPH requires a stepwise approach, starting with symptom evaluation, functional evaluation, screening imaging, and progressing to interventional hemodynamic assessment. On the backbone of anticoagulation, CTEPH management necessitates a multidisciplinary approach. Surgical pulmonary thromboendarterectomy (PTE) is the only potentially curative option. In nonoperable disease or residual disease after PTE, interventional balloon pulmonary angioplasty and/or pulmonary-vasodilator therapies can be offered, in collaboration with interventional and vascular pulmonary colleagues. As it is a disease that can cause high morbidity and mortality, CTEPH requires a high index of suspicion to diagnose and treat in patients following PE.
Learning Objectives
Understand that CTEPH is a rare complication of acute PE that has high morbidity and mortality, necessitating a high index of suspicion
Apply diagnostic algorithms to evaluate patients for CTEPH and understand multidisciplinary treatment strategies, including anticoagulation, surgical and interventional treatments, and pulmonary hypertension therapy
CLINICAL CASE
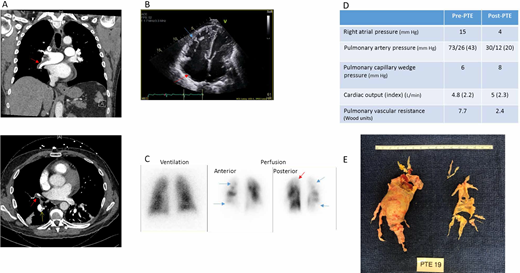
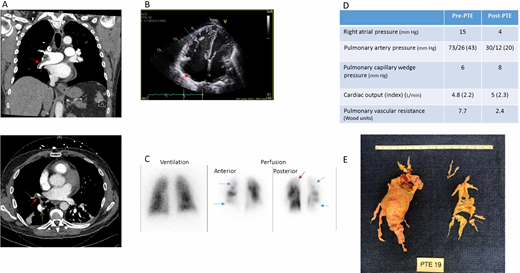
A 55-year-old man with a history of unprovoked pulmonary embolism (PE) 18 months earlier sought treatment for shortness of breath. Although he initially improved, he never returned to baseline functional status. For the past 6 months, dyspnea on exertion has progressed despite appropriate anticoagulation. His oxygen saturation is 92%. Computed tomography (CT) angiogram is obtained and reported to show PE (Figure 1A). Subsequent echocardiogram shows a dilated right ventricle (RV) with severely reduced right ventricular function (Figure 1B). He was diagnosed with submassive PE and underwent catheter directed thrombolysis. Following thrombolysis, his hypoxemia worsened, and he was transferred to our institution for consideration of embolectomy.
Clinical Imaging from a representative case. PTE, pulmonary thromboendarterectomy.
Clinical Imaging from a representative case. PTE, pulmonary thromboendarterectomy.
Introduction
As many as 50% of patients have a chronic functional limitation up to 1 year after PE, termed the “post-PE syndrome,” that is associated with impaired quality of life, dyspnea, and reduced exercise tolerance.1,2 Post-PE syndrome is defined as the presence of functional or cardiac impairment (without another non-PE explanation), chronic thromboembolic disease (CTED), or chronic thromboembolic pulmonary hypertension (CTEPH), occurring after at least 3 months of effective anticoagulation for acute PE.3 CTED and CTEPH share common features of exertional dyspnea and persistent thromboembolic material in the pulmonary artery tree. However, CTED lacks pulmonary hypertension (PH) at rest, whereas in CTEPH, resting PH is present, as defined by a mean pulmonary artery pressure (mPAP) of 25 mm Hg or more, and a pulmonary capillary wedge pressure of 15 mm Hg or less.3 Of note, a change to decrease mPAP to more than 20 mm Hg to define PH has been proposed but is not yet incorporated into diagnostic criteria of CTEPH.4,5
Although a significant number of patients are diagnosed with CTEPH without a known prior acute PE (estimates vary from 25% to 67%),6,7 hematologists are more likely to encounter CTEPH following acute PE. CTEPH affects approximately 1% to 5% of PE survivors.8 One meta-analysis demonstrated the pooled incidence of CTEPH following acute PE to be 0.56% (95% CI, 0.1%-1.0%) for “all-comers,” 3.2% (95% CI, 2.0%-4.4%) for “survivors,” and 2.8% (95% CI, 1.5%-4.1%) for “survivors without major comorbidities.”8 However, when the expected incidence of CTEPH based on the number of incident acute PE cases per year is compared with the number of pulmonary thromboendarterectomy (PTE) surgeries performed each year, it is highly likely that CTEPH is underdiagnosed and therefore undertreated. A study modeling epidemiologic data from Europe, Japan, and the United States, for example, estimated that only 16% of CTEPH cases would be diagnosed in 2015, and 70% or more of those diagnosed would be in the setting of advanced heart failure.9 Furthermore, data from both a survey of international physicians and retrospective claims study showed that diagnostic tests to evaluate CTEPH are underused.10,11 As CTEPH has high morbidity and mortality—causing premature death in more than 50% of untreated patients within 5 years of diagnosis—but has potentially curative interventions, a high index of suspicion is critical to identify the disease.12
When to suspect CTEPH
CTEPH and/or CTED should be considered in PE survivors with persistent dyspnea for more than 3 months after a diagnosis of acute PE, despite adequate anticoagulation, or in those who initially improve but subsequently develop worsening functional limitations and dyspnea with exercise between 3 and 24 months following acute PE. Onset of CTEPH is rare after 24 months following initial PE diagnosis.13 In addition, CTEPH should be suspected if echocardiogram obtained for suspected acute PE shows increased RV wall thickness or tricuspid valve peak systolic gradient more than 60 mm Hg, both of which suggest changes beyond acute RV overload.14 Symptoms of CTEPH can be insidious, such as exercise intolerance and dyspnea on exertion, or more severe, such as leg swelling, chest pain, and syncope that can occur with right heart (RH) failure. As screening echocardiogram in all PE survivors has a high false-positive rate,15 evaluation for CTEPH should be reserved for those with symptoms.16
Numerous risk factors have been historically associated with CTEPH, including splenectomy, chronic inflammatory disorders, indwelling catheters, elevated factor VIII, and unprovoked or recurrent venous thromboembolism (VTE), among others, reviewed elsewhere.17 More recently, in a post hoc patient-level analysis of 3 large prospective cohorts of more than 700 PE survivors, of whom 2.8% developed CTEPH, predictors of CTEPH were unprovoked PE (odds ratio [OR], 20; 95% CI, 2.7 to >100), onset of symptoms more than 14 days prior to diagnosis (OR, 7.9; 95% CI, 3.3-19), hypothyroidism (OR, 4.3; 95% CI, 1.4-13), and RV dysfunction on presentation (OR, 4.1; 95% CI, 1.4-12), whereas diabetes mellitus and thrombolytic therapy had infinitely low OR for developing CTEPH.18
How to identify and diagnose CTEPH
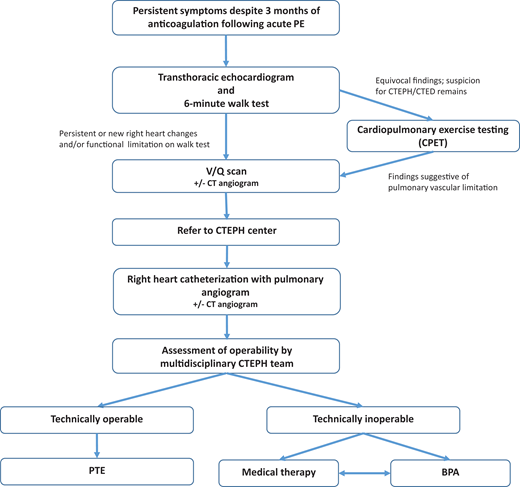
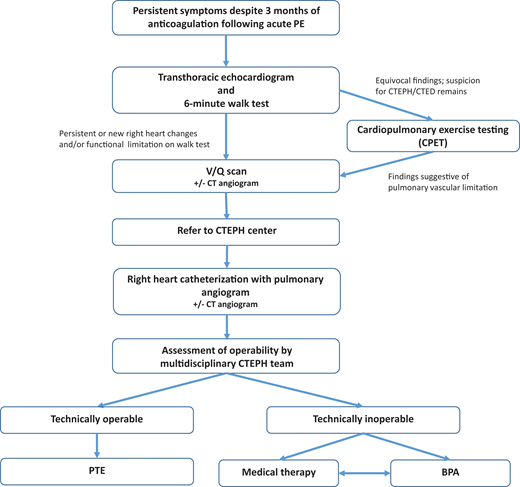
When CTEPH/CTED is suspected, a stepwise evaluation aims to identify pulmonary vascular disease related to nonresolving thrombus (Figure 2). The diagnostic evaluation also concurrently allows for assessment of treatment options and surgical candidacy.
Although no universally agreed-on diagnostic algorithm exists to evaluate for CTEPH in a patient post-PE with dyspnea, we start with an echocardiogram coupled with a basic functional test such as the 6-minute walk test (6MWT).19 Several guidelines also propose diagnostic strategies.4,14 Notably, however, if PH is strongly suspected, then we eliminate the 6MWT. Echocardiogram is used to estimate probability of PH, with intermediate to high probability suggested by estimated peak tricuspid valve regurgitation velocity more than 2.8m/s, or 2.8 m/s or less associated with at least one of the following: RV end diastolic diameter (EDD) more than 30 mm or RVEDD/left ventricular EDD more than 0.9, hypokinesia of the RV free wall, or exertional dyspnea.3,14 Importantly, echocardiogram allows for screening without exposure to radiation or contrast dye.20 The 6MWT is a simple test that can be performed in any clinic. As a patient walks for 6 minutes, distance walked, heart rate, and oxygen saturation by pulse oximetry are measured. The 6MWT provides a basic assessment of cardiopulmonary function and fitness, along with information on functional, cardiovascular, and gas exchange response to exercise. Abnormalities in either echocardiogram or 6MWT in a symptomatic patient more than 3 months beyond acute PE should trigger further evaluation.
Occasionally, more advanced exercise testing is needed to evaluate persistent symptoms. Formal cardiopulmonary exercise testing plays a key role in the evaluation of dyspnea in a patient post-PE, and guidelines suggest using cardiopulmonary exercise testing in patients with low probability of PH on echocardiography but continued suspicion for CTEPH/CTED based on symptoms.16 The pattern of increased dead space ventilation with a widening A-a gradient and flattened stroke volume in response to exercise suggests a pulmonary vascular limitation and need for further invasive testing.
Once a functional limitation and/or appropriate echocardiographic abnormalities raise sufficient concern for CTEPH/CTED, the next step in evaluation is documenting unresolved vascular occlusion. Although a full discussion of the roles of CT angiography (CTA) and ventilation-perfusion (VQ) scanning in the evaluation of CTEPH is beyond the scope of this article, both imaging modalities can add important information in the assessment and determining appropriate intervention (Table 1). While the gap between sensitivity of V/Q and CTA scan is narrowing, VQ scan remains the preferred test for screening for CTEPH/CTED,4 as nonocclusive changes to the vessels that occur in CTEPH (such as mural thrombi, webs, and strictures) can be missed on routine CTA reads but are identifiable on VQ because of their effect on perfusion to distal areas of the lung.21 Furthermore, VQ has lower exposure to radiation and no contrast dye exposure.21 Notably, however, VQ scans are underused: in 1 study, 43% of patients undergoing workup for PH did not get the recommended VQ scan.22
After associating ongoing symptoms, functional limitation, and/or echocardiographic changes to perfusion abnormalities on imaging, patients should be referred to an experienced CTEPH center for right heart catheterization (RHC) with pulmonary angiogram (PA gram).16 The diagnosis of PH of any kind, including CTEPH, relies on a comprehensive hemodynamic assessment completed during an RHC. These data are critical for confirming the correct PH diagnosis and providing prognostic information that informs treatment decisions.23 Coupling a diagnostic RHC with PA gram to better define chronic thromboembolic lesions not only allows for diagnostic and prognostic information but are the final data needed to determine surgical candidacy.
CLINICAL CASE (continued)
On arrival to our institution, the patient had persistence of his chronic dyspnea despite thrombolysis and anticoagulation, which raised suspicion for CTEPH. On review of CTA by experienced radiologists, findings of eccentric mural thrombi and webs were more consistent with CTEPH, and VQ scan confirmed marked ventilation perfusion mismatches (Figure 1A and 1C). He underwent RHC with PA gram, which confirmed CTEPH.
Management considerations of CTEPH
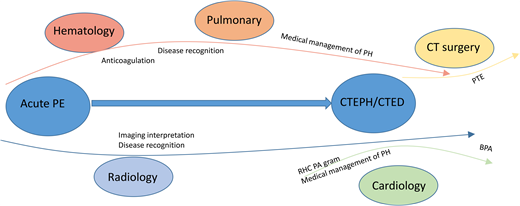
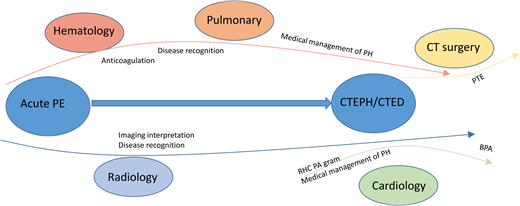
The backbone of treatment of CTEPH requires lifelong anticoagulation to prevent acute VTE recurrence. There are also 3 other treatment arms (detailed below) used to address the chronic vascular occlusions and PH to improve hemodynamics and quality of life: (1) surgical PTE, (2) interventional balloon pulmonary angioplasty (BPA), and (3) pulmonary vasodilator medications. Treatment strategy is selected based on an individual patient and hemodynamic characteristics. Although the focus of PTE and BPA is typically patients with CTEPH, it is important to note that patients with CTED may also be offered surgical or procedural interventions if symptoms affect quality of life.16 Although anticoagulation falls within the hematology expertise, definitive surgical, interventional, and PH medical therapy for CTEPH necessitates a multidisciplinary team involving hematology, cardiothoracic surgery, radiology, cardiology, and pulmonology.
Anticoagulation
Anticoagulation is recommended as standard of care for CTEPH (so long as minimal bleeding risk). Of note, whether chronic anticoagulation is indicated in CTED is not clear, and no guidelines offer formal recommendations; in our practice, we continue anticoagulation in symptomatic CTED to prevent VTE recurrence if low bleeding risk. Vitamin K antagonists (VKAs) are currently recommended as the anticoagulant of choice in patients with CTEPH given historical experience, as data on direct-acting oral anticoagulants (DOACs) are limited.16 A retrospective study compared outcomes following PTE in those treated with warfarin (n = 700) and DOACs (n = 200) and found significantly higher rates of VTE recurrence with DOACs (4.62%/person-year) compared with VKA (0.76%/person-year) (P = .008), with no difference in overall survival and similar rates of major bleeding (0.67%/person-year vs 0.68%/person-year for VKA and DOAC, respectively).24 Furthermore, VTE recurrence occurred at a median of 5.8 months (interquartile range 5.4 months) after PTE, suggesting choice of anticoagulant in the first 6 months may be particularly important, although this requires further evaluation. In the absence of robust data, in our practice, we treat with VKA for 6 months following PTE and then allow for switch to DOAC per patient preference. Prospective studies are needed to determine optimal anticoagulation strategy following PTE. In addition, pulmonologists and/or cardiothoracic surgeons may need hematology expertise on optimal anticoagulants in distinct clinical situations, such as heparin-induced thrombocytopenia and antiphospholipid syndrome (APS). Reported rates of APS in CTEPH cohorts range from 3% to 7%,25 and although routine thrombophilia testing does not affect management in patients with CTEPH who require lifelong anticoagulation, the presence of APS would, given evidence of higher rates of recurrent thrombosis in patients with APS taking rivaroxaban compared with VKA.26,27
Pulmonary thromboendarterectomy
PTE remains the only potentially curative option for CTEPH and thus is considered the gold standard where appropriate. All patients should be referred to an experienced center to determine whether obstructive pulmonary artery lesions are amenable to surgical removal (“technically operable”) and whether the patient is a surgical candidate. As PTE is technically challenging, patients should be referred to a program with an experienced surgeon, considered to be more than 30 to 50 PTEs performed annually.16 Between 10% and 50% of patients are deemed nonoperable,6 and these patients should be referred for a second opinion at a center with significant experience. Current in-hospital mortality rates following PTE are less than 5% (<2% in experienced centers),28 and survival exceeds more than 90% at 1 year and more than 70% at 10 years.29 Hematologists are frequently asked about the role of inferior vena cava (IVC) filters perioperatively. Although IVC filters have fallen out of favor prior to most surgeries given a lack of clear mortality benefit and increased complication risks, there are no dedicated prospective or observational studies specifically in the CTEPH population.30 However, given that this patient population is committed to lifelong anticoagulation, the routine placement of IVC filters is not currently recommended.31
Balloon pulmonary angioplasty
BPA is available for (1) inoperable CTEPH, because of the presence of either distal lesions not amenable to surgical intervention (“technically inoperable”) or comorbidities that preclude surgery, or (2) persistent or recurrent CTEPH following PTE. BPA is performed by catheterization via femoral or jugular access and does not require general anesthesia or circulatory arrest. Using image guidance, a balloon is inflated at the site of the obstructive lesion to compress the intravascular fibrotic occlusion against the wall, in an attempt to restore the vascular lumen. Most patients require multiple sessions for BPA, with an number of sessions between 4 and 8, typically over several months.32 Studies have shown efficacy of BPA in reducing pulmonary vascular resistance (PVR) and increasing functional activity, with low rates of complications.33,34 Postprocedural complications occur in approximately 10% of patients, including pulmonary vascular injury (eg, wire perforation), reperfusion injury, and pulmonary hemorrhage. Critically, hematologists should be aware of BPA as a treatment option and refer patients with nonoperable CTEPH to a center with BPA expertise.
Medical therapy of PH
Medical therapy of PH should be addressed by pulmonary vascular specialists with expertise in PH. Currently, riociguat is the only approved therapy for the treatment of inoperable or residual/recurrent CTEPH following PTE. A soluble guanylate cyclase stimulator, riociguat works via the nitric oxide pathway to promote vasodilatation of the pulmonary arterial bed and has been shown to improve 6-minute walk distance and decrease PVR.35 Published case series and clinical trials have explored the role of other pulmonary vasodilators such as synthetic prostacyclins and endothelin receptor antagonists for the treatment of CTEPH, but riociguat currently remains the only approved therapy.36-38 Although the role of targeted PH therapies in patients with operable CTEPH remains unclear, limited available data do not support a role for “pretreatment” pending PTE surgery. Further research is needed to determine whether high-risk subsets of patients, such as those with very elevated PVR, could benefit.39,40
How to manage patients with CTEPH after PTE
Long-term management of CTEPH involves anticoagulation and monitoring for recurrent symptoms. Currently, all patients who have a CTEPH diagnosis are maintained on indefinite anticoagulation to prevent recurrent VTE. The optimal intensity is unclear; currently, most are maintained on therapeutic doses if bleeding risk is low. Although PTE is potentially curative, up to 35% of patients have a mPAP of 25 mm Hg or more post-PTE.41-43 In addition, most studies follow patients for only 5 years after PTE, and recurrent PH may occur as late as 10 years after surgery, suggesting incidence of recurrent PH may be even higher.41 Therefore, long-term monitoring is essential, with particular attention on assessing for symptoms suggestive of recurrent disease. If recurrent CTEPH occurs, options include BPA and/or medical PH therapy. In rare instances of recurrent severe disease, repeat PTE may be performed.
Conclusion
CTEPH is a rare but significant complication in PE survivors, with high rates of morbidity and mortality. Therefore, it requires awareness of and attentiveness to symptoms, which, if present, should prompt an appropriate diagnostic evaluation. Once CTEPH is diagnosed, management requires multidisciplinary care. Although PTE remains the only potentially curative option, BPA and medical therapy for PH are options for nonoperable CTEPH that can improve symptoms and cardiopulmonary function. Further studies should evaluate optimal long-term anticoagulant agent and intensity for both CTEPH and CTED, the preferred strategy (CTEPH vs BPA) for different patient risk profiles, and role of combination therapy.
CLINICAL CASE (continued)
Upon review of the patient's case in a multidisciplinary CTEPH conference, he was deemed a PTE candidate based on clot distribution, associated perfusion abnormalities and hemodynamic changes, age, and lack of comorbidities. He underwent successful PTE with excellent hemodynamic response (Figure 1D and 1E). Two years after PTE, he continues on warfarin. He has no symptoms of dyspnea on exertion or functional limitations.
Conflict-of-interest disclosure
Karlyn A. Martin reports funding from Janssen Scientific Affairs for an investigator-initiated study outside the submitted work.
Michael J. Cuttica reports grants and consulting fees from Bayer, United Therapeutics, and Actelion.
Off-label drug use
Karlyn A. Martin: nothing to disclose.
Michael J. Cuttica: nothing to disclose.