Abstract
The combination of frequently abnormal hemostatic markers and catastrophic bleeding as seen with variceal hemorrhage has contributed to the longstanding misperception that chronic liver disease (CLD) constitutes a bleeding diathesis. Laboratory studies of hemostasis in liver disease consistently challenge this with global coagulation assays incorporating activation of the protein C pathway demonstrating rebalanced hemostasis. It is now recognized that bleeding in CLD is predominantly secondary to portal hypertension (rather than a coagulopathy) and additionally that these patients are at increased risk of venous thrombosis, particularly in the portal venous system. This narrative review describes the current understanding of hemostasis in liver disease, as well as the periprocedural management of hemostasis and anticoagulation for management of venous thromboembolism in patients with CLD.
Learning Objectives
Describe the changes leading to rebalanced hemostasis in CLD
Recognize factors influencing periprocedural bleeding risk in patients with CLD
Evaluate the need for anticoagulation of PVT in patients with liver disease and select the most suitable agent
CLINICAL CASE
A 46-year-old woman with a new diagnosis of presumed alcohol-related cirrhosis presents to her local hospital with increasing abdominal swelling, pain, and jaundice. She had been admitted 1 year prior with alcohol withdrawal but did not attend planned hepatology outpatient reviews. She has continued to drink 0.5 L of vodka daily. Initial laboratory investigation reveals the following: hemoglobin, 90 g/L; platelets, 43 × 109/L; bilirubin, 199 µmol/L; albumin, 26 g/L; international normalized ratio (INR), 2.2; prothrombin time (PT), 33 seconds; and creatinine, 126 µmol/L. The Child-Turcotte-Pugh class is C and Model for End Stage Liver Disease score is 25. She commences spironolactone but there is no improvement in ascites or weight, and her renal function further deteriorates. Therapeutic paracentesis is planned and the medical team requests authorization of fresh-frozen plasma (FFP) (aiming for an INR of <1.5) to proceed.
The natural history of chronic liver disease (CLD) is characterized by phases of stable cirrhosis progressing to acute decompensation (AD), from which patients may recover or progress to multiorgan failure, known as acute-on-chronic liver failure (ACLF).1 Most patients admitted to the hospital are in a state of AD, often following a precipitating event. Common complications pertain to liver failure or portal hypertension and comprise jaundice, encephalopathy, ascites, and variceal bleeding. Although bleeding is common in liver disease, it is now recognized that most spontaneous bleeding events are predominantly gastrointestinal (GI) and secondary to portal hypertension (refer to Table 1 for incidence).2-4
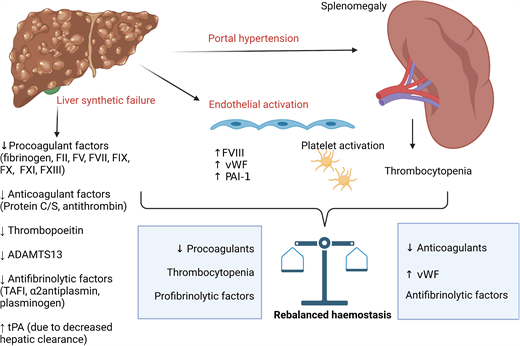
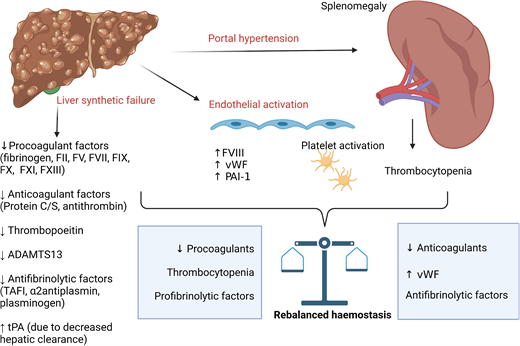
The liver has a key role in the synthesis of both pro- and anticoagulant proteins, along with pro- and antifibrinolytics and thrombopoietin.9 Portal hypertension in CLD leads to splenomegaly and thrombocytopenia. Progression of CLD with synthetic failure is accompanied by prolongation of PT.10 As PT simply measures time to first detection of clot in plasma following activation with tissue factor and calcium, it does not reflect in vivo hemostasis in patients with CLD in which natural anticoagulants (protein C, protein S, and antithrombin) are reduced in parallel, and it does not detect increased factor VIII and von Willebrand factor. Global coagulation assays, such as thrombin generation incorporating activation of the protein C pathway, demonstrate that patients with CLD have rebalanced hemostasis with some evidence of hypercoagulability. With increasing disease severity (as in ACLF), wide interindividual variation in thrombin generation potential is seen with a more precarious hemostatic balance.10-12 Similarly, thrombocytopenia and platelet dysfunction are countered by increased von Willebrand factor, with increased high molecular weight multimers due to reduced ADAMTS13.13,14 The changes in hemostasis associated with CLD are summarized in Table 2.
Periprocedural bleeding risk
It has been repeatedly demonstrated over the past 40 years that neither prolonged PT/INR nor thrombocytopenia independently predict periprocedural bleeding.9,15-17 These parameters continue to be measured with attempts at correction common in this setting, despite potential for harm associated with transfusion.18,19
Procedural bleeding risk in patients with CLD is not well defined; variable practice in attempting to correct hemostatic markers and a lack of consistent definition for major bleeding are key contributory factors.20 It is proposed that procedures with an incidence of major bleeding of more than 2% or bleeding with potential to cause organ damage/death be considered high risk.20 Ascites is a frequent complication of CLD, developing in approaching two-thirds of patients within 10 years of cirrhosis diagnosis.21 Therapeutic paracentesis is recommended as first-line treatment for large-volume ascites, and this scenario is commonly encountered. The risk of bleeding following paracentesis is low, estimated at 0.2%.22 Acute kidney injury is a recognized independent risk factor for postparacentesis hematoperitoneum.23
Prophylactic hemostatic interventions
Despite evidence that major surgery such as liver transplantation can be safely performed without correcting abnormal hemostatic markers,24 attempts at correction remain common in the procedural setting.18,19 In Spain, a national survey (of predominantly hepatologists, 69%) from 2017 reported the majority of respondents would attempt to correct PT prior to major procedures, with 17% also attempting to correct prior to low-risk procedures.19 Almost all respondents reported attempting to correct thrombocytopenia prior to major surgery, with 35% also attempting to correct prior to low-risk procedures, predominantly using a threshold for platelets of less than 50 × 109/L. However, attempts to correct thrombocytopenia and coagulopathy with transfusion are associated with additional risks. Administration of a “therapeutic” volume of FFP predisposes to both transfusion-associated circulatory overload and transfusion-related acute lung injury.25 Furthermore, due to subsequent increases in portal venous pressure,26 FFP administration may paradoxically increase the risk of bleeding (eg, during endoscopic variceal band ligation or transjugular portosystemic shunt placement). In addition, laboratory studies demonstrate that patients with CLD have preexisting normal to hypercoagulable profiles, with FFP transfusion having a minimal impact on improving PT but increasing hypercoagulability.27,28 Platelet transfusion results in variable platelet count increment,28,29 and randomized controlled trials of platelet transfusion in other populations (eg, intracerebral bleeding) are associated with worse outcomes.30 Thrombopoietin receptor agonists have been demonstrated to better correct platelet count prior to elective procedures.31,32 However, the seminal studies included both low- and high-risk procedures and lacked clinically important primary outcomes. Meta-analysis suggests their use may reduce periprocedural bleeding.33
Given the risks association with plasma transfusion and lack of evidence to demonstrate benefit, correction of prolonged PT prior to paracentesis has been advised against since 2003 by international societies. Recommendations for high-risk bleeding procedures are summarized in Table 3.20,34-36 Vitamin K deficiency may be relevant in those with poor diet and/or malabsorption, and a single dose of 10 mg more than 12 hours prior to interventions in such patients is advocated by some.35,36
CLINICAL CASE (Continued)
This patient received a single dose of vitamin K on admission with no improvement in the PT. After reassuring the medical team that the procedural bleeding risk of paracentesis is low and that PT/INR and platelet count are not a measure of bleeding risk, therapeutic paracentesis is performed under ultrasound guidance without complication.
She recovers from this acute episode of decompensation and remains abstinent from alcohol following hospital discharge. On routine screening ultrasound for hepatocellular carcinoma 2 years later, she is diagnosed with occlusive portal vein thrombosis (PVT) extending to the superior mesenteric venous junction. The spleen is enlarged (20 cm), and the patient has the following laboratory values: hemoglobin, 110 g/L; platelets, 57 × 109/L; bilirubin, 16 µmol/L; albumin, 40 g/L; PT, 18 seconds; INR, 1.3; sodium, 138 µmol/L; and creatinine, 116 µmol/L. She is Child-Turcotte-Pugh class A, and Model for End Stage Liver Disease score is 13. An upper GI endoscopy performed during the previous admission revealed mild portal hypertensive gastropathy with no esophageal varices. The medical team seek advice on the role for anticoagulation in the context of thrombocytopenia.
Venous thromboembolism in CLD
PVT is the most common thrombotic event affecting patients with CLD, with its prevalence reported in up to 26% in those listed for liver transplantation and increasing in parallel with disease severity.38 CLD is also associated with an increased risk of deep vein thrombosis (DVT) and pulmonary embolism. A meta-analysis of predominantly retrospective cohort studies reported cirrhosis was associated with an increased odds ratio for venous thromboembolism (VTE) of 1.7 (95% CI, 1.3-2.2).39 The incidence of PVT in recent prospective studies is summarized in Table 1. The Italian observational cohort of 753 patients with cirrhosis reported an incidence rate of 6 per 100 patient years.5 Of note, half of the events were asymptomatic and detected on routine screening for hepatocellular carcinoma. Those with prior PVT at study entry had a significantly higher rate of PVT compared with those without (18.9 vs 4.0 per 100 patient years).5 The only independent risk factors for PVT identified in this study were previous PVT and degree of thrombocytopenia (with lower counts associated with increased risk). A further prospective observational cohort of 241 patients with cirrhosis reported a cumulative incidence of 3.7% and 7.6% at 1 and 3 years, respectively.8 Thrombocytopenia and previous decompensation were identified as independent predictors of PVT. Of note, in both cohorts, most patients were Child-Pugh class A (see Table 1).
This scenario illustrates again that conventional laboratory tests do not reflect the underlying hemostatic milieu in patients with liver disease. The paradoxical increase in PVT risk associated with thrombocytopenia likely reflects increasing severity of both liver disease and portal hypertension with reduced portal venous flow.8,40 Only a single, small retrospective study (n = 53) has examined the influence of hypercoagulability measured with thrombin generation on PVT risk.41 Patients with baseline “thrombomodulin resistance” (defined as an endogenous thrombin potential ratio >95th percentile of normal controls) had an 8-fold increase in risk of PVT over 4 years. Local hypercoagulability mediated by bacterial translocation, inflammation, and endotoxemia are proposed as additional contributors but have not been confirmed in prospective studies.42
Anticoagulation in CLD
The need for anticoagulation in this scenario of incidental PVT in the absence of intestinal ischemia or planned liver transplantation (in which anastomosis may be compromised) is uncertain. A recent meta-analysis of 33 studies comprising 1696 patients with PVT reported spontaneous complete recanalization in 18% of patients (n = 33/180) not receiving anticoagulation. However, anticoagulation was associated with a significant 2-fold improvement in overall and complete recanalization rates compared with no anticoagulation. The risk of thrombus progression was also significantly reduced (risk ratio [RR], 0.26; 95% CI, 0.14-0.49). Six studies reported overall survival, with anticoagulation associated with improvement (RR, 1.11; 95% CI, 1.03-1.21).43 There are clearly a number of limitations to this analysis. In our case, given the thrombus is occlusive and extends to the mesenteric junction, anticoagulation is likely to be beneficial in preventing further extension to the mesenteric vasculature and achieving recanalization, with potential reduction in portal hypertension (given the lack of portosystemic collateral circulation). Guidelines recommend obtaining cross-sectional imaging (computed tomography/magnetic resonance imaging) to confirm the diagnosis and extent of thrombosis, as well as to exclude malignant obstruction prior to treatment initiation.20 There is a clear role for anticoagulation in patients with symptomatic PVT to prevent progression and intestinal ischemia.
The principal concern in anticoagulating patients with liver disease is the concomitant increased risk of bleeding. In the aforementioned meta-analysis, the pooled overall rate of bleeding associated with anticoagulation from 19 studies was 2.8%, with fatal bleeding 0.7% (from 25 studies).43 The pooled rate of esophageal variceal bleeding from 17 studies was 2%. The use of anticoagulation did not increase the risk of bleeding (RR, 0.78; 95% CI, 0.47-1.3) from 4 studies reporting outcomes in those both on and off anticoagulation, and interestingly, anticoagulation was associated with a reduced risk of both upper GI and variceal bleeding (RR, 0.26; 95% CI, 0.11-0.65).43 These data suggest anticoagulation does not increase the risk of variceal hemorrhage (and may in fact reduce the risk, potentially by reducing portal hypertension). It is recommended that upper GI endoscopy be performed to identify and enable treatment of high-risk varices.20 In practice, anticoagulation can be initiated while awaiting further endoscopic evaluation. Endoscopic variceal banding is a low bleeding risk procedure,20 with procedural bleeding uncommon and the majority of bleeding secondary to band ulceration, presenting some days later.15,44 A single observational study found no increased bleeding risk associated with use of low molecular weight heparin (LMWH) (169 procedures in 80 patients) at the time of variceal band ligation.45 However, proceduralists may temporarily interrupt anticoagulation following variceal banding when the perceived bleeding risk due to secondary band ulceration is thought to outweigh the risk of thrombosis extension.
Thrombocytopenia is a recognized risk factor for bleeding with anticoagulation; a small observational cohort reported a platelet count of less than 50 × 109/L as a predictor for bleeding.46 In these patients, I consider initiating LMWH at prophylactic to intermediate doses and titrate the dose as tolerated. I favor intermediate doses for symptomatic and/or extensive PVT. For limited extent/asymptomatic events in which a decision for anticoagulation is made, I favor prophylactic doses at initiation. Severe thrombocytopenia (<30 × 109/L) is uncommon, and in such cases, I would look for and treat contributing factors but still consider prophylactic LMWH on a case-by-case basis. If the platelet count improves, therapeutic doses can then be used. As this patient has a platelet count more than 50 × 109/L, full-dose anticoagulation should be used.
For venous thromboembolism in patients without CLD, direct oral anticoagulants (DOACs) are now the agents of choice due to their predictable pharmacokinetics, eliminating the need for routine drug monitoring and favorable safety profile.47 Characteristics of available anticoagulants are summarized in Table 4. Of note, patients with CLD were excluded from the randomized controlled trials establishing their efficacy in VTE, and there is little evidence for use of DOACs in treatment of PVT in cirrhosis. The use of DOACs is not recommended in patients with increased severity of CLD (see Table 4). A recent systematic review identified 5 retrospective cohort studies comprising 239 patients with CLD treated for DVT, splanchnic vein thrombosis, or atrial fibrillation.48 The analysis suggests comparable efficacy and safety but is limited by the small sample size and retrospective nature of the studies. A small randomized controlled trial (n = 80) in Egypt compared rivaroxaban 10 mg daily with warfarin for treatment of acute PVT in patients with compensated hepatitis C cirrhosis (predominantly after splenectomy) for a variable treatment duration based on thrombus extent and recanalization.49 Patients were monitored fortnightly with ultrasound imaging with a primary outcome of complete recanalization. The primary outcome was achieved in significantly more patients receiving rivaroxaban (85% vs 45%), with recanalization occurring at a mean of 2.5 months. There was no major bleeding reported in the rivaroxaban arm and no recurrent events at 12 months. A further small study comparing outcomes in those treated with edoxaban (n = 20) to warfarin (n = 30) following 2 weeks of danaparoid also reported superior clot regression with edoxaban.50 Although these findings are promising, it is important to note that neither warfarin arm provided standard of care in that there was no bridging until therapeutic INR was reached, with a subtherapeutic INR target (1.5-2.0) in 1 study. This is important as recanalization rates are improved with early anticoagulation.46 Further adequately powered studies with the use of LMWH bridging until therapeutic INR is achieved in the comparator arm are required. In practice, LMWH is often initiated until completion of upper GI endoscopy, following which a vitamin K antagonist (VKA) can be initiated. DOACs may be a reasonable alternative in cases with difficulty complying to variable dosing of VKA or the monitoring requirements (eg, limited venous access and/or unable to use a point-of-care INR device). For patients with significantly prolonged PT at baseline, I favor extended LMWH provided continued parenteral administration is acceptable to the patient, given the optimal INR target is unknown in those with significantly raised baseline INR.
For PVT, in which a decision to initiate anticoagulation is made, the minimum recommended duration of anticoagulant therapy is 6 months.20,37 Guidelines suggest reimaging to evaluate recanalization to guide the ongoing need for anticoagulation.20 There are, however, no high-quality data to inform whether incomplete recanalization is associated with a higher risk of recurrence or other adverse outcomes related to portal hypertension. Small observational studies suggest the risk of early recurrent PVT is high (up to 38%) following discontinuation of anticoagulation in patients with advanced CLD.42,46 In addition, previous PVT is a strong predictor for recurrence in prospective observational cohorts.5 However, given the lack of randomized controlled trials to definitively inform the risk of both PVT recurrence and bleeding, the feasibility of a multicenter prospective study to determine the optimal duration of anticoagulation should be evaluated. Currently, those listed for liver transplantation frequently remain on extended anticoagulation to reduce the risk of recurrent PVT, particularly as the presence of occlusive PVT at transplantation affects both technical success and posttransplant survival.51 For patients with DVT/pulmonary embolism, the minimum duration of anticoagulation is 3 months. All patients should be reviewed prior to completing 3 months of treatment to consider the need for extended anticoagulation in the presence of persistent risk factors (eg, inflammatory bowel disease) and/or for events not associated with a major provoking factor (eg, major surgery/hospitalization).52
CLINICAL CASE (continued)
Therapeutic anticoagulation with LMWH was initiated while further endoscopy was planned (unchanged from previous, with no esophageal varices seen with mild portal hypertensive gastropathy). Following this, warfarin was initiated with a target INR of 2.5. LMWH was continued until an INR of more than 2 was achieved. She remained on warfarin for 12 months, and reimaging confirmed partial recanalization of the portal vein. Apart from minor traumatic bruising, there were no significant bleeding complications during treatment.
Conclusion
In summary, despite frequently deranged hemostatic markers in patients with CLD, there is a dual propensity to bleeding and thrombosis (with a preponderance of portal vein thrombosis). Variceal bleeding secondary to portal hypertension is the most frequent bleeding manifestation. There is little evidence to support prophylactic correction of hemostatic markers in the periprocedural setting, and use of preemptive transfusion support should be avoided. Anticoagulation is likely to improve outcomes following occlusive portal vein thrombosis, even in the presence of hemostatic abnormalities. However, the optimal duration of anticoagulation and role of DOACs remains uncertain.
Conflict-of-interest disclosure
Lara N. Roberts: no competing financial interests to declare.
Off-label drug use
Lara N. Roberts: nothing to disclose.