Abstract
Clinicians generally counsel patients with a history of heparin-induced thrombocytopenia (HIT) to avoid heparin products lifelong. Although there are now many alternative (nonheparin) anticoagulants available, heparin avoidance remains challenging for cardiac surgery. Heparin is often preferred in the cardiac surgery setting based on the vast experience with the agent, ease of monitoring, and reversibility. To “clear” a patient with a history of HIT for cardiac surgery, hematologists must first confirm the diagnosis of HIT, which can be challenging due to the ubiquity of heparin exposure and frequency of thrombocytopenia in patients in the cardiac intensive care unit. Next, the “phase of HIT” (acute HIT, subacute HIT A/B, or remote HIT) should be established based on platelet count, immunoassay for antibodies to platelet factor 4/heparin complexes, and a functional assay (eg, serotonin release assay). As long as the HIT functional assay remains positive (acute HIT or subacute HIT A), cardiac surgery should be delayed if possible. If surgery cannot be delayed, an alternative anticoagulant (preferably bivalirudin) may be used. Alternatively, heparin may be used with either preoperative/intraoperative plasma exchange or together with a potent antiplatelet agent. The optimal strategy among these options is not known, and the choice depends on institutional experience and availability of alternative anticoagulants. In the later phases of HIT (subacute HIT B or remote HIT), brief intraoperative exposure to heparin followed by an alternative anticoagulant as needed in the postoperative setting is recommended.
Learning Objectives
Recognize the phases of HIT and implications for heparin reexposure for CV surgery
Understand the indications and potential alternative (nonheparin) anticoagulants for use in CV procedures and surgeries
Introduction
Heparin-induced thrombocytopenia (HIT) is a highly prothrombotic state resulting from pathogenic antibodies to platelet factor 4/heparin (PF4/H) complexes.1 Clinicians generally counsel patients who experience this potentially life-threatening adverse reaction to never receive heparin again. With the development of many alternative (nonheparin) anticoagulants, avoiding heparin in most circumstances (eg, venous thromboembolism treatment) is not difficult.2 Cardiovascular (CV) surgery is a unique scenario in which heparin is highly preferred given the vast experience with the drug, the ease of monitoring with a point-of-care assay (activated clotting time), and a readily available reversal agent (protamine).3 It is not uncommon for hematologists to be asked to “clear” a patient with a history of HIT for CV surgery. Here we present our approach to evaluating and managing such patients.
CLINICAL CASE
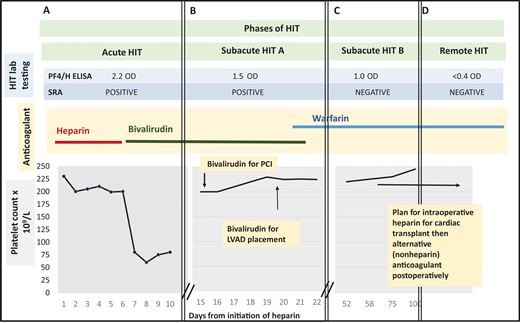
A 45-year-old man with ischemic cardiomyopathy and a history of left ventricular thrombosis receiving warfarin is admitted with worsening dyspnea. Warfarin is held and an unfractionated heparin infusion is started. He develops acute thrombocytopenia on hospital day 7, and a lower extremity ultrasound reveals a new popliteal vein thrombosis (Figure 1A). A 4Ts score is calculated to be 7 points (high probability). The clinical team switches the heparin to bivalirudin and sends HIT laboratory testing. The immunoglobulin G–specific PF4/H enzyme-linked immunosorbent assay (ELISA) is 2.2 optical density (OD) units (positive result ≥0.4 units). A few days later, the serotonin release assay (SRA) returns positive.
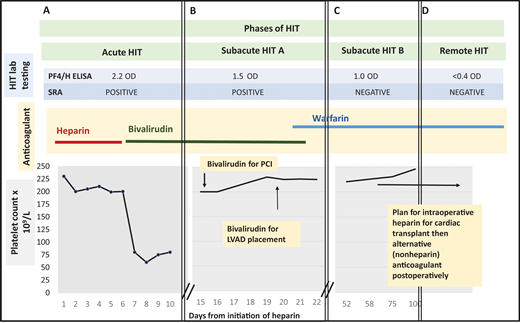
Management of a patient undergoing PCI and cardiac surgery during multiple phases of HIT. (A) Patient develops a fall in platelet count and lower extremity deep vein thrombosis 7 days after initiation of unfractionated heparin. The 4Ts score is 7. Heparin is stopped and the patient is started on bivalirudin. HIT laboratory testing reveals a positive PF4/H ELISA and positive SRA. Acute HIT is diagnosed. (B) At hospital day 15, the platelet count has recovered. The PF4/H ELISA and SRA remain positive, meeting criteria for subacute HIT A. The patient undergoes left heart catheterization with bivalirudin. At hospital day 20, he remains in subacute HIT A and requires LVAD placement that cannot be delayed. He receives bivalirudin during LVAD placement. Postprocedurally, he continues receiving bivalirudin and is bridged to warfarin for discharge to home. (C) The patient is subsequently referred to a hematology clinic for cardiac transplant evaluation. Repeat anti-PF4/H testing remains positive by ELISA (1.0 OD units) 45 days post-HIT diagnosis, but the SRA is now negative, satisfying criteria for subacute HIT B. (D) Approximately 3 months after index admission for HIT, both PF4/H ELISA and SRA are negative. The patient is listed for cardiac transplant with planned brief intraoperative heparin exposure followed by treatment with an alternative anticoagulant postoperatively. PCI, percutaneous cardiac intervention; LVAD, left ventricular assist device; PF4/H ELISA, Platelet factor-4/heparin Enzyme linked immunoassay; SRA, serotonin release assay.
Management of a patient undergoing PCI and cardiac surgery during multiple phases of HIT. (A) Patient develops a fall in platelet count and lower extremity deep vein thrombosis 7 days after initiation of unfractionated heparin. The 4Ts score is 7. Heparin is stopped and the patient is started on bivalirudin. HIT laboratory testing reveals a positive PF4/H ELISA and positive SRA. Acute HIT is diagnosed. (B) At hospital day 15, the platelet count has recovered. The PF4/H ELISA and SRA remain positive, meeting criteria for subacute HIT A. The patient undergoes left heart catheterization with bivalirudin. At hospital day 20, he remains in subacute HIT A and requires LVAD placement that cannot be delayed. He receives bivalirudin during LVAD placement. Postprocedurally, he continues receiving bivalirudin and is bridged to warfarin for discharge to home. (C) The patient is subsequently referred to a hematology clinic for cardiac transplant evaluation. Repeat anti-PF4/H testing remains positive by ELISA (1.0 OD units) 45 days post-HIT diagnosis, but the SRA is now negative, satisfying criteria for subacute HIT B. (D) Approximately 3 months after index admission for HIT, both PF4/H ELISA and SRA are negative. The patient is listed for cardiac transplant with planned brief intraoperative heparin exposure followed by treatment with an alternative anticoagulant postoperatively. PCI, percutaneous cardiac intervention; LVAD, left ventricular assist device; PF4/H ELISA, Platelet factor-4/heparin Enzyme linked immunoassay; SRA, serotonin release assay.
Diagnosis of HIT in patients with CV disease
HIT is a highly feared iatrogenic complication of CV surgery, during which patients are nearly universally exposed to heparin.4 Indeed, when HIT occurs post-CV surgery, it is associated with excess morbidity and mortality. In a propensity score–matched study of 11 820 CV surgery patients, 29.1% of patients who developed HIT after cardiac surgery had a thromboembolic event (compared with 2.9% who did not develop HIT), and postoperative mortality was 21.8% in patients with HIT (vs 5.3% in patients who did not develop HIT). Fortunately, in this study, as in others, HIT was uncommon, occurring in 1.1% of CV surgery patients.5
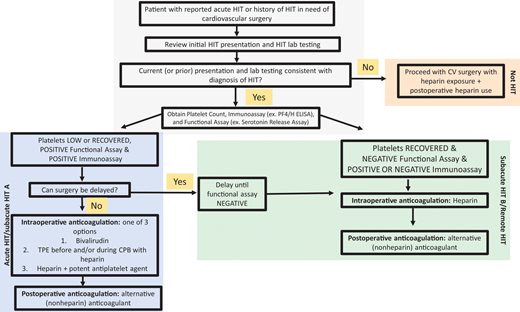
Educational initiatives have increased awareness of what can be a horrendous complication following even the most routine of CV surgeries. However, they have also contributed to an epidemic of overdiagnosis.6 The ramifications of overdiagnosis in patients with CV disease are serious; as such, patients frequently require surgeries or procedures necessitating heparin use. A patient with a history of HIT may have urgent procedures delayed or canceled. In extreme cases, HIT may be the comorbidity that deters clinicians from proceeding with lifesaving measures such as listing for cardiac transplant. Although the diagnosis of HIT alone should never be an absolute contraindication to necessary urgent cardiac interventions, it undoubtedly complicates operative management. Thus, it is of utmost importance to get the diagnosis “right.” The first step of any evaluation for “history of HIT” is to obtain and review the clinical history and laboratory testing that led to the diagnosis. Heparin allergies placed on the chart for suspected HIT are often not removed after HIT has been ruled out.7 Clarifying the HIT diagnosis is vital to determining the anticoagulant strategy for surgery.
In cardiac intensive care unit or CV surgery patients, caution is needed when calculating the 4Ts score (Table 1). First, most patients will have a fall in platelet count following placement of intravascular devices, cardiopulmonary bypass (CPB), or extracorporeal membrane oxygenation that can mimic the degree of fall typical of HIT (≥30%-50% from baseline).8 With intra-aortic balloon pump placement, the platelet count falls a mean 40% from baseline shortly after placement.9 Left ventricular assist devices (LVADs) also cause platelet activation and shear stress, leading to thrombocytopenia.10 Following CPB surgery, 30% to 50% of patients develop thrombocytopenia, with a typical decrease of 50% and a predictable nadir 48 to 72 hours postoperatively.8 Thrombocytopenia that occurs soon after cardiac surgery and persists is rarely indicative of HIT in cardiac surgery patients.11 Rather, a “biphasic fall,” in which the platelet count recovers after the 48- to 72-hour post-CPB fall and then suddenly drops around days 5 to 10 after heparin exposure is more indicative of HIT.12 Second, “dusky digits” are commonly due to prolonged vasopressor use, arterial line placement, peripheral arterial disease, non-HIT disseminated intravascular coagulation, and/or poor cardiac output, not thrombosis.4 There are other prediction scores devised for the CV surgery population (Lillo–Le Louet score)13 or that incorporate features such as the use of an intra-aortic balloon pump or CPB within the past 96 hours (HIT Expert Probability [HEP] score).14 These scores have not been consistently proven to have higher diagnostic accuracy than the 4Ts score in prospective studies.14 However, they may be useful in conjunction with the 4Ts score, particularly in highlighting “other” causes of thrombocytopenia.
Determining an accurate pretest probability of HIT is crucial as HIT antibody testing can be misleading in the CV surgery population. Up to 20% of patients screened before CPB surgery had detectable anti-PF4/H antibodies (most were the nonpathogenic non–immunoglobulin G type). Detection of these antibodies was not associated with an increase in adverse events.15 PF4/H antibodies also frequently develop postoperatively; a multicenter study of approximately 1000 CV surgery patients reported a 50% seroconversion rate as measured using a polyspecific PF4/H ELISA at 30 days after surgery.16 Seroconversion was not associated with increased death or thromboembolism. Thus, it is essential to send HIT laboratory testing only in patients with sufficiently high clinical suspicion of HIT.
CLINICAL CASE (continued)
The patient continues taking bivalirudin for confirmed HIT. His platelet count recovers to baseline approximately 1 week after diagnosis. Unfortunately, the patient's cardiac status worsens. An echocardiogram shows new left ventricular wall motion abnormalities. Cardiology recommends a left heart catheterization (Figure 1B).
Phases of HIT: selection of anticoagulant for cardiac procedures/surgery
HIT generally follows a predictable course, with the platelet count returning to baseline in most patients within 7 days following discontinuation of heparin, followed by negative functional assay at a median of 50 days, and disappearance of anti-PF4/H antibodies at a median of 85 days.17,18 The risk of heparin reexposure depends on the “phase of HIT” (Table 2).19,20 The highest risk is before platelet count recovery and when the functional assay remains positive. Recurrence of HIT with heparin reexposure in the “remote HIT” phase after the immunoassay becomes negative appears to be low.19 However, as most patients avoid heparin after a HIT diagnosis, this risk is not well characterized.
PCI: acute HIT or history of HIT
There are robust data for alternative anticoagulants, particularly bivalirudin, for percutaneous cardiac interventions (PCIs) (Table 3). The American College of Cardiology/American Heart Association guideline gives both heparin and bivalirudin a class I indication for use during primary PCI in patients with ST-elevation myocardial infarction (STEMI).21 A recent unpublished meta-analysis identified 8 randomized controlled trials comparing bivalirudin to heparin for PCI for STEMI or non-ST elevation myocardial infarction. In the pooled data, bivalirudin was associated with a reduction in serious bleeding rates compared with heparin and, in the STEMI subgroup, a 30-day mortality benefit as well.22,23 One prospective study of bivalirudin during PCI exclusively in patients with HIT reported “procedural success” in 98% of patients; only 1 of 52 patients (1.9%) had major bleeding.24 There are also data supporting the use of bivalirudin in other minimally invasive cardiac procedures, including transcathether aortic valve replacement. The effect of Bivalirudin on Aortic Valve Intervention 3 trial randomized 802 patients (HIT and non-HIT) to bivalirudin vs heparin during transcathether aortic valve replacement and found similar rates of major bleeding and adverse CV outcomes.25 Among patients with a history of HIT, there was no significant difference in bleeding rates in the heparin and bivalirudin groups (9.0% vs 10.4%).25
Other alternative anticoagulants studied for PCI include argatroban and danaparoid (not available in the United States). These agents have less data than bivalirudin but may be considered when there is a lack of availability or procedural experience with bivalirudin. In 1 open-label study of 91 patients with HIT who received argatroban for PCI, the “procedural success” rate was 94%.26 There are reports of 61 cases of danaparoid use for invasive vascular procedures (mostly PCI), but outcomes were not reported.27
In acute HIT/subacute HIT A, American Society of Hematology (ASH) 2018 HIT guidelines suggest using bivalirudin over other alternative anticoagulants for PCI. If bivalirudin is not available, argatroban may also be considered.2 In later phases of HIT, subacute HIT B/remote B, the panel also suggests bivalirudin over heparin use for PCI because multiple studies show similar safety and efficacy to heparin. However, this is subject to the availability of the agent and institutional experience.
CLINICAL CASE (continued)
The patient undergoes cardiac catheterization while receiving bivalirudin. No intervenable coronary lesion is identified. The patient remains critically ill. The cardiac team recommends a LVAD. On hospital day 18, HIT laboratory testing is repeated. The PF4/H ELISA is 1.5 OD units, and the SRA remains positive (Figure 1B).
Cardiac surgery: acute HIT/subacute HIT A
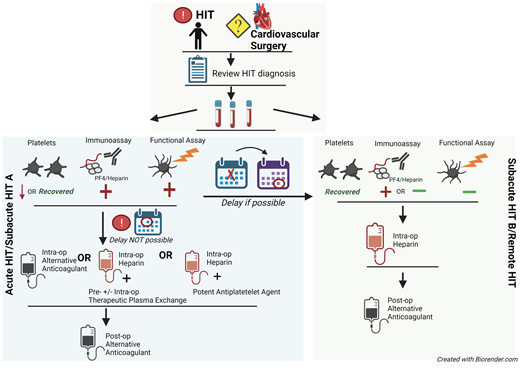
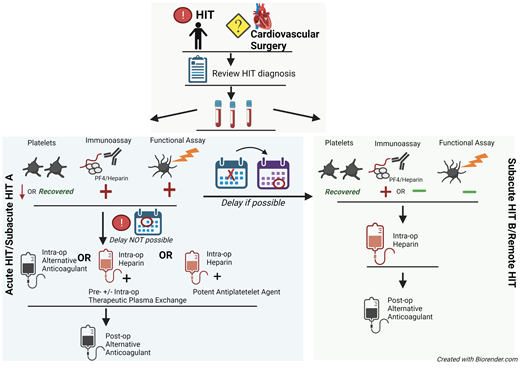
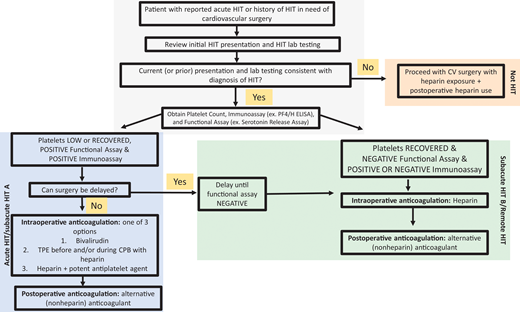
Although data support bivalirudin for PCI, heparin remains the highly preferred agent for most other CV procedures/surgeries. Thus, hematologists are asked to determine the safety of reexposure to heparin. Figure 2 describes our approach to selecting an anticoagulant strategy for CV surgery in patients with HIT. Due to the preference for heparin in cardiac surgeries, ASH HIT guidelines recommend delaying CV surgery until patients enter at least the subacute HIT B phase, if feasible. For cardiac surgery that cannot be delayed, these guidelines suggest 1 of 3 options2 :
intraoperative anticoagulation with bivalirudin,
intraoperative heparin after treatment with preoperative and/or intraoperative therapeutic plasma exchange (TPE), and
intraoperative heparin with a potent antiplatelet agent (eg, prostacyclin/tirofiban).
Approach to the management of HIT or history of HIT in cardiac surgery patients. For patients with a history of HIT who require cardiac surgery, the records should first be reviewed to confirm the diagnosis. The platelet count, anti-PF4/H immunoassay, and functional assay (e.g., SRA) determine the “phase of HIT” and the risks of heparin reexposure.
Approach to the management of HIT or history of HIT in cardiac surgery patients. For patients with a history of HIT who require cardiac surgery, the records should first be reviewed to confirm the diagnosis. The platelet count, anti-PF4/H immunoassay, and functional assay (e.g., SRA) determine the “phase of HIT” and the risks of heparin reexposure.
There is growing experience with direct thrombin inhibitors (DTIs), particularly bivalirudin, in CV surgery (Table 3). The Evaluation of Patients during Coronary Artery Bypass (Evolution-ON) study compared heparin with bivalirudin for patients undergoing coronary artery bypass graft (CABG) with CPB (history of HIT was not required).28 “Procedural success” was not significantly different between study arms, nor was cumulative median blood loss within the first 24 hours. Notably, 6.1% of patients who received bivalirudin required repeat exploratory operations compared with 1.9% of patients who received heparin. Limitations of the study were small sample size and variation in transfusion thresholds between institutions. Similarly, the EVOLUTION-OFF study showed similar rates of major adverse events in patients undergoing elective “off-pump” cardiac surgery with bivalirudin compared with heparin.29 A prospective single-arm study of bivalirudin only in patients with a history of HIT who required CPB surgery enrolled 49 patients (43 with acute HIT and 6 with a history of HIT). “Procedural success” was achieved in 42 of 49 patients (85.7%).30 In a “real-world” retrospective study, 13 patients received a DTI for CPB surgery, mostly CABG and/or valve surgery.31 Patients who received DTI for CPB had no difference in mortality, thrombosis, or hemorrhage compared with those who received heparin. The authors cautioned that patients treated with a DTI may have been at intrinsically lower bleeding risk or may have undergone lower-risk procedures than those reexposed to heparin.31
Another strategy is to reexpose patients with acute HIT/subacute HIT A to heparin intraoperatively following TPE +/− intravenous immunoglobulin (IVIG). In a clinical cohort of 24 patients undergoing TPE for HIT before heparin use for CPB, 3 non-HIT-related deaths and 3 thromboembolic events occurred.32 Notably, a National Inpatient Sample database study identified 90 patients with HIT who received TPE; less than one-fourth underwent TPE for cardiac surgery (CPB or non-CPB). Outcomes following cardiac surgery were not reported, but overall, TPE with HIT compared with not receiving TPE with HIT was associated with a higher likelihood of major bleeding (mostly gastrointestinal bleeding) and higher length of stay (20.5 vs 10 days).31 Other centers have successfully used IVIG and TPE before emergent CV surgery.33,34
A third strategy for patients with acute/subacute HIT A requiring CV surgery is intraoperative heparin in combination with platelet inhibition. Multiple case studies have described this approach, using a variety of potent antiplatelet agents.35,36 A recent report describes successful heparin reexposure in a patient with acute HIT for CPB surgery combining antiplatelet therapy and IVIG.37
As the 3 aforementioned strategies have not been directly compared, the ASH guidelines do not recommend 1 strategy over the others. Rather, the selection of strategy should depend on institutional experience and surgical preference. If the second or third strategy is chosen, patients' exposure to heparin should be strictly limited to the intraoperative setting. Before and after surgery, they should receive an alternative anticoagulant.
CLINICAL CASE (continued)
With a positive immunoassay and functional assay but normal platelet count, the patient is diagnosed with “subacute HIT A.” After discussion with CV surgery and anesthesia, the patient undergoes LVAD placement with bivalirudin infusion. Postprocedurally, he continues on bivalirudin and is ultimately discharged on warfarin. The patient presents to the outpatient hematology clinic for cardiac transplant evaluation. Repeat testing 45 days post-HIT diagnosis shows PF4/H ELISA remains positive (1.0 OD units), but the SRA is now negative (Figure 1C). Two months later, both PF4/H ELISA and SRA are negative (Figure 1D). The patient is listed for cardiac transplant with planned brief intraoperative heparin exposure.
Reexposure in subacute B/remote HIT
After a patient with HIT no longer has a positive functional assay and/or no longer has PF4/H ELISA antibodies, the risk of developing HIT with intraoperative heparin exposure appears to be low.38 Some groups have hypothesized that the very high doses of intraoperative intravenous heparin do not invoke the same platelet-activating response as lower concentrations, even if HIT antibodies are present.39 Warkentin and Sheppard38 described 17 patients with a history of HIT who received intraoperative heparin (but no postoperative heparin) for cardiac or vascular surgery between 8 weeks and 13.5 years after HIT diagnosis. All patients had a negative PF4/H ELISA and SRA preoperatively. Anti-PF4/H antibody positivity and SRA positivity were common following surgery (9/17 [53%] and 8/17 [47%], respectively). Despite the high rate of seroconversion, recurrent HIT occurred in only 1 patient, and this appeared to be a case of “autoimmune HIT” characterized by strong HIT antibodies that also activated platelets in the absence of heparin.
Given this evidence and the vast experience with heparin in CV surgery, ASH HIT guidelines recommend using intraoperative heparin in patients with remote HIT/subacute HIT B.2 Again, heparin exposure should be limited to the intraoperative setting and scrupulously avoided before and after surgery.19,20 The expected period for HIT recurrence is 5 to 10 days after intraoperative heparin exposure, and thus the platelet count should be monitored during this time.20
Postoperative anticoagulation in patients with a history of HIT
Postoperatively, regardless of the phase of HIT, heparin should be avoided. Table 4 outlines alternative anticoagulants for venous thromboembolism prophylaxis and considerations in the postoperative setting.
Conclusions
Clinicians should be aware of the many challenges in diagnosing HIT in the CV population and order HIT laboratory testing judiciously. Ideally, CV surgery that requires heparin is delayed in a patient with acute HIT/subacute HIT A until the platelet count recovers and the functional assay becomes negative. If delay is not possible, options include intraoperative bivalirudin, TPE prior to and/or during surgery with intraoperative heparin, or intraoperative heparin in combination with a potent antiplatelet agent. Once the patient progresses to subacute HIT B and beyond, the benefits of intraoperative heparin use outweigh the risks of brief heparin exposure. In all cases, heparin should be avoided pre- and postoperatively.
Acknowledgment
Allyson M. Pishko is supported by a 2019 HTRS Mentored Research Award from the Hemostasis and Thrombosis Research Society (HTRS), which was supported by an educational grant from Sanofi Genzyme.
Conflict-of-interest disclosure
Allyson M. Pishko has received research funding from an educational grant from Sanofi Genzyme and Novo Nordisk. Adam Cuker has served as a consultant for Synergy and has received royalties from UpToDate, and his institution has received research support on his behalf from Alexion, Bayer, Novartis, Novo Nordisk, Pfizer, Sanofi, and Spark.
Off-label drug use
Allyson M. Pishko: Off-label use of fondaparinux and direct oral anticoagulants for treatment of heparin-induced thrombocytopenia are discussed in the manuscript. Off-label use of alternative anticoagulants in cardiac bypass surgery discussed.
Adam Cuker: Off-label use of fondaparinux and direct oral anticoagulants for treatment of heparin-induced thrombocytopenia are discussed in the manuscript. Off-label use of alternative anticoagulants in cardiac bypass surgery discussed.