Abstract
Delayed hemolytic transfusion reactions (DHTRs) in patients with sickle cell disease are underappreciated and potentially fatal. Patients with DHTRs typically have symptoms of pain or dark urine days to weeks following a red blood cell (RBC) transfusion. In instances of DHTRs with hyperhemolysis, the patient's hemoglobin (Hgb) may be significantly lower than it was pretransfusion, and the Hgb A may drop by more than 50%. In most cases, at least 1 RBC alloantibody and sometimes multiple RBC alloantibodies can be identified during the DHTR, with those antibodies presumably having fallen below the level of detection at the time of the implicated transfusion. However, in up to one-third of cases, no new RBC alloantibodies can be identified posttransfusion. Complement is increasingly being appreciated to play a role in DHTRs and hyperhemolysis, not only due to classic pathway activation (with complement fixed antibody bound to RBCs) but also due to alternative pathway activation (resulting in part from plasma free heme). As such, anti-C5 inhibition has recently been reported to be effective at mitigating hemolysis in the setting of some severe DHTRs. Transfusion avoidance during DHTRs is recommended if possible, with long-term transfusion support advice being less clear; for example, a history of a severe DHTR may lead to questions regarding the safety of transfusions prior to curative therapies such as stem cell transplantation or gene therapy. A better understanding of antibody-positive and antibody-negative DHTRs, including patient- or disease-specific risk factors, is necessary to improve transfusion safety.
Learning Objectives
Consider the pathophysiology of delayed hemolytic transfusion reactions in patients with sickle cell disease
Review potential therapeutic interventions to prevent or treat hemolytic transfusion reactions
CLINICAL CASE
A 12-year-old girl with sickle cell disease (SCD) sought treatment at the emergency department for whole-body pain, fatigue, and dark urine 15 days after being discharged from the hospital after an episode of acute chest syndrome. During that prior hospitalization, she had been transfused with 2 units of C/E/K phenotypically matched red blood cells (RBCs); her hemoglobin (Hgb) at the time of discharge was 11 g/dL (reference range, 12-15 g/dL). Results of a complete blood count drawn by the emergency room physician just returned with an alarmingly low Hgb (3.7 g/dL). As the consulting hematologist, what is in your differential diagnosis, and what else would you like to know?
Pathophysiology of delayed hemolytic transfusion reactions
Delayed hemolytic transfusion reactions (DHTRs), or the premature destruction of transfused RBCs, typically occur days to weeks following the transfusion of fully crossmatch-compatible RBCs.1 Although DHTRs may be tolerated without major adverse events in patients without SCD, they present unique pathophysiology and challenges in patients with SCD. In a study of 99 patients with SCD undergoing DHTRs, dark urine occurred in 94%, and vaso-occlusive crisis symptoms were present in 89%.2 In addition to a drop in Hgb, the Hgb A level drops, the lactic dehydrogenase (LDH) rises above baseline, and reticulocytopenia is commonly present.3 An algorithm to diagnose DHTRs has been proposed and validated in an adult cohort with SCD, with a drop in Hgb A of more than 50% and a drop in total Hgb of more than 30% posttransfusion making a DHTR likely.4,5 Clinical decision making in the absence of Hgb A decline is also important, given the fact that Hgb electrophoresis or high-performance liquid chromatography testing is rarely ordered in the United States after an episodic transfusion.6
It is important for physicians caring for patients with SCD, including those in emergency departments, to be aware that the symptoms of a DHTR may resemble a vaso-occlusive crisis.7 A high degree of suspicion for DHTR should be maintained in any patient transfused in the past month who has symptoms including pain. A complete blood count (CBC), reticulocyte count, chemistries, LDH, urinalysis, Hgb A and S quantification, type and screen (to evaluate for any new antibodies in the patient's serum), and direct antiglobulin test (DAT; to evaluate antibodies coating either the patient's own RBCs or past transfused RBCs) should be drawn. In addition, notifying the blood bank to initiate a delayed transfusion reaction evaluation aids in confirmatory testing of DHTR by allowing for crossmatching segments from the previously transfused RBC unit(s) (if available) with the patient's current serum sample.
In this 12-year-old patient's case, the antibody screen was now positive, and the DAT returned positive for immunoglobulin G and C3. Three new alloantibodies (anti-Fya, anti-Jkb, and anti-S) were detected in her plasma and in the eluate. Her absolute reticulocyte count was low at 20 000/µL, with a baseline near 300 000/µL (age-based reference range, 23 000-140 000/µL). Her LDH was 3-fold higher than her baseline. Her urinalysis showed heme/blood but no intact RBCs. Results of the transfusion reaction evaluation demonstrated that segments from the 2 crossmatch-compatible units transfused previously were now incompatible when crossmatched with the patient's current serum sample. The patient's family asks you why this is happening to their daughter and why her Hgb is now so far below her pretransfusion baseline of 8 g/dL.
Incidence of DHTRs
Given a lack of mandated reporting of nonfatal transfusion reactions in the United States, the incidence of DHTRs is unknown. In the general transfused patient population, the incidence of DHTRs has been estimated to occur in 1:500 to 1:10 000 transfusions by some authors8 and between 110:000 and 1:100 000 transfusions by others.9 These numbers likely underestimate the risk of DHTRs in patients with SCD.10,11 In fact, approximately 4% of episodic transfusions in adult patients with SCD may result in DHTRs,4,12 making such reactions more common than febrile and allergic reactions combined.13 DHTRs not only contribute to morbidity but also can be fatal.10,14 In the general transfused patient population, DHTRs are less common than delayed serologic transfusion reactions (DSTRs) (Figure 1), with DSTRs characterized by a new antibody identified by the blood bank in the absence of clinical sequelae; the ratio of DHTRs to DSTRs in patients with SCD has not been well studied.
DSTRs, DHTRs, and DHTRs with hyperhemolysis. In the general population, DSTRs are much more common that DHTRs, although the DSTR to DHTR ratio has not been well studied in patients with SCD. Bystander hemolysis occurs in an unclear percentage of DHTRs in patients with SCD.
DSTRs, DHTRs, and DHTRs with hyperhemolysis. In the general population, DSTRs are much more common that DHTRs, although the DSTR to DHTR ratio has not been well studied in patients with SCD. Bystander hemolysis occurs in an unclear percentage of DHTRs in patients with SCD.
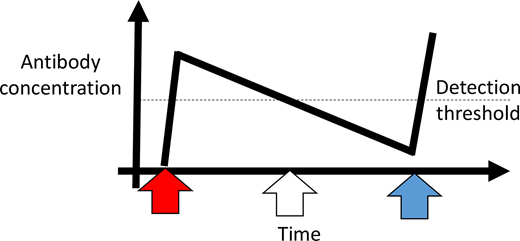
As the hematologist in the case example, you ask to speak to the family in a private room in the emergency department. You explain that the RBCs their daughter received during her admission for acute chest syndrome were indicated, given the severity of her symptoms and the degree of her hypoxia. You also explain that at the time of the transfusion, their daughter's antibody screen was negative and that it had always been negative. Furthermore, those 2 RBC units were phenotypically matched for C/E/K, as recommended by evidence-based guidelines,15,16 and crossmatch compatible. It is possible that the 3 newly detected antibodies developed de novo after the transfusions, although it is more likely that these antibodies developed in the distant past but were never detected because they fell below the level of detection by standard blood bank evaluations and have now returned in an anamnestic fashion (Figure 2). The family tells you the patient has never been transfused outside of your hospital; this makes it unlikely that medical record fragmentation is contributing to the current DHTR. However, she was transfused at 3 years of age for parvovirus-associated anemia and again at 8 years of age for acute chest syndrome.
Antibody detection and evanescence considerations. Upon initial RBC exposure (red arrow), 1 or multiple RBC alloantibodies may be generated. Once an antibody concentration gets above a certain level (dotted line), it can be detected by blood bank methods. Over time, that antibody may evanesce and fall below the level of detection (white arrow). If the patient needs a transfusion at a later time point (blue arrow), the antibody may not be detected. Of note, if an antibody screen was not completed within weeks of the initial transfusion, the antibody or antibodies may not have been detected at all. If the patient was seen at a different hospital in the United States at the time point indicated by the blue arrow, that hospital will most likely be unaware of an antibody or antibodies previously detected by the initial hospital. Figure generated with the assistance of R. George Hauser, MD.
Antibody detection and evanescence considerations. Upon initial RBC exposure (red arrow), 1 or multiple RBC alloantibodies may be generated. Once an antibody concentration gets above a certain level (dotted line), it can be detected by blood bank methods. Over time, that antibody may evanesce and fall below the level of detection (white arrow). If the patient needs a transfusion at a later time point (blue arrow), the antibody may not be detected. Of note, if an antibody screen was not completed within weeks of the initial transfusion, the antibody or antibodies may not have been detected at all. If the patient was seen at a different hospital in the United States at the time point indicated by the blue arrow, that hospital will most likely be unaware of an antibody or antibodies previously detected by the initial hospital. Figure generated with the assistance of R. George Hauser, MD.
It is critically important to obtain an accurate transfusion history and to contact the blood banks at prior sites of care, given antibody evanescence rates (which may be higher in patients with SCD than in the general population),17 multiple care sites, and the lack of linked blood bank electronic medical record systems between most US hospitals.18 It is also useful to obtain an antibody screen within 1 to 3 months after transfusion in patients with SCD, to increase the likelihood of identifying newly forming antibodies.10 With intermittent antibody screening (“real-world” experience), it is estimated that up to two-thirds of antibodies fail to be detected,19 with evanescent antibodies that are unknown to the treating hospital predisposing patients to adverse transfusion outcomes.
Antibody-positive and antibody-negative DHTRs
Most patients, including the patient described, have antibodies identified that contribute to the DHTR. If no new antibody or antibodies can be detected, repeating a crossmatch using RBCs in the segment(s) of the unit(s) previously transfused but now with a current plasma sample can aid in diagnosing an antibody against a low-incidence antigen that may not be obvious after an antibody screen alone. For example, an incompatible repeat crossmatch despite the lack of new antibodies identified in the antibody screen/panel can alert the blood bank or a reference laboratory to do a more complete evaluation for an antibody against a low-incidence antigen. At present, no “select screening cells” expressing low-incidence antigens specifically from donors of African descent exist, although using cells expressing antigens present in the donor population may be useful in working through what at first glance seems to be an antibody-negative DHTR. Despite extensive evaluation, new RBC alloantibodies still cannot be detected in some DHTRs.20 The incidence and prevalence of “antibody-negative” DHTRs are not known, but it has been estimated that up to one-third of DHTRs fall into this category and that complement activation likely plays a key role.10,21
Complement in SCD, DHTRs, and hyperhemolysis
Complement activation was shown to play a role in SCD more than 40 years ago,22 with defective complement regulation noted on RBCs from patients with SCD.23 Phosphatidyl serine expression on sickled RBCs activates the alternative pathway,24 and plasma free heme also activates the alternative complement pathway.25,26 Heme-triggered Toll-like receptor 4 signaling on endothelial cells activates the complement system, with complement deposits mediated by P-selectin expression on the endothelial cell surface.27 Reviewed more extensively elsewhere,28,29 it is likely that multiple stimuli activate complement pathways in patients with SCD, leading to a broad range of clinical sequelae.
Over the past few years, the importance of complement activation in DHTRs (both antibody positive and antibody negative) has increasingly been realized.28,30 Some antibodies, such as those against antigens in the Kidd or MNS families, are traditionally known to fix complement after binding to cognate antigen on transfused RBCs.31 However, complement activation can also occur through the alternative pathway independent of antibody-mediated processes, as described above.32 During a DHTR, increased complement activation may contribute to hyperhemolysis, also known as bystander hemolysis. Hyperhemolysis involves destruction of the patient's own RBCs, likely triggered by by-products of clearance of transfused RBCs; hyperhemolysis has primarily been reported in patients with SCD.30
In the case example, the anti-Jkb antibody could be responsible for the C3 detected on the positive DAT. If the patient was having a traditional DHTR and had just hemolyzed the 2 previously transfused units (presumed to be positive for the Fya, Jkb, and S antigens; antigen typing could be completed using residual segments from the units transfused), then her Hgb should have returned to near her pretransfusion baseline of 8 g/dL. With a Hgb below 4 g/dL, she has evidence of hyperhemolysis. Reticulocytopenia is also often seen in DHTRs with hyperhemolysis, for reasons that remain to be elucidated. A hemoglobinopathy evaluation was not ordered after the transfusions administered during her prior acute chest syndrome admission, and thus Hgb A/S changes since that transfusion cannot readily be evaluated.
Treatment of DHTRs
As the consulting hematologist, what treatment advice will you provide to the emergency department as they prepare to admit the 12-year-old girl?
Treatment options for DHTRs can be classified as described by Gardner et al33 into (1) supportive care, (2) optimization of erythropoiesis, (3) consideration of immunomodulatory therapies (including complement inhibition, steroids, intravenous immunoglobulin, and/or B-cell depletion5,16,30 ), and (4) minimizing future transfusions if possible. Of these treatment pillars (shown in the visual abstract, created using BioRender.com), transfusion avoidance may be most important as even fully crossmatch-compatible, antigen-negative RBC units may exacerbate the ongoing hemolysis. However, transfusion avoidance may not be feasible in extreme clinical conditions such as cardiac/respiratory failure. Emerging evidence suggests that treatment of severe DHTRs with anti-C5 antibody (eculizumab), which inhibits cleavage of C5 into C5a, thus inhibiting terminal complement pathway activation, may potentially reverse ongoing hemolysis; 600 to 900 mg weekly, up to 4 doses, has been described in adults weighing more than 40 kg.16,32,34,35 However, no controlled clinical trials data exist, and anticomplement therapies are not without risk. If vaccinations against meningococcus are not up to date, antibiotic prophylaxis for coverage is recommended in patients treated with anti-C5 antibodies. Other immunomodulatory therapies that have been used for severe DHTRs, also in the absence of clinical trials evidence and not without risk, include steroids (at doses ranging from 1 to 4 mg/kg/d of prednisone or methylprednisolone) and/or intravenous immunoglobulin (at doses ranging from 0.4 to 1 g/kg/d for 3-5 days, up to a total dose of 2 g/kg)16,30 ; rebound pain, hypertension, and hyperviscosity/neurologic complications have been reported.16 Rituximab (375 mg/m2 or 1000 mg total, dosed again in 1-2 weeks) has been used to prevent additional alloantibody formation in patients who require further transfusion; it is also not risk free and would rarely be used to treat an ongoing severe DHTR.16,30
In our case example, the 12-year old girl was admitted and put on a cardiac/respiratory monitor. The blood bank medical director put a “transfusion hold” on the girl's chart, asking that any RBCs ordered for transfusion required medical director approval for release; none ended up being ordered. The patient was given intravenous iron and erythropoietin along with fluids and treatment for her pain. Within 3 days of admission, her reticulocyte count increased to 200 000/µL, and a day later, her Hgb began to increase. Her pain improved as her hemolysis slowed. She was discharged 7 days after admission with an Hgb of 6 g/dL and a reticulocyte count near her baseline of 300 000/µL.
If the patient's Hgb had continued to downtrend or if she had developed end-organ dysfunction because of her severe anemia, then treatment with an anti-C5 antibody and/or additional RBC transfusions would likely have been considered next. The risk/benefit ratio of such therapies would need to be discussed with the patient and her parents at a multidisciplinary conference. Furthermore, any additional RBC transfusions would need to be as closely phenotypically matched to the patient's own RBCs as possible.
Prevention of DHTRs
DHTRs cannot entirely be prevented.36 However, their likelihood can be reduced by avoiding unnecessary transfusions, and their likelihood may be reduced by providing RBCs prophylactically antigen matched for at least C/c, E/e, and K (and possibly for Fya/Fyb, Jka/Jkb, and S/s as well).16 As described above, obtaining an accurate transfusion history and communicating with the blood banks where a patient was previously transfused will decrease the likelihood of an evanescent antibody from being transfused against and reinduced.19 Assuming the antibodies involved in this patient's case were anamnestic in nature, they were presumably initially formed after the patient's last transfusion (when she was 8 years old) but were never detected as she did not have a repeat antibody screen within 3 months of that prior transfusion. A widespread US RBC alloantibody registry, as exists in some countries,37 would increase transfusion safety for all patients; those with high alloimmunization prevalence rates (including patients with SCD) would likely benefit the most from such a registry.
Future transfusion considerations
If the patient in this case example sought treatment 3 years after this DHTR for an allogeneic hematopoietic stem cell transplant, do you think the risk/benefit ratio of a pretransplant RBC exchange transfusion would be favorable? Would you recommend a stem cell donor lacking the Fya, Jkb, and S antigens be selected?
There is no “right” answer to the risk/benefit question, but this scenario increasingly presents itself as the number of potentially curative therapies for SCD rises in number. The necessity of lowering the Hgb S prior to stem cell transplantation or peripheral blood stem cell collection (for gene therapy or for other indications) is not clear,7,38 although most protocols recommend lowering the Hgb S to decrease transplant-related complications, to decrease mobilization-induced vaso-occlusion, and/or to improve stem cell mobilization from the marrow. However, patients who have previously had DHTRs are at increased risk of having future (potentially life-threatening) DHTRs with RBC exposure even years later and even when extensively phenotypically matched RBCs are selected for transfusion. As such, educating the family and the involved care teams on the potential risks is important; case reports describe curative therapies being cancelled or delayed as a result of DHTRs following pretransplant transfusions.7,39 Furthermore, the optimal stem cell donor would lack the cognate RBC antigens against which the patient is alloimmunized, but HLA matching takes priority, and in some instances, RBC antigen avoidance is not possible.
Conclusions
DHTRs, with or without hyperhemolysis, although not well understood, recognized, or studied, are among the most common transfusion reactions in patients with SCD. Some steps that may theoretically decrease these reactions, including prophylactic antigen matching, have now been widely implemented. Other potentially life-saving steps, including establishing an RBC antibody registry in the United States, remain on a wish list. In addition to antibody identification/acknowledgment, determining risk factors for DHTRs on an individual and a disease basis will be an important next step in advancing treatment strategies; multiple other areas for study exist (Table 1). Continued multidisciplinary collaboration of hematologists, transfusion medicine physicians, transplant physicians, pharmacists, and others are necessary to mitigate the morbidity of DHTRs and to prevent mortality.
Conflict-of-interest disclosure
Jeanne E. Hendrickson: no competing financial interests to declare.
Ross M. Fasano: no competing financial interests to declare.
Off-label drug use
Jeanne E. Hendrickson: IVIg, eculizumab, and rituximab are discussed.
Ross M. Fasano: IVIg, eculizumab, and rituximab are discussed.