Abstract
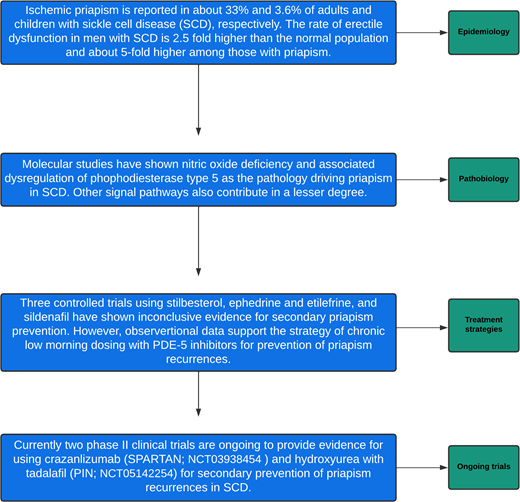
Ischemic priapism is a common but underrecognized morbidity affecting about 33% of adult men with sickle cell disease (SCD). The onset of priapism occurs in the prepubertal period and tends to be recurrent with increasing age. Significantly, priapism is associated with an unrecognized high burden of mental duress and sexual dysfunctions. The diagnosis of priapism is clinical. Many episodes of priapism will resolve spontaneously, but when an episode lasts longer than 4 hours, the episode is considered a urologic emergency requiring quick intervention with either corporal aspiration or shunt surgery. Only 3 randomized clinical trials (stilbesterol, ephedrine or etilefrine, and sildenafil) have been conducted for secondary priapism prevention in SCD. All 3 trials were limited with small sample sizes, selection biases, and inconclusive results after completion. The current molecular understanding of the pathobiology of priapism suggests a relative nitric oxide (NO) deficiency secondary to chronic hemolysis in SCD and associated phosphodiesterase type 5 dysregulation. We posit an increase in NO levels will restore the normal homeostatic relationship between voluntary erection and detumescence. Currently, 2 randomized phase 2 trials (1 double-blind, placebo-controlled trial and 1 open-label, single-arm intervention) are being conducted for secondary priapism prevention in men at high risk for recurrent priapism (NCT03938454 and NCT05142254). We review the epidemiology and pathobiology of priapism, along with mechanistic therapeutic approaches for secondary prevention of priapism in SCD.
Learning Objectives
To review the latest epidemiology and pathobiology of priapism in sickle cell disease
To explain the mechanistic therapeutic approaches for secondary prevention of priapism in sickle cell disease
Introduction
Sickle cell disease (SCD) is associated with priapism, a persistent painful erection occurring with or without sexual desire.1,2 Priapism-related pain is a cardinal feature of ischemia (low flow) due to compartment syndrome. In contrast, nonischemic (high flow) priapism results from a trauma- related arterial injury.3 In children and adults with SCD, ischemic priapism is the most common presentation (95%),4 reported at least once in approximately 33% of adolescents and adults with SCD.1 Approximately 74% of the priapism episodes are the stuttering (recurrent) type, lasting for a several-hour duration or less, which remits and relapses with or without medical intervention.1 Priapism occurs less commonly in children younger than 18 years (3.6%).1,2 Genome-wide association studies have reported an association between priapism and polymorphism in genes of transforming growth factor–β receptor (TGFBR3), subunit of factor VIII, integrin α-V, aquaporin, and Klotho gene.5
Distinguishing major priapism (lasting ≥4 hours) from stuttering priapism (<4-hour episode) is critical for risk stratification of time-dependent priapism-related erectile dysfunction and penile fibrosis.1,6,7 Individuals with SCD- related priapism also have a higher rate of penile deformities, lower sexual desire, lower intercourse satisfaction, and overall decreased satisfaction with sex life compared with those without a history of priapism.1 The burden of severe erectile dysfunction is 2.5-fold higher among men with SCD compared with men without SCD and about 5-fold higher among those with a history of priapism.1,4
Priapism is associated with mental health morbidity, including anxiety and depression.1,8 An individual's inadequate knowledge of priapism, in addition to the shame and embarrassment, may contribute to about 50% of affected individuals failing to seek medical intervention or even discuss the complication with their clinicians and relatives.1,8,9 Thus, the lack of knowledge of priapism may add to the underrecognition of the burden in a clinical setting.9,10 We review the epidemiology and pathobiology of priapism, along with mechanistic therapeutic approaches for secondary prevention of priapism in SCD.
Literature search method
We searched PubMed and MEDLINE databases using medical subject headings (MeSH) for full research articles published in the English language from January 2017 to April 2022 that reported on priapism in SCD: key search terms include priapism OR recurrent ischemic priapism OR stuttering AND sickle cell AND treatment. As per instructions for authors, we only include articles published within the past 5 years. A total of 12 articles were identified in PubMed. We also reviewed the clinicaltrials.gov for actively enrolling SCD priapism trials. We reviewed each of the 12 articles with their references. Two authors independently screened the publications before they were selected. We selected all articles that reported prevalence, incidence, pathogenesis, and treatment of SCD-related priapism. More articles on priapism published within the past 5 years were identified in the references included when they contained new relevant information. We excluded non-English-language publications. Based on the suggestion of the reviewers, we added more references that were published more than 5 years ago because of their relevance in clarifying some statements included in our first submission.
Consent was obtained from the patient whose case scenario was described, and the details were sufficiently altered to mask his identity.
CLINICAL CASE
A 22-year-old young adult with sickle cell anemia (SCA) posted on a Facebook health education group about his frustration with a recurrent painful erection, which disturbed him almost daily at night. He had visited several health care facilities with the same complaint, and medications were given to him without improvement. He confessed to the group that he was praying to die because of this painful and disturbing health challenge. The adult hematology physician was consulted. On presentation, the patient confirmed having a history of recurrent priapism daily, with each episode lasting about 2 to 3 hours at night. The last episode was the night before he came to the clinic. He confessed to having attempted suicide due to the priapism several times but could not because of his faith. He had no history of other triggers of priapism apart from SCA. His baseline Patient Health Questionnaire–9 score was 13, blood pressure was 100/60 mm Hg, and steady-state hemoglobin was 6.5 g/dL. He was not eligible for assessment with the International Index of Erectile Function (IIEF) because he had no history of sexual activity. After evaluation, he was counseled about his condition and started on hydroxyurea 20 mg/kg and tadalafil 5 mg (in the morning) daily. He was asked to chart the priapism incidences in a paper diary for the next 4 weeks. For his suicidal ideation and symptoms of depression, a psychiatrist evaluated him and commenced him on imipramine 25 mg once an evening. He came back 4 weeks later and had only 3 episodes of stuttering priapism with a maximum duration of 45 minutes since starting the treatment mentioned above, with the last episode being 3 weeks prior to the clinic visits. His Patient Health Questionnaire–9 score had dropped from 13 to 3. Subsequently, the priapism subsided to almost no episodes per month. The patient later believed that he was no longer at risk for recurrent priapism and elected to stop all therapy. Approximately a year after stopping the therapy, the symptoms recurred, and he developed a major priapism episode that lasted 48 hours at home. After 48 hours of an ischemic priapism episode, he presented to a private urology hospital, where he initially had penile aspiration with saline and phenylephrine irrigation. These strategies failed to result in detumescence. Subsequently, he was taken to the operating room for a penile shunt surgery to relieve compartment syndrome.
Pathobiology of priapism and a potential target for prevention
Nitric oxide (NO) is a critical molecule in the initiation and regulation of normal erection. Nitric oxide acts through the activation of guanylate cyclase to stimulate cyclic guanosine monophosphate (cGMP) production, which activates protein kinase G. The final result of these events includes blood vessel dilation and penile smooth muscle relaxation.11 Nitric oxide–induced cGMP also regulates the expression of phosphodiesterase type 5 (PDE-5), the key enzyme associated with detumescence by degrading cGMP. The expression of PDE-5 is directly correlated with the tonic level of cGMP. Thus, a low PDE-5 level is associated with abnormal detumescence. Chronic hemolysis in SCD results in endothelial NO depletion, associated with decreased cGMP level and corresponding low level of PDE-5, which cumulatively lead to an inability to terminate an erection. When neuronal NO is released, driving an erection response, the chronically low PDE-5 expression in the penile tissues fails to terminate the erection. Thus, the primary working hypothesis for priapism in SCD is related to NO depletion associated with PDE-5 dysregulation. Other molecular pathways implicated in the pathobiology of priapism include RhoA/Rho kinase, adenosine, and opiorphin.4,11
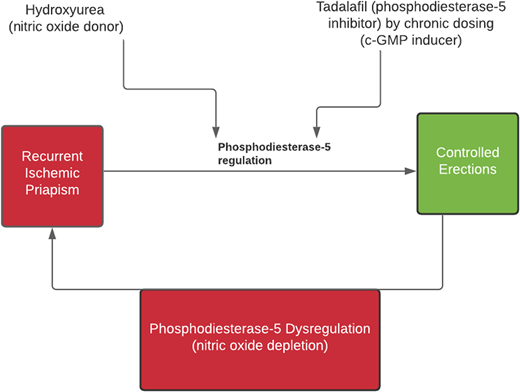
Based on the understanding of NO and PDE-5 homeostasis for normal erection and detumescence, we propose to test the hypothesis that a combination of hydroxyurea (potential NO donor) and a daily PDE-5 inhibitor increases the tonic cGMP levels and decreases the priapism incidence (see Figure 1).
A chart showing a potential synergy of combining hydroxyurea and PDE-5 in controlling recurrent priapism.
A chart showing a potential synergy of combining hydroxyurea and PDE-5 in controlling recurrent priapism.
Clinical evaluation and management of acute priapism
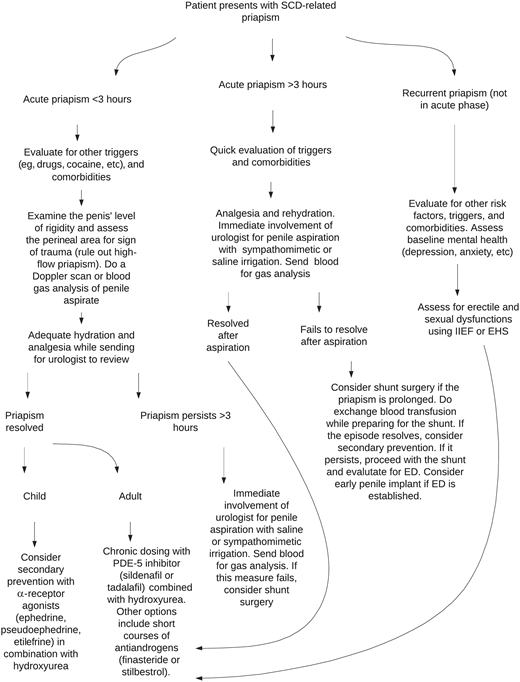
The typical presentation of priapism is stuttering, which is often prodromal for major priapism requiring surgical intervention. When a patient presents with acute priapism lasting <3 hours, a history of potential triggers should be taken. Infection, full bladder when going to sleep, and dehydration have been associated with the onset of priapism, but there is no yet well-established mechanistic relationship.1,6 Rapid eye movement sleep mechanism involves disinhibition of neural functions at the spinal cord level that regulate sleep-related erections and could trigger priapism in the absence of PDE-5 control.12
Some drugs, such as antidepressants, antipsychotics, anticoagulants, alcohol, and cocaine, have been reported to cause priapism (see Table 1).13 The medical history should address these factors and establish the duration, pain severity, and recurrence pattern of the episodes. Priapism in SCD is reportedly more common among patients with vasculopathy (leg ulcer, stroke, pulmonary hypertension, nephropathy, etc).3,14 Both the medical history of prior priapism events in the past year and physical examination should be thoroughly evaluated to identify risk factors. A history of trauma-related high-flow priapism should be assessed, especially in patients who had corporal aspiration recently.6 Penile examination may show deformity due to scarring or signs of injury in a rare case of high-flow priapism. The patient may be writhing in pain, with the priapic penis rigid and erect, whereas the glans penis appears soft, unlike in a normal erection. Aspiration of corporal blood for gas analysis can confirm ischemia (see Table 2) but is not routinely performed.6 A color Doppler ultrasound scan may serve as a substitute for blood gas analysis and reveal low or high flow in the arterial circulation, but the imaging is also typically not requested.
Optimal management of acute priapism requires multidisciplinary care. In some affected individuals, their priapism resolved after exercising, using a cold or warm compress, having a hot bath or shower, or self-penile injection with etilefrine or phenylephrine before reaching a hospital.15 Adequate analgesia and rehydration are the first measures in a hospital setting. If priapism resolves quickly, the patient should be counseled for secondary prevention with the available treatments. If priapism fails to resolve, early intervention by the urologist is critical to salvaging the erectile function. In most situations, corporal aspiration with or without irrigation using sympathomimetics (phenylephrine, epinephrine, norepinephrine, etilefrine) or saline could be effective; however, a shunt surgery is often required to resolve some recalcitrant episodes.6,16
For major priapism or any episode lasting for >3 hours, immediate involvement of a urologist is recommended for managing this urologic emergency so as not to allow the event to last beyond 4 hours. The rationale for prioritizing 3 hours is because ischemic priapism is associated with a time-dependent risk of erectile dysfunction. Hence, early intervention before 4 hours would likely curtail the risk of permanent sequelae. Recently, the American Urology Association guidelines for priapism management have indicated that surgical management should occur between 1 and 4 hours after onset of the ischemic priapism episode depending on the patient's circumstance.7 Typically, the stepwise approach involves starting with corporal aspiration before a shunt surgery.6,17 However, in a situation where the priapism is prolonged (see Figure 2), the likelihood of corporal aspiration failing to resolve the episode is high. Thus, shunt surgery should be the immediate next step to relieve pain. Both prolonged major priapism and a shunt surgery are associated with irreversible erectile dysfunction. Therefore, an early decision on using a penile implant should be part of the management (see Figure 3).1,18 Sexual and erectile function assessments using an IIEF or Erection Hardness Score should be considered for all patients with priapism due to the associated high rate of erectile dysfunction.1,2
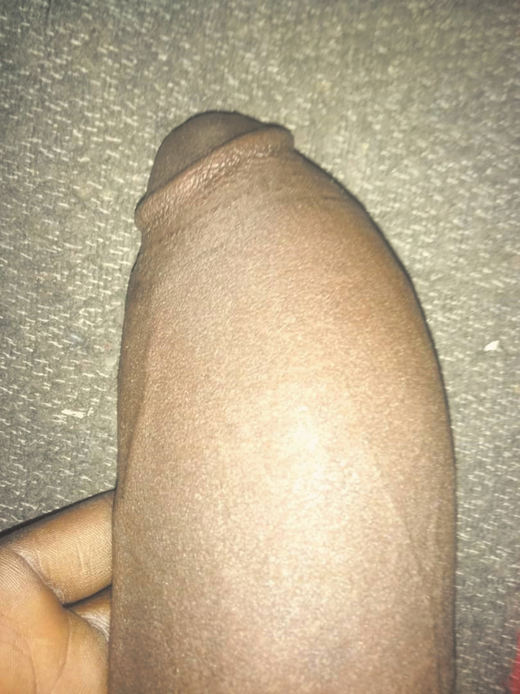
Edematous priapic penis, an episode that lasted for 8 days in a young adult with SCD.
Edematous priapic penis, an episode that lasted for 8 days in a young adult with SCD.
Flowchart for evaluation and treatment of priapism in SCD. ED, erectile dysfunction; EHS, Erection Hardness Score.
Flowchart for evaluation and treatment of priapism in SCD. ED, erectile dysfunction; EHS, Erection Hardness Score.
In acute priapism management, exchange blood transfusion is sometimes used, but limited evidence supports its efficacy. We reserve exchange transfusion for a recalcitrant priapism episode not responding to corporal aspiration while preparing for shunt urologic surgery intervention. Apart from lack of proven efficacy of exchange transfusion in resolving priapism, if individuals with priapism are transfused, caution should be taken to avoid hyperviscosity syndrome associated with cerebral hemorrhage, ischemia, or both.19,20 When red cell exchange transfusions are performed according to a protocol to decrease the likelihood of hyperviscosity syndrome for preventing priapism recurrence or cerebral infarcts recurrence, the incidence of neurologic complications is rare following the transfusions.21,22
Secondary priapism prevention is the key to reducing pain and further complications in men
There are no clinical algorithms to predict who would have priapism; however, a history of recent priapism in the past 12 months is a risk factor for recurrence in SCD.23 Men with SCD benefit from secondary prevention to forestall catastrophic complications associated with priapism, such as erectile dysfunction, penile scarring, and mental health morbidity.8 There is currently no evidence-based treatment strategy for preventing priapism recurrence. However, the 3 classes of drugs already tried in this patient population have some anecdotal clinical benefits.24,25
The double-blind, placebo-controlled trial comparing a 50-mg morning dose of sildenafil with equivalent placebo failed to demonstrate the primary efficacy end point of 50% reduction in priapism frequency. A secondary analysis of the open-label phase of the same trial demonstrated that among the participants receiving sildenafil, 62.5% (5 of 8) and 66.7% (2 of 3) had decreased incidence rates of priapism based on intention-to-treat and per protocol analysis, respectively. Sildenafil treatment was associated with a 4-fold decrease in major priapism events among participants adherent to sildenafil therapy.26
The Walk-PHaSST trial assessed whether treatment with sildenafil 20 to 80 mg orally, 3 times daily, would improve exercise tolerance in patients with SCD and tricuspid regurgitation velocity ≥2.7 m/s compared with placebo. The trial was stopped for safety because there was a significantly higher rate of hospitalization (45% vs 22%, P = .022) among participants taking sildenafil compared with the placebo group.27 Both the sildenafil trial for priapism and Walk-PHaSST provided no definitive evidence that PDE-5 inhibitors would prevent priapism episodes or improve exercise tolerance in SCD.24,28
In a recent retrospective observational study, regimented dosing of either sildenafil or tadalafil (median duration 3 months, interquartile range 2-7 months) showed that 92% (22 of 24) of the participants reported improvement on a PDE-5 inhibitor, with an observed reduction in the emergency visit by 4.4-fold compared with the period before the intervention.25 However, when the participants stopped the drug, their priapism recurred with significantly increased frequency (P < .001) and duration (P < .001). For those men receiving tadalafil daily, there was an improvement in the rate of priapism in 71.4% (5 of 7) and a self-report of improved erectile function.29
The US Food and Drug Administration approved hydroxyurea for preventing vaso-occlusive events in children and adults with SCA. Additionally, hydroxyurea has been reported to reduce priapism events in small case series.30,31 Potential mechanisms of action include hydroxyurea as an NO donor (improves PDE-5 expression), an inducer of hemoglobin F production (reduces polymerization, sickling, and hemolysis), a myelosuppressive agent (reduces sterile inflammation and adhesion and possibly decreases blood viscosity), its ability to decrease endothelial adhesion of red blood cells, and its effect on endothelin 1 (possibly reducing penile fibrosis).31 The investigators proposed pharmacologic synergism between hydroxyurea and the PDE-5 inhibitor in preventing priapism episodes (NCT05142254).
Hormonal therapies such as 5-α reductase inhibitor (finasteride), gonadotrophin-releasing hormone inhibitors, and estrogen analogue (stilbesterol) could also be used for priapism prevention, but their adverse effects (gynecomastia, thromboembolism, etc) are intolerable for many men with priapism.16,32 These drugs possibly counter the androgen effect on penile tissue, reduce nocturnal tumescence, and inhibit testosterone-mediated NO release from the nerve terminals.33,34
Unique challenges of secondary priapism prevention in children
Priapism in children with SCD is less common than among men with SCD.10 However, the complications can be just as devastating. Unfortunately, priapism is poorly reported in children with SCD and is likely due to an underdiagnosis. The prior and current randomized controlled secondary priapism prevention trials have excluded children.24 Thus, all therapies for secondary priapism prevention in children are based on anecdotal evidence. Antiandrogens are one class of drugs often used to treat priapism in SCD. However, antiandrogens could have a negative impact on the growth and development of secondary sexual characteristics in children. Hence, antiandrogens are not generally considered an option. Alternatively, children are offered α-receptor agonists such as etilefrine and pseudoephedrine/ephedrine.24 This class of drugs works by increasing vasoconstriction to induce detumescence. However, sympathomimetics are associated with potential strokes, mainly due to cerebral hemorrhage reported with intracavernosal injections for treatment of SCD-related priapism.35,36 In the general population, an oral sympathomimetic was also associated with stroke.37,38 PDE-5 inhibitors have not been explored as a treatment option in children with recurrent priapism.
Physical therapy as a coping mechanism to relieve stuttering priapism
In adults with SCD, the most commonly reported strategy to cope with priapism is exercise alone (approximately 90%) or in combination with other strategies such as a warm or cold compress.1 A report on drug-related priapism shows that climbing a staircase was associated with immediate resolution of an episode, within 7 minutes, at the emergency room.39 Exercise likely helps to resolve priapism by increasing oxygen demand and diversion of blood flow to the skeletal tissues of the lower extremities.39 Other potential mechanisms of exercise include a distraction for pain, increases sympathetic response, and activation of endothelial NO synthase.40,41 Other physical therapies, with limited evidence, for individuals with SCD-related priapism include a hot bath, shower, warm or cold compress, masturbation, and voiding.1
Mental health challenge needs to be addressed in priapism
Priapism is associated with a high rate of unrecognized mental health challenges, including suicidality, depression, and anxiety.1,8 These factors heighten the poor quality of life associated with priapism and could preclude patients from seeking medical intervention.1,8 In our clinical case, the patient presented with depression and suicidal ideation, which warranted referral to a psychiatrist and treatment with a tricyclic antidepressant (imipramine). When treating mental health conditions such as depression, drugs such as fluoxetine should be avoided because of a potential synergy in precipitating priapism by interfering with the sympathetic response (see Table 1).13 A substantial number of patients with priapism have erectile and sexual dysfunctions,1,4 which may compound their mental health challenges.1 Therefore, regular assessment with IIEF and Erection Hardness Score would help to identify this subset of patients.
Challenges and prospects of priapism secondary prevention
Three controlled clinical trials were conducted in SCD to demonstrate evidence for secondary prevention (Table 3). None of the trials addressed the treatment effect on acute priapism. All 3 trials had small sample sizes and suffered from high attrition and biases.23 Currently, 2 trials are ongoing to address previous shortcomings. In the United States, a phase 2 trial is using crizanlizumab, a humanized monoclonal P-selectin inhibitor licensed by the Food and Drug Administration to prevent vaso-occlusive pain,42 for prevention of priapism recurrences (SPARTAN; NCT03938454). In Nigeria, a phase 2 single-center internal feasibility pilot trial comparing moderate-dose hydroxyurea with tadalafil vs moderate-dose hydroxyurea with a placebo. (PIN trial; NCT05142254) is currently recruiting participants to assess the potential effect of this strategy in preventing priapism recurrences in adults with SCA.
Acute medical treatment and primary prevention of ischemic priapism are lacking
To our knowledge, no clinical trial has been conducted on primary prevention or acute treatment of priapism in SCD. However, a small case series has shown a potential role of nitrous oxide in relieving acute priapism in the emergency department.27 The absence of a trial focused on a medical rather than a surgical strategy for managing acute priapism is a significant gap in evidence- based medical care.
Conclusion
Priapism is an underreported and underrecognized serious complication of SCD among males of all ages, with no evidence-based primary and secondary prevention. While efforts are ongoing to identify effective secondary prevention strategies, prompt recognition by both clinicians and patients is required to reduce the likelihood of potentially catastrophic sequelae of erectile dysfunction and improve the affected individuals' quality of life.
Funding
Ibrahim M. Idris was supported by the American Society of Hematology Global Research Award and the Phillips Family donation.
Conflict-of-interest disclosure
Michael R. DeBaun and Arthur L. Burnett are involved in the clinical advisory board of the industry-sponsored SPARTAN trial. As investigators, Michael R. DeBaun, Arthur L. Burnett, and Ibrahim M. Idris are involved with the PIN trial. Michael R. DeBaun is a paid consultant for Novartis and Forma Pharmaceutical and previously a paid consultant for Vertex for designing a cost-effective model for curative therapy, 2020. Ibrahim M. Idris is on the Agios pharmaceuticals' mitapivat trial (RISE UP) clinical advisory committee.
Off-label drug use
The article discusses off-label uses of phosphodiesterase-5 inhibitors (tadalafil and sildenafil) and hydroxyurea for prevention of recurrent ischemic priapism in sickle cell disease. Other off-label uses of drugs such as sympathomimetics and hormones have also been discussed in the article.