Abstract
Recent advances in our understanding of the molecular basis of inherited neutrophil disorders and complementary studies in transgenic mouse models have provided new insights into the normal mechanisms regulating myelopoiesis and the functional responses of mature neutrophils. Neutrophil specific granule deficiency is a rare disorder of neutrophil function characterized by a lack of neutrophil secondary granule proteins and associated with recurrent bacterial infections. The CCAAT/enhancer binding protein (C/EBP) ϵ, a leucine zipper transcription factor expressed primarily in myeloid cells, and C/EBPϵ-deficient mice generated by gene targeting lack specific granules and have impaired host defense are discussed by Dr. Lekstrom-Himes in Section I. The similarity between these phenotypes led to the identification of a loss of function mutation in the C/EBPϵ gene in a subset of patients with specific granule deficiency. Dr. Dale reviews the clinical features and management of congenital neutropenia and cyclic hematopoiesis in Section II. Inherited mutations in the neutrophil elastase gene have recently been identified in both disorders. Specific mutations identified in cyclic and congenital neutropenia are described along with possible mechanisms for regulation of hematopoiesis by neutrophil elastase. In Section III, Dr. Dinauer reviews the molecular genetics of chronic granulomatous disease and studies in knockout mouse models. This work has revealed important features of the regulation of the respiratory burst oxidase and its role in host defense and inflammation. Results from preclinical studies and phase 1 clinical trials for gene therapy for CGD are summarized, in addition to alternative approaches using allogeneic bone marrow transplantation with nonmyeloablative conditioning.
I. Transcriptional Regulation of Neutrophil Granule Proteins: Using the C/EBPϵ Knockout Mouse to Understand the Human Disease, Neutrophil Specific Granule Deficiency
Julie A. Lekstrom-Himes, M.D.*
Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bldg. 10, 11N103, 10 Center Drive, Bethesda MD 20892
The orchestrated synthesis, sequestration and release of neutrophil granule proteins comprise an integral component of the neutrophil-mediated innate immune response. Terminally differentiated neutrophils are non-dividing effector cells of innate immunity, densely populated with heterogeneous granules containing a variety of proteases, enzymes, and antibacterial proteins. Granule membranes are studded with adhesion molecules, receptors, and the membranous components of the NADPH oxidase apparatus, permitting rapid upregulation of cell membrane proteins with neutrophil activation and granule-cell membrane fusion. The sequence of granule release is well regulated but poorly understood. The synthesis and trafficking of granule proteins into membrane-bound granules during neutrophil myelopoiesis appears to be closely tied to precise transcriptional events occurring at different stages of neutrophil maturation. These findings are salient in the discussion of the granule protein disorder, neutrophil specific granule deficiency, which has been linked to a defect in the transcription factor CCAAT/enhancer binding protein (C/EBP) ϵ.
Neutrophil Granule Proteins and Synthesis During Myelopoiesis
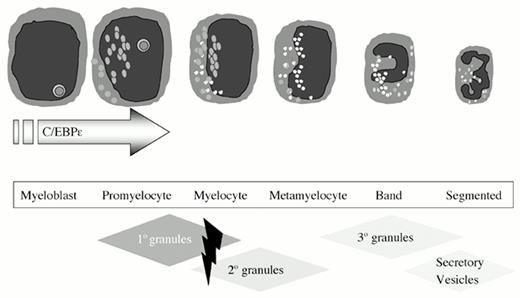
Neutrophil granules can be categorized into azurophil (primary) granules, specific (secondary) granules, gelatinase (tertiary) granules, and secretory vesicles.1,2 Azurophil granule proteins are synthesized early in myelopoiesis, during the myeloblast to promyelocyte transition (Figure 1) and are easily identified upon Giemsa-Wright staining of promyelocytes as large azurophil granules.1 Azurophil granule contents include myeloperoxidase (MPO) and defensins, among others (Table 1).1 MPO is essential for the conversion of hydrogen peroxide, a product of NADPH oxidase, into hypochlorous acid (bleach), a potent antimicrobial agent.3 Defensins are small, cysteine- and arginine-rich microbicidal proteins that undergo multiple proteolytic cleavages before assuming their mature form.4
Neutrophil granule expression during myelopoiesis.
Granule protein synthesis and granule packaging accompany the sequential stages of myeloid cell development. Loss of C/EBPϵ expression, predominantly expressed during promyelocytic differentiation, results in the loss of azurophil granule defensins, and specific and tertiary granules (hatched diamonds).
Neutrophil granule expression during myelopoiesis.
Granule protein synthesis and granule packaging accompany the sequential stages of myeloid cell development. Loss of C/EBPϵ expression, predominantly expressed during promyelocytic differentiation, results in the loss of azurophil granule defensins, and specific and tertiary granules (hatched diamonds).
Membrane and matrix components of neutrophil granules.
. | Azurophil Granules . | Specific Granules . | Gelatinase Granules . | Secretory Vesicles . |
|---|---|---|---|---|
| Membrane proteins | CD63 | CD11b | CD11b | Alkaline phosphatase |
| CD68 | fMLP-R (formyl peptide receptor) | Cytochrome b558 | CD11b | |
| cytochrome b558 | fMLP-R | CD14 | ||
| VAMP-2 | VAMP-2 | CD16 | ||
| fMLP-R | ||||
| VAMP-2 | ||||
| Matrix proteins | β-glucuronidase | Collagenase | Gelatinase | Plasma proteins (including albumin) |
| Defensins | Gelatinase | Lysozyme | ||
| Elastase | Lactoferrin | |||
| Lysozyme | Lysozyme | |||
| Myeloperoxidase | NGAL |
. | Azurophil Granules . | Specific Granules . | Gelatinase Granules . | Secretory Vesicles . |
|---|---|---|---|---|
| Membrane proteins | CD63 | CD11b | CD11b | Alkaline phosphatase |
| CD68 | fMLP-R (formyl peptide receptor) | Cytochrome b558 | CD11b | |
| cytochrome b558 | fMLP-R | CD14 | ||
| VAMP-2 | VAMP-2 | CD16 | ||
| fMLP-R | ||||
| VAMP-2 | ||||
| Matrix proteins | β-glucuronidase | Collagenase | Gelatinase | Plasma proteins (including albumin) |
| Defensins | Gelatinase | Lysozyme | ||
| Elastase | Lactoferrin | |||
| Lysozyme | Lysozyme | |||
| Myeloperoxidase | NGAL |
Specific granule proteins are synthesized during the promyelocyte to myelocyte transition of myelopoiesis.1 Lactoferrin is the predominant component, whose function is not known. Myeloid lactoferrin synthesis, however, differs from salivary gland production, as shown in an individual with neutrophil specific granule deficiency, who lacked myeloid but not salivary lactoferrin.5 Specific granule membrane proteins include CD11b (β2 integrin), receptors for formyl peptides and fibronectin, and cytochrome b558, the membranous proteins of the NADPH oxidase enzyme.1
Gelatinase granules are similar in content to specific granules; however, synthesis occurs later, during the metamyelocyte to band stages.6 Like specific granules, they are myeloperoxidase negative and are considered by some to be a subset of specific granules.
Secretory vesicles are highly mobilized granules that are notable for the high density of membrane receptors they carry including CD11b, CD14, CD16, and formyl peptide receptors.1 They contain plasma proteins including albumin, which becomes secreted with vesicle membrane fusion with the cell membrane.1 Although synthesized late during myelopoiesis, the mechanism of formation is not known.
Regulation of granule protein synthesis during neutrophil myelopoiesis is primarily transcriptional rather than translational.7 Transcription factors prevalent in early myeloid progenitors include AML, GATA-1, Pu.1, and C/EBPα.7,8 Granule protein promoters that have been cloned and studied possess cognate sites for many of these factors. However, gene knockout mouse models with targeted disruption of specific transcription factors have revealed more concerning the regulation of granule protein synthesis. C/EBPα nullizygous neutrophils fail to develop beyond the myeloblast stage and do not synthesize azurophil granules or their content proteins.9 In the case of C/EBPϵ-deficient mice, myeloid cells develop into mature neutrophils but fail to synthesize specific or gelatinase granule proteins or granules.10
Trafficking of granule proteins into granules is not well understood. Precise patch clamp studies of granule protein membranes suggest that granule proteins are packaged in the trans-Golgi network into small unit vesicles that aggregate by homotypic fusion into larger mature granules.11 The heterogeneity of protein content and function of neutrophil granules suggests a precise mechanism of protein sorting during granule formation. Experiments using myeloid cell lines engineered to asynchronously express the specific granule protein NGAL during the myeloblast/promyelocyte stage resulted in incorrect packaging of NGAL into azurophil granules.12 These experiments demonstrated that granule protein packaging is dependent in part on time of synthesis during the course of myelopoiesis. Alternative factors and post-granule transport are also likely involved. For example, defensins, synthesized later than other azurophil granule proteins during the promyelocyte-myelocyte transition, are targeted to azurophil granules subsequent to their formation.13
Function of Neutrophil Granule Proteins
The content and kinetics of degranulation of neutrophil granules reflect their role in response to inflammation and infection.1 Secretory vesicles are exocytosed rapidly in response to neutrophil rolling upon endothelium and may be signaled by selectins and their ligands.14 Fusion of secretory vesicle membranes with the neutrophil cell membrane upregulates surface expression of β2 integrins, facilitating neutrophil-endothelial adhesion and further responses to inflammatory stimuli.15 Specific and gelatinase granules are released with neutrophil activation and contain pro-enzymes requiring further proteolytic modification following secretion.1 The sequence of degranulation may be a stochastic phenomenon, simply reflecting the density of VAMP-2, the neutrophil v-SNARE protein, on the granule surface (secretory→gelatinase→specific), which directs granule release.1 Azurophil granules do not usually undergo exocytosis.1,2 They contain mature, processed proteins and active enzymes that fuse with phagosomes and elicit intracellular microbicidal killing.1,2
Neutrophil Specific Granule Deficiency and the C/EBPϵ Knockout Mouse
Neutrophil specific granule deficiency is a rare disorder characterized by a lack of specific or secondary granules in developing mature neutrophils. The five reported cases16 described patient presentations of early and frequent bacterial infections with abnormal neutrophil migration and disaggregation, atypical nuclear morphology with bilobed nuclei, and absent specific granules (Table 2). Abnormalities in patient eosinophils (deficient eosinophil specific granules) and platelets (abnormal α granules) are also reported, suggesting an underlying defect in myelopoiesis.17,18 Patient neutrophils lack lactoferrin, a neutrophil specific granule maker. Interestingly, lactoferrin was detected in normal amounts in patient salivary glands, suggesting a defect in transcriptional regulation of myeloid cell granule proteins.5
Comparative analysis of the phenotypic characteristics of neutrophils from patients with specific granule deficiency and mice deficient for C/EBPϵ protein.
| Phenotypic Characteristics . | Specific Granule Deficiency . | C/EBPϵ Knockout Mouse . |
|---|---|---|
| Clinical Presentation | Multiple bacterial infections | Bacterial infections and MDS-like disorder |
| Neutrophil morphology | Bilobed nuclei | Bilobed nuclei |
| Absent 20 granules by Wright staining | Absent 20 granules by Wright staining | |
| Absent eosinophils by Wright staining | Absent eosinophils by Wright staining | |
| Neutrophil migration | Decreased (by Rebuck skin window) | Decreased (by thioglycollate challenge) |
| Disaggregation | Decreased | Not tested |
| Phagocytosis | Normal | Decreased |
| Superoxide generation: | ||
| PMA | Decreased | Decreased |
| A23187 | Decreased | Not tested |
| Fmet-leu-phe | Normal | Not tested |
| Bactericidal activity | Decreased | Decreased |
| Phenotypic Characteristics . | Specific Granule Deficiency . | C/EBPϵ Knockout Mouse . |
|---|---|---|
| Clinical Presentation | Multiple bacterial infections | Bacterial infections and MDS-like disorder |
| Neutrophil morphology | Bilobed nuclei | Bilobed nuclei |
| Absent 20 granules by Wright staining | Absent 20 granules by Wright staining | |
| Absent eosinophils by Wright staining | Absent eosinophils by Wright staining | |
| Neutrophil migration | Decreased (by Rebuck skin window) | Decreased (by thioglycollate challenge) |
| Disaggregation | Decreased | Not tested |
| Phagocytosis | Normal | Decreased |
| Superoxide generation: | ||
| PMA | Decreased | Decreased |
| A23187 | Decreased | Not tested |
| Fmet-leu-phe | Normal | Not tested |
| Bactericidal activity | Decreased | Decreased |
Generation and analysis of C/EBPϵ knockout mice have revealed a phenotype remarkably similar to patients with neutrophil specific granule deficiency.10,19 C/EBPϵ is a member of the basic zipper family of transcription factors and is expressed nearly exclusively in myeloid lineage cells.20 The highest level of C/EBPϵ expression occurs in promyelocyte and late-myeloblast cell lines. Retinoid treatment of NB4 or HL60 promyelocytic cell lines that promote granulocyte differentiation induces C/EBPϵ expression.21,22
Although C/EBPϵ nullizygous mice are fertile and offspring develop normally, they are highly susceptible to infection with Pseudomonas aeruginosa. Additionally, 40% of these mice succumb to polyclonal accumulations of myeloid cells in vital organs without apparent antecedent infections. Mice typically survive only 2-5 months after birth.19 C/EBPϵ-deficient neutrophils possess an abnormal nuclear morphology with bilobed nuclei and a depressed respiratory burst in response to PMA stimulation.19 Furthermore, C/EBPϵ-deficient neutrophils lack secondary granules and a variety of granule proteins including defensins,23 gelatinase, and secondary granule proteins.19,24 Loss of these proteins suggests that C/EBPϵ functions during the promyelocyte to myelocyte transition in myelopoiesis, a hypothesis further supported by in vitro differentiation experiments on C/EBPϵ-deficient colony-forming units, which show developmental blockade at the promyelocyte stage (Figure 1).19
Because of the similarity of C/EBPϵ knockout mice and human specific granule deficiency, the C/EBPϵ gene from one specific granule deficiency patient was sequenced and found to possess a five-base pair deletion in the second exon of C/EBPϵ, resulting in a frameshift and premature termination codon.24 Loss of C/EBPϵ mRNA and protein expression was further confirmed by RNA blotting and immunoblotting of patient samples. In vitro functional studies of the defective gene product demonstrated a profound loss of transcriptional activation, consistent with the deletion of the DNA-binding domain and dimerization domains required for transactivating function.24
Summary
Analysis of the C/EBPϵ knockout mouse was essential to the identification of a causative gene defect in neutrophil specific granule deficiency. Delineation of the abnormalities in the knockout mouse model and identification of a C/EBPϵ mutation in a patient with neutrophil specific granule deficiency has provided insight into the role of C/EBPϵ in myelopoiesis. The multiplicity of C/EBPϵ target genes at varying stages of myeloid development suggests that C/EBPϵ acts upon a set of early stage-specific genes, inducing promyelocyte development and granule formation.24 Loss of primary granule defensins, gelatinase, all secondary granule proteins, and blocked lineage maturation highlights the importance of C/EBPϵ early in myelopoiesis.
II. Cyclic Neutropenia and Severe Congenital Neutropenia
David C. Dale, M.D.*
University of Washington, Box 356422, 1959 NE Pacific, Seattle WA 98195-6422
Cyclic and congenital neutropenia are rare diseases, primarily affecting neutrophil production. The estimated frequency is about one or two cases per million population. Interest in these disorders increased substantially a decade ago when clinical trials demonstrated the effectiveness of granulocyte colony-stimulating factor (G-CSF) in their treatment.1 More recently, interest has focused on the risk of patients with congenital neutropenia for evolution to myelodysplasia and acute myelogenous leukemia2 and genetic and molecular studies indicating that both cyclic and congenital neutropenia are attributable to mutations in an enzyme found in the primary granules of neutrophils, neutrophil elastase.3,4
Clinical Features of Cyclic and Congenital Neutropenia
Cyclic neutropenia occurs sporadically and by autosomal-dominant inheritance. The diagnosis of cyclic neutropenia is suggested when a child begins to have regularly recurring episodes of fever and oropharyngeal and skin infections in the first year of life.5 Usually these events occur at 21-day intervals. When the child is febrile, the neutrophil count is usually extremely low. Serial observations will reveal oscillations of neutrophil counts, but the counts often remain below the lower limit of normal, i.e. less than 1.5 × 109/L, except with severe illness. Both blood monocytes and reticulocytes cycle out of phase with neutrophils; oscillations in other cell types, including platelets, usually can be seen with long periods of serial observations.
Numerous studies point to periodic interruption of cell production in the bone marrow as a cardinal feature of this disease, which causes the cycling phenomenon.5 Within families with autosomal dominant cyclic neutropenia, however, there is substantial variation in the phenotype.6 Some individuals are severely affected, with counts that oscillate quite dramatically, whereas other affected individuals have a chronic neutropenia, without obvious cycling of these neutrophil counts. In general, oscillations are more obvious in children than adults.
Longitudinal studies indicate that deep tissue cellulitis, as an extension of cutaneous infections, and bacteremias, frequently due to Clostridium species, are the most serious medical complications of cyclic neutropenia. The latter complication has often been fatal. For this reason, an episode of fever and severe abdominal pain in the patient with cyclic neutropenia requires urgent evaluation.
Congenital neutropenia is a more severe disorder and probably occurs 3 to 4 times more frequently than cyclic neutropenia.7 It occurs sporadically and as both an autosomal recessive and an autosomal dominant disorder. Usually the diagnosis is made within the first months of life, based on the findings of severe neutropenia, fevers and recurrent infections often involving the upper respiratory tract, lungs and skin. Deep tissue infections including chronic pneumonia and lung and liver abscesses are much more common than in cyclic neutropenia. Typically, the neutrophil count is less than 0.2 × 109/L and rarely rises much higher than this, even with severe infections. Typically, monocytes and platelets are elevated, and there is mild anemia. Bone marrow examination is helpful in the diagnosis, particularly the finding of promyelocytic maturation arrest. There may be an increase in the percentage of marrow myeloblast and diminished or atypical granulation of the developing promyelocytes. Marrow eosinophilia is also often observed.
Treatment of Cyclic and Congenital Neutropenia
Clinical trials demonstrating the effectiveness of G-CSF for the treatment of cyclic and congenital neutropenia were a major advance for these patients in the late 1980s.1,8,9 Clinical trials using glucocorticosteroids, androgens, lithium, intravenous gammaglobulin and granulocyte macrophage colony-stimulating factor had shown that these therapies are often ineffective. In cyclic neutropenia, G-CSF does not eliminate cycling; however, it increases the amplitude of the oscillations in blood neutrophils and shortens the cycle length. More importantly, G-CSF treatment reduces the duration and severity of the neutropenic periods. As a result, fever, mouth ulcers, and infections are markedly reduced. In congenital neutropenia, there is often a delay of 7 to 10 days in the response to G-CSF,8 but most patients gradually increase their neutrophil levels, and there is a concomitant decrease in the occurrence of fever and infections. In the largest trial, the dose of G-CSF for cyclic neutropenia was approximately 3 μg/kg/day and for congenital neutropenia 6 μg/kg/day. For congenital neutropenia, there were wide differences in the dose, and some patients were treated with more than 100 μg/kg/day. Observations conducted through the Severe Chronic Neutropenia International Registry indicate that most patients with cyclic neutropenia will respond to about 2 μg/kg/day of G-CSF, administered either daily or on alternate days. Intermittent therapy at longer intervals is generally less effective and is associated with more frequent side effects. For congenital neutropenia, it is best to start with approximately 5 μg/kg/day and to titrate the dose to achieve a neutrophil level of 1-2 × 109/L. Long-term observations have shown that patients will maintain their neutrophil responses and that the development of antibodies and loss of effectiveness of the therapy due to “marrow exhaustion” does not occur. Usually, platelet counts, which are often elevated before treatment, fall to the normal range on G-CSF therapy. About 5% of patients develop mild to moderate thrombocytopenia, which usually responds to either no change in therapy or modest reductions of G-CSF. Most patients are mildly anemic at the start of therapy; the hematocrit and hemoglobin usually rise to normal on G-CSF treatment. Mild bone pain and headache are the most frequent acute adverse effects. Growth and development of children is not altered by treatment. A major effect is improved school attendance and work performance due to less severe illness. Some patients have developed osteoporosis on long-term G-CSF; understanding of the risks of this adverse event and its relationship to the primary disease or treatment is still incomplete.
Risk of Leukemia
Before the availability of G-CSF, children with congenital neutropenia were known to develop acute myelogenous leukemia, but probably most affected children died from severe infections.2 Therefore, the risk of their evolution to AML was never determined before G-CSF became available. There is no recognized risk of evolution to AML in cyclic neutropenia. Over the last decade, it has become quite clear that myelodysplasia and acute leukemia are complications of severe congenital neutropenia, but the relationship between treatment and evolution to AML remains unclear. The Severe Chronic Neutropenia Registry (the Registry) now includes more than 350 patients with congenital neutropenia; 31 of these patients have evolved to myelodysplasia or acute leukemia.2 The annual rate of transformation is approximately 2% per year. None of the Registry patients with well-documented cyclic neutropenia have developed MDS or AML, despite a similar duration of treatment. In 18 of the 31 cases, the transformations occurred in association with partial or complete loss of chromosome 7. In nine patients, there was an associated abnormality of chromosome 21. Within the congenital neutropenic patients, there is no recognized relationship of age, gender, G-CSF dose, or the duration of treatment with malignant transformation. The Registry recommends monitoring patients with congenital neutropenia with regular clinical assessments, blood counts, and annual bone marrow examinations. Bone marrow transplantation is the only alternative therapy and its risks depend largely upon the histocompatibility match of the donor and the recipient.
Recent Pathophysiological Studies
Clinical and laboratory studies now suggest that the primary defect in cyclic and congenital neutropenia is a defect in the hematopoietic cascade either at the stem cell level or early in the process of differentiation of these cells to the neutrophil lineage. The finding of normal or near normal mean levels of erythrocytes, platelets, and other leukocytes supports this idea. The elevated monocyte levels in both congenital and cyclic neutropenia also suggest that there may be preferential or compensatory increases in production of the cells. A series of recent investigations now suggest that cyclic and congenital neutropenia have overlapping features but are distinctive disease entities. The results of these studies can be summarized as follows:
Cyclic and congenital neutropenia are not due to a deficiency or dysfunction of G-CSF. In response to neutropenia and infections, both groups of patients elevate G-CSF levels.10
The receptor for G-CSF is mutated in some patients with congenital neutropenia, but not in patients with cyclic neutropenia.11 However, these mutations of the G-CSF receptor are acquired abnormalities associated with the evolution of these patients to MDS and AML.12 These mutations are in the cytoplasmic domain of the receptor.
Patients with both cyclic neutropenia and congenital neutropenia have mutations of neutrophil elastase (ELA-2), a serine protease formed early in the differentiation of cells to the neutrophil lineage.3,4 This abnormality was discovered through linkage analysis in 13 families with autosomal dominant cyclic neutropenia. The disease mapped to chromosome 19 p 13.3, the locus for several enzymes found in the primary granules of neutrophils.3 Extension of this work showed that sporadic cases of cyclic neutropenia have ELA-2 mutations, predominantly at the junction of exons 4 and 5.4 This location is proximate to the binding site for the enzyme with its inhibitors and substrates. A recent report shows that 22 of 25 patients with congenital neutropenia had mutations of neutrophil elastase involving more diverse locations within the elastase gene.4 In this series, there were five families with multiple affected members, suggesting an autosomal dominant pattern of inheritance. The same mutation was found in all the affected members of each of these families, indicating a germline, rather than a somatic, mutation. This evidence suggests that ELA-2 mutations are the primary cause of cyclic neutropenia and most cases of congenital neutropenia. ELA-2 mutations may provide the background for the occurrence of the G-CSF-R mutations that occur in the pathway for development of AML.
Accelerated apoptosis of neutrophil precursors is a common feature of both cyclic and congenital neutropenia. The abnormality is more severe in congenital neutropenia. Accelerated apoptosis can be demonstrated by electron microscopic studies, staining of separated precursor populations with an annexin V and in vitro culture studies.13 From these investigations, it can be inferred that the mutations of ELA-2 cause the premature death of these cells by activating the apoptotic process, either directly or indirectly.
Many aspects of the pathophysiology of cyclic and congenital neutropenia are still poorly understood. We do not yet know precisely why cycling occurs. Studies by Mackey et al14,15 suggest that cycling may occur when the rate of apoptosis of precursors is increased to a moderate, but not an extreme, degree. Extreme levels of cell loss are predicted to lead to very severe reductions of counts as occurs in severe congenital neutropenia. Thus, the differences in the phenotype may depend upon the specific mutations and the relative rates of apoptosis of early precursors.
The evolution for congenital, but not cyclic, neutropenia to AML is also not yet understood. It has been hypothesized that in congenital neutropenia there is a higher compensatory flux of cells from the stem cell compartment in response to more severe loss of the developing neutrophil precursors. This could result in a stem cell population that is more vulnerable to leukemic transformation.
Summary
The last decade has seen remarkable progress both in our understanding of the pathophysiology and in the treatment of cyclic and congenital neutropenia. Several investigators are working to refine our understanding of these and related conditions through the Severe Chronic Neutropenia International Registry. G-CSF has proven to be a very effective therapy for these conditions; further unraveling of the pathophysiological mechanisms should lead to even better therapies.
III. Chronic Granulomatous Disease: Lessons from Murine Models and Potential New Therapies
Mary C. Dinauer, M.D., Ph.D.*
James Whitcomb Riley Hospital for Children, Wells Center for Pediatric Research, Indiana School of Medicine, 1044 W Walnut Street, R4 466, Indianapolis IN 46202-5225
Chronic granulomatous disease (CGD) is an inherited disorder of phagocyte function in which generation of superoxide by the phagocyte nicotinamide dinucleotide phosphate (NADPH) oxidase (also referred to as the respiratory burst oxidase) is absent or markedly deficient.1,2 CGD, which has an estimated incidence of between 1/200,000 and 1/250,000 live births,3 results from mutations in any of the four genes encoding essential subunits of the NADPH oxidase. Respiratory burst-derived oxidants are an important component of the innate immune response, and their absence results in recurrent, often life-threatening bacterial and fungal infections and is also associated with formation of inflammatory granulomas. Knockout mouse models for CGD have provided additional insight into the role of superoxide in the host response to infection and inflammation, and new treatment approaches based on stem cell therapies hold promise for the future management of patients with CGD.
Clinical Features of CGD
The clinical manifestations of CGD typically begin in infancy or early childhood. Superoxide generated during the phagocyte respiratory burst is the precursor to numerous microbicidal oxidants, including hydrogen peroxide and hypochlorous acid. Lacking this pathway, CGD patients are particularly susceptible to Staphylococcus aureus, Aspergillus species, and a variety of gram-negative enteric bacilli including Serratia marcescens, Salmonella species and Burkholderia cepacia. Many of these organisms contain catalase, which prevents CGD phagocytes from utilizing microbe-generated hydrogen peroxide to promote killing of ingested organisms. Frequent sites of infection include skin and its draining lymph nodes, lungs, bone and gastrointestinal tract (including the liver, which can often be the site of abscesses). Although S. aureus is the most frequently isolated organism overall, the most common causes of death reported in a recent series were pneumonia and/or sepsis due to Aspergillus or B. cepacia.3 Many CGD patients also develop chronic inflammatory granulomas, which are a distinctive hallmark of this disorder. Symptomatic disease can include colitis/enteritis or granulomatous obstruction of either the gastric outlet or urinary tract. In some cases, granuloma formation is a response to active infection, but may also reflect a dysregulated inflammatory response and/or inefficient degradation of debris in the absence of respiratory burst-derived oxidants.4,5 A registry of CGD patients in the United States, established in 1993, is maintained through the Immune Deficiency Foundation and should aid in further characterizing the epidemiologic and clinical features of CGD.3
Because of X-linked inactivation, typically 35-65% of neutrophils from female carriers of X-CGD are oxidase-positive, indicating that there is no selective advantage for oxidase-positive versus oxidase-negative cells. Recurrent stomatitis or significant gingivitis, or both, have been noted in as many as half of X-CGD carriers.2 Photosensitivity and discoid lupus erythematosus has also been described. Rarely, because of an unusually skewed X-inactivation, some X-CGD carriers have a smaller percentage of oxidase-positive neutrophils. Some women with only 5-10% nitroblue tetrazolium test (NBT)-positive cells are healthy, yet others have experienced recurrent bacterial infections similar to those seen in classic CGD.1,2
The NADPH Oxidase and the Molecular Genetics of CGD
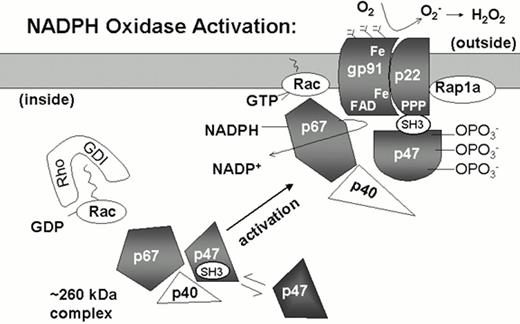
The NADPH oxidase is a phagosomal and plasma membrane-associated enzyme complex that is dormant in the resting neutrophil and rapidly assembled when neutrophils are activated by a variety of inflammatory stimuli (Figure 2).1,2,6 Four polypeptide subunits are essential for NADPH oxidase activity and mutations in the corresponding genes are responsible for the four different genetic subgroups of CGD (Table 3). The oxidase subunits are referred to by their apparent molecular mass (kDa) and have been given the designation phox, for phagocyte oxidase.
Model of NADPH oxidase activation.
In unstimulated neutrophils, the subunits of the NADPH oxidase are separated in the cytoplasmic and membrane compartments. The plasma and specific granule membranes contain flavocytochrome b with its p22phox and glycosylated gp91phox subunits and the small GTPase Rap1a. The flavin and heme groups (Fe) that mediate the transfer of electrons from NADPH to molecular oxygen appear to be localized in the gp91phox subunit. The cytosolic components p47phox and p67phox may exist as a preformed complex of 260 kDa, which also includes at least one additional protein, p40phox. The small GTPase Rac is also present in the cytosol in its inactive GDP bound state, in a complex with a GDP dissociation inhibitor (Rho-GDI). With neutrophil activation, the cytosolic phox subunits translocate to the membrane, which is dependent upon phosphorylation of p47phox and exposure of an SH3 domain that binds to p22phox. Other binding sites between p47phox and gp91phox are also likely to be important. Neutrophil activation also triggers the conversion of Rac-GDP to Rac-GTP and dissociation from Rho-GDI. Rac-GTP is membrane-associated, interacting with p67phox and another undetermined site in the oxidase complex.
Model of NADPH oxidase activation.
In unstimulated neutrophils, the subunits of the NADPH oxidase are separated in the cytoplasmic and membrane compartments. The plasma and specific granule membranes contain flavocytochrome b with its p22phox and glycosylated gp91phox subunits and the small GTPase Rap1a. The flavin and heme groups (Fe) that mediate the transfer of electrons from NADPH to molecular oxygen appear to be localized in the gp91phox subunit. The cytosolic components p47phox and p67phox may exist as a preformed complex of 260 kDa, which also includes at least one additional protein, p40phox. The small GTPase Rac is also present in the cytosol in its inactive GDP bound state, in a complex with a GDP dissociation inhibitor (Rho-GDI). With neutrophil activation, the cytosolic phox subunits translocate to the membrane, which is dependent upon phosphorylation of p47phox and exposure of an SH3 domain that binds to p22phox. Other binding sites between p47phox and gp91phox are also likely to be important. Neutrophil activation also triggers the conversion of Rac-GDP to Rac-GTP and dissociation from Rho-GDI. Rac-GTP is membrane-associated, interacting with p67phox and another undetermined site in the oxidase complex.
Genetic classification of chronic granulomatous disease.
| Component Affected . | Gene Locus . | Subtype* . | Flavocytocrome b558 Spectrum . | NBT Score (% Positive) . | Frequency (% of Cases) . |
|---|---|---|---|---|---|
| gp91phox | Xp21.1 | X91 | 0 | 0 | 56 |
| X91- | Low | 80-100 (weak) | 5 | ||
| X91- | Low | 5-10% | 2 | ||
| X91+ | N | 0 | 2 | ||
| p22phox | 16q24 | A220 | 0 | 0 | 6 |
| A22+ | N | 0 | 1 | ||
| p47phox | 7q11.23 | A470 | N | 0 | 23 |
| p67phox | 1q25 | A670 | N | 0 | 6 |
| Component Affected . | Gene Locus . | Subtype* . | Flavocytocrome b558 Spectrum . | NBT Score (% Positive) . | Frequency (% of Cases) . |
|---|---|---|---|---|---|
| gp91phox | Xp21.1 | X91 | 0 | 0 | 56 |
| X91- | Low | 80-100 (weak) | 5 | ||
| X91- | Low | 5-10% | 2 | ||
| X91+ | N | 0 | 2 | ||
| p22phox | 16q24 | A220 | 0 | 0 | 6 |
| A22+ | N | 0 | 1 | ||
| p47phox | 7q11.23 | A470 | N | 0 | 23 |
| p67phox | 1q25 | A670 | N | 0 | 6 |
In this nomenclature, the first letter represents the mode of inheritance (X-linked [X] or autosomal recessive [A]). The number indicates the phox component that is genetically affected. The superscript symbols indicate whether the protein expression of the affected component is undetectable (0), diminished (-), or normal (+). X91+ and A22+ represent crossreactive but defective enzymes. Abbreviations: N, normal; NBT, nitroblue tetrazolium test. Frequency of cases represents data from 121 families.65,66
Approximately two-thirds of CGD cases result from defects in the X-linked gene encoding the gp91phox subunit of flavocytochrome b558, a membrane-bound heterodimer that is the redox center of the oxidase. A rare autosomal recessive form of CGD is caused by mutations in the gene encoding p22phox, the smaller subunit of flavocytochrome b558. The remaining cases of autosomal recessive CGD involve genetic defects in either p47phox or p67phox, which are located in the cytosol of unstimulated cells but translocate to the membrane with oxidase activation (Figure 2). A fifth phox protein, p40phox, is associated with p67phox in resting neutrophils and plays an unknown but dispensable role in superoxide production.
Regulation of superoxide formation also involves the small GTP-binding protein Rac and possibly Rap 1 a, which act as molecular switches through conformational changes between the GDP and GTP-bound forms.6 Rac-GTP can bind to p67phox and is required for respiratory burst oxidase activity.6 Genetic defects in Rap1a, Rac, or p40phox have not been reported in CGD. However, a dominant-negative mutation in the hematopoietic-specific Rac2 GTPase has recently been identified in an infant with recurrent, deep-seated soft tissue infections and poor formation of pus despite peripheral neutrophilia. This clinical picture is more reminiscent of leucocyte adhesion deficiency than CGD. Neutrophil function assays showed normal leukocyte β2 integrin expression but severe defects in chemotaxis and NADPH oxidase activation in response to some but not all agonists, along with impaired L-selectin-mediated adhesion.7,8 Of interest, this phenotype is similar to that reported for Rac2 knockout mice.9,10
gp91phoxand p22phox
The NADPH oxidase flavocytochrome b is comprised of gp91phox and p22phox. Heterodimer formation is important for stable expression of gp91phox and p22phox in phagocytes, and both polypeptides are typically absent with null mutations in either flavocytochrome b subunit.
The gp91phox subunit is the product of the gene affected in the X-linked form of CGD. gp91phox is the redox center of the oxidase and contains two heme prosthetic groups embedded within the membrane in the hydrophobic N-terminus of the protein; the C-terminus contains a flavoprotein domain with binding sites for flavin and NADPH binding.6 The gp91phox protein is the first example of a growing family of flavocytochromes with similar structural features. Homologous proteins were first identified in yeast, which acts as a ferric iron reductase for transmembrane iron transport,11 and in plants,12 which may generate oxidants for intracellular signaling and host defense.13 In the past year, mammalian homologs of gp91phox have also been discovered. One form is highly expressed in colon,14,15 and a second has been identified in renal tubular epithelial cells.16 These gp91phox homologs have superoxide-generating activity (albeit at a much lower level compared to the phagocyte NADPH oxidase) and may also act as proton channels. Although their physiologic functions are currently unknown, it has been proposed that these homologs produce superoxide anions that act as signals for regulation of gene expression, cell proliferation and apoptosis.
In X-CGD, more than 60 distinct mutations have been identified in the gp91phox gene. These include deletions, frameshifts, splice site, nonsense, and missense mutations that are distributed throughout the gene.17,18 A database is accessible at http://www.helsinki.fi/science/signal/databases/x-cgdbase.html.19 In some patients, missense mutations affect histidine residues in the N-terminus of gp91phox that are candidate heme-binding ligands. Flavocytochrome b is absent in these patients, consistent with a strict requirement for heme incorporation in flavocytochrome b biosynthesis.20,21 Rarely, X-CGD patients express a stable but non-functional form of gp91phox (X91+). Some cases are due to mutations in important consensus sequences for NADPH or flavin binding in the C-terminus of gp91phox; others affect translocation of the cytosolic subunits p47phox and p67phox.
The p22phox subunit of the flavocytochrome b heterodimer has multiple hydrophobic regions in its N-terminus that probably intercalate in the membrane, and an intracellular, proline-rich C-terminus. Mutations that have been identified in A22 CGD are heterogeneous and range from large interstitial gene deletions to point mutations associated with missense, frameshift, or RNA splicing defects.17 Analogous to cases of X91 + CGD is one A22+ patient who is homozygous for a proline to glutamine missense substitution in the N-terminus, an important binding site for an SH3 domain in p47phox.22 SH3 domains, first described in the src tyrosine kinase family, mediate protein-protein interactions by binding to proline-rich motifs in target proteins. The proline substitution in p22phox is the second example of a genetic disease caused by disrupted protein interactions mediated by SH3 domains. A kindred with X-linked agammaglobulinemia due to deletion of the SH3 regions of the Bruton's tyrosine kinase has also been reported.23
p47phoxand p67phox
The cytosolic oxidase subunits, p47phox, p67phox and p40phox, each contain a pair of SH3 domains and at least one proline-rich SH3-binding motif; p40phox also contains an SH3-binding domain.6 In resting neutrophils, these three polypeptides can be isolated as a complex, whose formation is mediated at least in part by interactions between their SH3 and proline-rich domains. Phagocyte activation results in translocation of this complex to the membrane to assemble the catalytically active oxidase, although p40phox, as already noted, is dispensable for enzyme function. Neither p47phox nor p67phox appears to participate directly in electron transfer but probably act in a regulatory role. Substantial amounts of superoxide can be generated from neutrophil membranes even in the absence of p47phox, provided that high concentrations of p67phox and Rac-GTP are supplied.6 Hence, p47phox may function as an “adaptor” protein to position p67phox correctly in the active NADPH oxidse complex. Translocation of p47phox to the membrane requires phosphorylation of multiple serine residues in its C-terminus.6 One effect of phosphorylation is to unmask the p47phox SH3 domain so it can bind to the p22phox subunit of flavocytochrome b (Figure 2).24,25
Patients with p47phox-deficient CGD account for approximately one-fourth of cases in the Unites States and Europe, but only about 7% of cases in Japan.2 Virtually all A47 patients are either homozygotes or compound heterozygotes for a mutant allele with a GT deletion at the beginning of exon 2 that predicts a premature stop codon.2 The high frequency of the p47phox GT deletion mutation appears to reflect the existence of highly conserved and closely linked p47phox pseudogenes.26 This close physical proximity leads to recombination events between the wild-type gene and pseudogenes.27
A heterogenous group of mutations has been found in the p67phox gene in A67 CGD.2 These include missense mutations in the N-terminus of p67phox, which contains four α-helical tetratectopeptide (TPR) motifs that interact with both the Rac GTPase and another, unknown, site within the active NADPH oxidase complex.28 All but one of these mutations disrupt protein stability. In one patient, where a triplet nucleotide deletion results in an in frame deletion of lysine 58, a non-functional form of p67phox was expressed, which failed to translocate and also was unable to bind to Rac.29
Correlation between genetic defect and clinical course
As a group, patients with X-CGD, A22 CGD, and A67CGD tend to have a more severe clinical course compared to patients with A47 CGD.1,2,3 This may reflect residual superoxide formation by p47phox-deficient neutrophils.30 X91- patients (see Table 3), who have low levels of NADPH oxidase activity due to a partially functional flavocytochrome b558, can have a variable clinical course.1 Some have a milder form of CGD, while others have had numerous infectious complications. Polymorphisms in oxygen-independent antimicrobial systems or other components regulating the innate immune response are also likely to play an important role in modifying disease severity. Specific polymorphisms in the myeloperoxidase, mannose binding lectin, and FcγRIIa genes have recently been shown to be associated with a higher risk for granulomatous or autoimmune/rheumatologic complications in CGD.31
Mouse Models of CGD
Gene targeting has been used to develop mouse models for both X-linked (gp91phox-/-) and an autosomal recessive (p47phox-/-) form of CGD.32,33 CGD mice have abnormalities in both host defense and inflammation that are similar to their human counterparts, confirming the importance of the respiratory burst in innate immunity.
CGD mice exhibit a marked increase in susceptibility to the opportunistic pathogens B. cepacia and Aspergillus species,5,32,34,35,36 two organisms that are particularly problematic in CGD patients. Interestingly, whereas catalase-negative bacteria can be killed effectively by CGD phagocytes due to the release of bacterial hydrogen peroxide in the phagocytic vacuole, a catalase-negative derivative of A. nidulans was still lethal in CGD mice.36 This suggests that catalase does not increase fungal pathogenicity in the setting of CGD. Other organisms showing increased virulence in CGD patients and, similarly, in CGD mice include S. aureus32 and Salmonella typhimurium.37 Partial defects in the host response to Mycobacterium tuberculosis38,39 and Listeria monocytogenes have also been found in CGD mice.40 Although cases of atypical mycobacteria have only been occasionally reported in CGD patients in the US, M. tuberculosis was not uncommon in a cohort of CGD patients followed in Hong Kong.41
Studies of Salmonella infection in gp91phox-/- mice have uncovered a novel mechanism of microbial resistance to phagocyte oxidants.42 Salmonella typhimurium contain a cluster of genes, designated Salmonella pathogenicity island 2 (SPI2), which translocates bacterial proteins into the host cell cytosol and are required for virulence and intracellular survival in macrophages. Salmonella with mutations at the SPI2 locus are no longer virulent in wild-type mice but cause lethal infection in gp91phox-/- mice, suggesting that the SPI2 genes normally prevent bacterial killing by the NADPH oxidase. In wild-type macrophages, NADPH oxidase activity was detected in phagosomes containing SPI2-mutant Salmonella. In contrast, macrophages infected with wild-type Salmonella, while still able to produce superoxide at the plasma membrane, had very little phagosome-associated NADPH oxidase activity. Hence, one strategy used by Salmonella and perhaps other intracellular pathogens to limit exposure to respiratory burst-derived oxidants may involve prevention of NADPH oxidase from trafficking to Salmonella-containing phagocytic vacuoles.
The generation of reactive nitrogen intermediates via inducible nitric oxide synthase (NOS2) is a second oxygen-dependent phagocyte antimicrobial system that plays an important role in host defense in mice and also likely in humans.43 Mice with a double deficiency in both the NADPH oxidase (gp91phox-/-) and NOS2 have a high rate of spontaneous infection with commensal organisms, mostly enteric bacteria, when raised under specific pathogen-free conditions, whereas parental mice with a single-enzyme deficiency rarely exhibit spontaneous infections under these conditions.44 This observation suggests that these two enzyme systems are able to compensate for each other in providing resistance to indigenous bacteria, at least in the mouse. Nitric oxide production has been detected in neutrophils from CGD patients.45 Superoxide production from xanthine oxidase, which is expressed in endothelial cells and other tissues, appears to be another source of antioxidants that can function as a backup to the phagocyte NADPH oxidase.35
Both gp91phox- and p47phox-deficient CGD mice also have abnormalities in the inflammatory response.32,33 CGD mice have a marked increase in exudate neutrophils compared to wild-type mice in response to peritoneal instillation of thioglycollate.32,33 In a study done using gp91phox-/ mice, an exaggerated acute inflammatory response occurred after instillation of sterilized hyphae into the lungs, which then evolved into a chronic granulomatous infiltrate.5 These studies support the hypothesis that respiratory burst products play an important role in inflammation outside of their function in microbial killing.
Whether absence of phagocyte NADPH oxidase activity has a protective effect in diseases with an inflammatory component has also been investigated using knockout CGD mice. gp91phox-/- mice had a significant decrease in infarct size compared to wild type mice in stroke injury induced by transient occlusion of the carotid artery.46 However, no decrease in acute injury to pulmonary endothelium following intravascular activation of complement was seen in gp91phox-/- mice, although numerous previous studies had found that this injury was attenuated by anti-oxidants.47 Superoxide production has also been implicated in the pathogenesis of atherosclerosis, and macrophages, an important cellular constituent of early atherosclerotic lesions, have been postulated to be an important source of superoxide. However, no decreases in atherosclerotic lesions were observed in either gp91phox-/- or p47phox-/- crossed onto the hypercholesterolemic ApoE-/- strain, or for gp91phox-/- mice fed a high fat diet.48,49
Treatment of CGD
Current approaches
The use of prophylactic antibiotics and interferon-γ, coupled with aggressive treatment of acute infections and prolonged courses of antimicrobial treatment, has markedly improved the clinical course of patients with CGD. Trimethoprim/sulfamethoxazole (or, for patients allergic to sulfa, dicloxacillin) is in standard use for antibiotic prophylaxis,50 and at least one study has suggested that prophylactic itraconazole may be useful in preventing pulmonary Aspergillus infection.51 Prophylactic interferon-γ is another mainstay of current management, although its use is not accompanied by any measurable improvement in phagocyte NADPH oxidase activity in the majority of CGD patients.52 Hence, the clinical benefit of interferon-γ is probably related to enhanced phagocyte function and killing by nonoxidative mechanisms and perhaps the NOS2 and xanthine oxidase pathways. Corticosteroids are used to treat clinically significant granulomatous complications of CGD, although with caution, given the underlying microbial killing defect.
The prognosis of CGD has improved dramatically in the past two decades. In one single-institution study of 21 British children with CGD diagnosed since 1990, prophylactic antibacterial and antifungal prophylaxis were routinely instituted at the time of diagnosis of CGD.53 No invasive or fungal infections occurred after diagnosis, nor were there any deaths. The overall mortality for patients with CGD in the United States has recently been recently estimated to be about 2% per year.34 Data from the above-mentioned CGD Registry will be very helpful in monitoring the changing outlook for patients with this disease, which is important for weighing the risks and benefits of more experimental therapeutic approaches.
Stem cell transplantation in CGD
Because CGD is due to a defect in myeloid lineage cells, allogeneic stem cell transplantation (SCT) has been curative, even when the outcome is a mixed chimeric state with only about 10-15% of circulating neutrophils being of donor origin.1,2,54,55 However, allogeneic bone marrow transplantation (BMT) has rarely been used in CGD because of its associated risks and often lack of suitable donors.
The recent development of nonmyeloablative conditioning regimens for allogeneic hematopoietic stem cell (HSC) transplantation may offer a new approach for stem cell therapy in CGD. Malech and colleagues at the National Institutes of Health have conducted a phase I study targeted at high-risk patients with CGD who had a history of two or more serious infections.56 In this trial, patients were conditioned with cyclophosphamide, fludarabine and antithymocyte globulin prior to transplantation with T cell-depleted CD34+ cells from matched sibling donors. Donor lymphocyte infusions were given on days +30 and +60 if the recipients' T cells were less than 60% donor in origin. In the initial report of preliminary results from this trial, all patients survived and tolerated the transplants reasonably well.56 Only one patient never engrafted with donor cells and had complete autologous recovery. Three patients had significant (≥ 30%) donor chimerism 100 days post transplant. Clinically significant skin graft-versus-host disease was seen in only one patient. These initial results are very promising, although questions about long-term durability of the allogeneic graft and other complications remain. Many of these should be answered with longer follow-up of these and other patients with inherited blood disorders or malignant marrow diseases who have recently been treated using allogeneic SCT with similar conditioning. Trials such as these will also help bridge the use of less intensive conditioning regimens in conjunction with gene therapy of autologous hematopoietic stem cells.
Gene therapy of CGD
CGD is also a candidate disease for gene therapy targeted at HSCs.54,55 Numerous reports have shown that NADPH oxidase activity can be restored by gene transfer to human CGD leukocytes cultured in vitro. Clinical observations have suggested that even partial reconstitution of NADPH oxidase activity may be of some benefit, as discussed above. Women who are X-CGD carriers with only 5-10% oxidase-positive neutrophils often have few or no symptoms, and partial chimerism following allogeneic BMT has been beneficial for CGD. X91-CGD patients with a partially functional flavocytochrome b and low levels of NADPH oxidase activity often have a milder clinical course. Taken together, these observations suggest that complete correction of respiratory burst activity in ∼10% of circulating neutrophils would lead to clinically relevant improvements in host defense. However, the relative level of superoxide within individual cells may be an important factor, and only partial correction of cellular NADPH oxidase activity may not restore full antimicrobial activity.
Preclinical studies of gene therapy in murine CGD
Animal models are very useful in developing strategies for gene therapy of inherited disorders. The relative level of expression of the transferred genetic sequences required to prevent or reverse disease manifestations can be tested directly. Evaluation for any untoward effects of this therapy over the lifetime of the animal can also be evaluated, such as the consequences of constitutive expression of transferred sequences in target cells and the potential immunogenicity of transgenic proteins.
In murine CGD, retroviral-mediated gene transfer into bone marrow cells can correct respiratory burst oxidase activity in phagocytes in vivo and improve the defect in host defense against bacterial and fungal pathogens.34,57,58 These studies were some of the first to show that gene therapy could ameliorate the clinical symptoms of an inherited disorder, using an animal model that closely resembles the human disease.
In studies using X-CGD mice, bone marrow cells were transduced with a murine stem cell virus-based retrovirus containing the murine gp91phox cDNA and transplanted into lethally irradiated syngeneic X-CGD recipients.57,58 Fifty to eighty percent of peripheral blood neutrophils were oxidase positive by NBT testing 12-14 weeks after primary BMT. Oxidase-positive neutrophils persisted for at least 18 months and were also detected in secondary transplant recipients. Although gp91phox protein expression in transduced neutrophils was less than 10% of wild-type, superoxide-generating activity was approximately one-third of normal mouse neutrophils. There were no obvious adverse consequences to the long-term, constitutive expression of these levels of recombinant gp91phox in bone marrow cells.57,58 These results show that long-term reconstituting HSC were successfully transduced and that this vector provides stable long-term expression of gp91phox in vivo.
Even this modest level of correction in neutrophil NADPH oxidase activity improved host defense. Gene therapy-treated X-CGD mice with ∼50% NBT-positive circulating neutrophils were resistant to respiratory challenge with A. fumigatus.57 As few as ∼5% wild-type neutrophils protected against A. fumigatus challenge in X-CGD mice transplanted with mixtures of wild-type and X-CGD marrow.57 However, for chimeric X-CGD mice generated by transplantation with mixtures of retrovirus-transduced and mock-transduced X-CGD bone marrow, at least 10% corrected neutrophils were needed to prevent A. fumigatus infection (Dinauer, unpublished observations). Increased superoxide generation using vectors with higher expression of recombinant gp91phox should prove even more effective.
Retroviral-mediated gene transfer has also been studied in p47phox-/- knockout mice that were conditioned with a sublethal dose of radiation (5 Gy), a regimen with potentially decreased toxicity in the clinical setting.34 One month post transplantation of p47phox-/- transduced p47phox-/- bone marrow, the percentage of oxidase-positive peripheral blood neutrophils ranged from ∼8% to 17% in individual mice, declining to ∼3% fourteen weeks after transplantation, and continuing to fall thereafter. Oxidase activity in individual neutrophils was similar to individual wild-type cells. Gene therapy-treated mice had prolonged survival after intraperitoneal injection with a dose of B. cepacia that was lethal in 100% of untreated p47phox-/- mice; two of nine mice treated with gene therapy had apparent spontaneous resolution of peritonitis. Wild-type mice had no mortality even with a two-log higher dose of B. cepacia. These data show that correction of a limited number of neutrophils improves host defense in p47phox-/- mice, but that reconstitution of NADPH oxidase activity in greater than 5-10% of cells is likely to be required for more complete restoration of host defense.
Human phase I clinical trials
Several phase I CGD gene therapy clinical trials using cytokine-mobilized peripheral blood CD34+ cells have been either completed or are ongoing. In one trial by Malech and colleagues involving five p47phox-deficient CGD patients, autologous peripheral blood CD34+ cells were collected by apheresis, transduced with a retroviral vector containing the p47phox cDNA over a 3-day period ex vivo, and then reinfused.59 Oxidase-positive neutrophils were first detected in peripheral blood after 3 weeks using a sensitive flow cytometric assay and persisted for the next several months. The maximum percentage of corrected cells represented only 0.004-0.05% of the circulating neutrophils. This group has also undertaken a second phase I trial with X-CGD patients60 utilizing a fibronectin-assisted retroviral transduction method for inserting a retrovirus containing a gp91phox-gene into autologous peripheral blood CD34+ cells.61 The manipulated cells were reinfused after a four-day ex vivo transduction process. The patients received multiple infusions of transduced cells 50 days apart, with no marrow conditioning. Similar to the p47phox gene therapy trial, oxidase-positive neutrophils were first detected 3 to 4 weeks after each infusion cycle. The frequency of circulating oxidase-corrected neutrophils ranged from 0.06-0.2%, but again, the number diminished over time. Similar results have been obtained in an ongoing phase I trial at our institution, using a transduction protocol that includes stem cell factor (SCF), megakaryocyte growth and development factor (MGDF), and granulocyte-colony stimulating factor (G-CSF) in the presence of fibronectin fragment CH-296 (Dinauer, unpublished observations).
These phase I clinical studies suggest that marrow conditioning prior to reinfusion of transduced cells may be required to achieve higher level engraftment of corrected stem/progenitor cells, along with more efficient methods of gene transfer into human long-term repopulating HSC. In vivo selection for transduced HSC expressing a drug resistance gene linked to the therapeutic replacement gene is another potential approach, as suggested by murine studies using the multidrug resistance (MDR) protein or dihydrofolate reductase (DHFR).62,63,64
Summary
The analysis of specific gene defects in CGD patients has shed light on important structural and regulatory features of the NADPH oxidase. Mouse knockout models have recently been developed for two genetic subgroups of CGD and have proved useful for investigating the pathophysiology of CGD and for the assessment of new treatments. Encouraging results have been obtained in a recent trial targeted at high-risk CGD patients using allogeneic bone marrow transplantation with non-myeloablative conditioning. Phase I clinical trials based on retroviral-mediated gene transfer into hematopoietic stem cells are ongoing and, although the numbers of gene-corrected cells seen to date are still well below therapeutic range, have established a foundation for further development of this approach.