Abstract
This review focuses on polycythemia vera (PV)—its diagnosis, cellular and genetic pathology, and management. In Section I, Dr. Pearson, with Drs. Messinezy and Westwood, reviews the diagnostic challenge of the investigation of patients with a raised hematocrit. The suggested approach divides patients on their red cell mass (RCM) results into those with absolute (raised RCM) and apparent (normal RCM) erythrocytosis. A standardized series of investigations is proposed for those with an absolute erythrocytosis to confirm the presence of a primary (PV) or secondary erythrocytosis, with abnormal and normal erythropoietic compartments respectively, leaving a heterogenous group, idiopathic erythrocytosis, where the cause cannot be established. Since there is no single diagnostic test for PV, its presence is confirmed following the use of updated diagnostic criteria and confirmatory marrow histology.
In Section II, Dr. Green with Drs. Bench, Huntly, and Nacheva reviews the evidence from studies of X chromosome inactivation patterns that support the concept that PV results from clonal expansion of a transformed hemopoietic stem cell. Analyses of the pattern of erythroid and myeloid colony growth have demonstrated abnormal responses to several cytokines, raising the possibility of a defect in a signal transduction pathway shared by several growth factors. A number of cytogenetic and molecular approaches are now focused on defining the molecular lesion(s).
In the last section, Dr. Barbui with Dr. Finazzi addresses the complications of PV, notably thrombosis, myelofibrosis and acute leukemia. Following an evaluation of published data, a management approach is proposed. All patients should undergo phlebotomy to keep the hematocrit (Hct) below 0.45, which may be all that is required in those at low thrombotic risk and with stable disease. In those at high thrombotic risk or with progressive thrombocytosis or splenomegaly, a myelosuppressive agent should be used. Hydroxyurea has a role at all ages, but 32P or busulfan may be used in the elderly. In younger patients, interferon-α or anagrelide should be considered. Low-dose aspirin should be used in those with thrombotic or ischemic complications.
I. Evaluation of Current Diagnostic Pathways in the Erythrocytoses
Thomas C. Pearson, M.D.,*Maria Messinezy, FRCP,
Haematology Department, St. Thomas Hospital, Lambeth Palace Road, London SE1 7EH, UK
There is a need to clarify the basic nomenclature to describe an increased proportion of red cells in the peripheral blood. Polycythemia has traditionally been used but lacks precision. Erythrocytosis conveys the meaning in one word. Polycythemia rubra vera, used for the myelo-proliferative disorder (MPD), means a true increase in red cells, a situation that applies in other forms of erythrocytosis. However, there is merit in retaining the term polycythemia vera (PV) since in this MPD all cell lines may be involved. Using the term erythrocytosis rather than polycythemia avoids the incorrect assumption that `polycythemia' equates with PV.
A satisfactory robust approach to investigation should be used to take into account that often there are no precise diagnostic tests to establish a causal relationship, that patients may have evolving disease processes and that particularly in the elderly dual pathology may exist. The start-point is the result of the complete blood count (CBC). The selection process of patients for further investigation will be described first.
The Hematocrit
It is more appropriate to define an erythrocytosis in terms of the hematocrit (Hct) rather than Hb value since in the erythrocytoses iron deficiency can occur and then the Hct may be disproportionately higher than the Hb value. There is a small variation between types of analyzer. Ideally, every laboratory should determine its own reference range. Beckman-Coulter analyzers underestimate the true Hct value in the presence of low mean cell volume (MCV) and mean cell hemoglobin (MCH) values,1 and a correction should be used when appropriate. When using these analysers the upper end of the reference range for Hct is 0.51 for males and 0.48 for females. Thus, by definition those with a Hct above these values could be said to have an erythrocytosis. However, patients should not be investigated further until a minimum of two separate CBCs have been done. In some cases previous CBC results are available to help assessment of current values. In those with iron-deficient red cell indices and high normal Hct values, iron therapy has been advocated to see if the Hct rises. In practice, this puts the patient at vascular occlusive risk; it is safer to presume that they have an absolute erythrocytosis.
Individuals with a stable raised Hct should generally be investigated further by measurement of their red cell mass (RCM). However, males and females with Hct values above 0.60 and 0.56, respectively, can be assumed to have an absolute erythrocytosis.2 Patients with splenomegaly and normal Hct values should have a RCM measurement to exclude an absolute erythrocytosis.
Red Cell Mass
A standardized method of RCM measurement3 produces very reproducible results, with as little as ±5% variation on repeat study. The single factor leading to inter-laboratory discrepancy is the Hct value, which should be determined by micro-hematocrit. Results expressed in terms of total body weight lack precision in the obese patient, since fat tissue is relatively avascular. The radio-nuclide panel of the International Council for Standardization in Haematology (ICSH), appreciating the problem, re-examined published normal RCM data and used surface area derived from the height and weight. The scatter of results showed that 98% of males and 99% of females fell within ± 25% of the mean value at any given surface area. These limits were therefore chosen as the reference range. Thus, a diagnosis of absolute erythrocytosis is made when an individual's measured RCM is more than 25% above their mean predicted value,4 while an apparent erythrocytosis (AE) applies to those whose results fall within the reference range. The RCM result, therefore, provides a separation of individuals with a raised Hct value. In some centers RCM measurements are no longer performed, but then all patients must be investigated equally comprehensively as if they had an absolute erythrocytosis. In addition, the demonstration of an absolute erythrocytosis is the hallmark that separates PV from other MPD. In an unselected group of 322 males, it has been shown that the incidence of an absolute erythrocytosis rises as the Hct rises above normal. Thus, an absolute erythrocytosis is present in only 18% with a Hct of 0.50-0.52, rising to 65% at 0.56-0.58 and to 100% at 0.60 and above.2
The Absolute Erythrocytoses
A classification for the absolute erythrocytoses is shown in Table 1.5 In the primary type, the erythropoietic compartment is defective, whereas in the secondary form, intrinsically normal erythropoiesis is increased as a response to increased erythropoietin secretion. The only acquired primary type is the clonal MPD, PV. Some families with more than one affected individual have been described. The likely sequence of events in these individuals is an acquired mutation occurring additionally to a congenital predisposition to develop PV. The only congenital type of primary erythrocytosis is a mutation in the erythropoietin receptor gene, leading to truncation of the cytoplasmic portion of the erythropoietin receptor, which is necessary for switching off signal transduction following erythropoietin binding. Several different mutations have been described. These are generally familial, but occasional individuals with a spontaneous mutation are found. There are a larger number of causes of secondary congenital and acquired erythrocytoses (SE) (Table 2). Idiopathic erythrocytosis (IE) is used for a heterogenous group of patients who at initial investigation cannot be defined as either primary or SE.
The classification of the absolute erythrocytosis.
|
|
The commoner causes of secondary erythrocytosis.
|
| Abbreviations: 2,3-DPG, 2,3-diphosphoglycerate |
|
| Abbreviations: 2,3-DPG, 2,3-diphosphoglycerate |
Rather than using complicated algorithms, a standard approach makes certain that key investigations are done in all patients. The start point is knowledge of the causes of a SE (Table 2) and the diagnostic criteria of PV (Table 3).
Proposed modified criteria for the diagnosis of polycythemia vera (PV).
| A1 | Raised red cell mass (> 25% above mean normal predicted value, or PCV ≥ 0.60 in males or 0.56 in females) |
| A2 | Absence of cause of secondary erythrocytosis |
| A3 | Palpable splenomegaly |
| A4 | Clonality marker, i.e. acquired abnormal marrow karyotype |
| B1 | Thrombocytosis (platelet count > 400 × 109/L) |
| B2 | Neutrophil leucocytosis (neutrophil count > 10 × 109/L; > 12.5 × 109/L in smokers) |
| B3 | Splenomegaly demonstrated on isotope or ultrasound scanning |
| B4 | Characteristic BFU-E growth or reduced serum erythropoietin |
| A1 + A2 + A3 or A4 establishes PV | |
| A1 + A2 + two of B establishes PV | |
| A1 | Raised red cell mass (> 25% above mean normal predicted value, or PCV ≥ 0.60 in males or 0.56 in females) |
| A2 | Absence of cause of secondary erythrocytosis |
| A3 | Palpable splenomegaly |
| A4 | Clonality marker, i.e. acquired abnormal marrow karyotype |
| B1 | Thrombocytosis (platelet count > 400 × 109/L) |
| B2 | Neutrophil leucocytosis (neutrophil count > 10 × 109/L; > 12.5 × 109/L in smokers) |
| B3 | Splenomegaly demonstrated on isotope or ultrasound scanning |
| B4 | Characteristic BFU-E growth or reduced serum erythropoietin |
| A1 + A2 + A3 or A4 establishes PV | |
| A1 + A2 + two of B establishes PV | |
Laboratory and Clinical Investigations of Absolute Erythrocytoses
Two stages of tests are given in Table 4. Selected tests in stage 2 are undertaken following an evaluation of the results of stage 1 investigations, which should be performed in all patients. Comments on these investigations and others are listed below.
Investigation in patients with an absolute erythrocytosis.
| Stage 1 . | Stage 2 . |
|---|---|
| CBC | Bone marrow aspirate/trephine |
| Arterial oxygen saturation | Marrow karyotype |
| Ferritin | BFU-E culture |
| B12 | Oxygen dissociation curve (p50) |
| Folate | Sleep study |
| Creatinine | Lung function tests |
| Liver function tests | Chest X-ray |
| Uric acid | Echocardiogram |
| Abdominal ultrasound | Erythropoietin receptor mutation analysis |
| Serum erythropoietin |
| Stage 1 . | Stage 2 . |
|---|---|
| CBC | Bone marrow aspirate/trephine |
| Arterial oxygen saturation | Marrow karyotype |
| Ferritin | BFU-E culture |
| B12 | Oxygen dissociation curve (p50) |
| Folate | Sleep study |
| Creatinine | Lung function tests |
| Liver function tests | Chest X-ray |
| Uric acid | Echocardiogram |
| Abdominal ultrasound | Erythropoietin receptor mutation analysis |
| Serum erythropoietin |
CBC
The red cell indices should be examined for evidence of iron deficiency. A neutrophilia supports a diagnosis of PV, but smokers have higher neutrophil counts than non-smokers (Table 3). An eosinophilia and/or basophilia are occasionally found in PV, but these counts are relatively inaccurate and therefore not useful diagnostic markers. Platelets > 400 × 109/1 is a useful minor criterion for PV. Platelet anisocytosis reflected in an increased platelet distribution width (PDW) is also often seen in PV but not sufficiently standardized for diagnostic use. Other qualitative platelet changes are discussed below.
Arterial oxygen saturation and carbon monoxy-hemoglobin level
Pulse oximetry is the most convenient method of measuring the arteral oxygen saturaton (SaO2); however, in cigarette smokers the carbon monoxyhemoglobin (COHb) level must be determined directly and subtracted to give an accurate value for SaO2. An SaO2 below 92% has been taken to indicate a causal relationship with an absolute erythrocytosis.6 A value below 92% due solely to a raised COHb level is less likely to cause a significant erythrocytosis than a similar desaturation due to a fall in arterial pO2. In the latter situation the pO2 is already markedly reduced and any further small fall in pO2 will result in a marked reduction in SaO2 in view of the shape of the oxygen dissociation curve. The SaO2 is not static and an erythrocytosis can result from intermittent reductions in SaO2. Nocturnal arterial oxygen desaturation with normal daytime values were observed in 20% of patients who would otherwise have been classified as idiopathic erythrocytosis. An inquiry into symptoms suggesting the sleep-apnea syndrome, such as snoring, waking unrefreshed and daytime somnolence, should be made, particularly in the obese patient. COHb reduces oxygen carriage and delivery. Smokers typically have raised values of COHb and Hcts of ± 0.02 higher than non-smokers. Rarely is smoking per se a cause of an absolute erythrocytosis. Usually raised COHb levels are an additional erythropoietic stimulus in those with hypoxemic lung disease.
Serum ferritin, B12, and folate
Low ferritin values are more common in PV than SE. B12 levels may be elevated in PV due to transcobalamin release from an increased granulocyte mass. A raised B12 level and unsaturated B12-binding capacity (UB12 BC) were used in the original Polycythemia Vera Study Group (PVSG) PV criteria.5 However, B12 assays are generally designed to be most sensitive to identify low values and UB12 BC is not a routine investigation. Folate deficiency has been reported in PV.
Renal and liver function tests
Mild renal impairment has been associated with a modest absolute erythrocytosis.7 In older patients, the two findings are generally unrelated. Renal ultrasound (see below) is an essential investigation in all patients. Cirrhosis and excessive alcohol intake with impaired liver function rarely may cause an absolute erythrocytosis due to associated hypoxemia, diminished erythropoietin catabolism or an increased basal hepatic production.8
Abdominal ultrasound
This is an essential investigation in all patients. An abdominal CT scan is warranted in some patients. Simple renal cysts are common and found with increasing frequency with age but, unlike polycystic kidneys, are uncommonly a cause of erythrocytosis. Occasionally, massive cysts, like hydronephrosis, may cause sufficient renal ischemia to produce this complication. There is no value in measuring the erythropoietic activity of cyst fluid or blood obtained by selective renal artery cannulation. However, cytological examination of cyst fluid is indicated to rule out malignancy when the ultrasound appearance is suspicious.
Relevant liver pathology and splenic size can be established by ultrasound. Palpable splenomegaly in the absence of liver pathology is a reliable major criterion of PV (Table 3). Splenic enlargement occurs before it is clinically palpable. Scanning techniques have demonstrated that two-thirds of PV patients have splenomegaly at diagnosis. However, there is inter- and intra-observer measurement error in ultrasound splenic sizing and spleen size varies with age and size of the individual.9 Thus, splenic enlargement established in this way can only be taken as a minor criterion (Table 3).
Serum erythropoietin
In PV, erythropoietin (Epo) values are characteristically reduced and remain low in the majority following normalization of their Hb values.10 However, Epo values below the reference range are seen in AE and IE at presentation with raised Hb levels.11 This limits the specificity of low Epo values for PV in patients with raised Hb levels, but generally a low Epo level is a reliable minor criterion of PV. The erythroid burst forming unit (BFU-E) hypersensitivity to various growth factors, including Epo, and low serum Epo in PV are almost certainly related; therefore, they are linked minor criteria for PV (Table 3). In SE due to arterial hypoxemia, Epo values are usually raised although in other forms of SE rather variable results are obtained, and a normal value is not against SE. In recessive or dominant familial erythrocytosis due to autonomous high Epo production different patterns of Epo serum levels are seen. Some have high values with raised Hb values while others only show clearly elevated Epo levels following Hb reduction. Clearly different, as yet uncharacterized, genetic mechanisms are involved. An abnormal oxygen sensing pathway, involving hypoxia inducible factor-1, has been proposed.12
Bone marrow aspirate/trephine
These should be performed in all patients with an absolute erythrocytosis, except where a clear causal relationship has already been established. There are characteristic marrow appearances in PV. These include hypercellularity with trilineage hyperplasia, pleomorphic megakaryocytes with giant forms with increased ploidy and clustering. The reticulin is normal or modestly increased and classically iron stores are absent. These findings have led to the proposal that marrow histology should be used as a diagnostic criterion for PV.13 Unfortunately, there are some inter-observer discrepancies, and histopathological opinions are not uniformly satisfactory in this specialized area. Thus, for the time being it is more appropriate to place marrow histology in a confirmatory diagnostic position. Confirmatory marrow histology should always be established before embarking on cytoreductive therapy. Marrow histology taken with clinical and peripheral blood findings may enable the recognition of the presence or a predisposition to myelofibrotic/leukemic transformation.
Marrow karyotype
An acquired abnormal marrow karyotype confirms the presence of a clonal disorder and holds a key diagnostic position in PV (Table 3). At presentation, between 10 and 20% have karyotypic changes, typically 20q-, trisomy 8, trisomy 9 and 13q-. Recently, interphase fluorescent in situ hybridization (FISH) on granulocytes has been shown to be useful in recognizing PV karyotypic abnormalities.14 Occasionally abnormalities can be found that have not been found by conventional karyotypic analysis.15
X-chromosome-linked DNA probes to demonstrate clonality
This technique, which is obviously only useful in females, requires the demonstration that granulocytes show only one active parental X-chromosome compared to control T-lymphocytes. By using the human androgen receptor (HUMARA) gene probe, the parental genes can be identified in 90% of subjects tested. However, 20% normally show skewing in their X-chromosome inactivation patterns, making the technique unsuitable. In addition, apparent clonal granulocytes are found in 20-30% of normal women over 60 years old. Thus, it is not useful as a routine test in support of a diagnosis of PV. There are insufficient data in young women to attest to its role in the confirmation of clonal proliferation.
Neutrophil alkaline phosphatase score
A raised neutrophil alkaline phosphatase (NAP) score was taken as a diagnostic marker of PV by the PVSG.6 However, the technique is only semi-quantitative, liable to considerable inter-observer and inter-laboratory error, and there are no standardized controls. Since there are much more reliable markers of PV, there is no good reason to use the NAP score as traditionally performed in this context. Flow cytometric analysis have confirmed the elevated NAP values in PV, but at present this approach is not used.
Burst-forming unit erythroid and endogenous erythroid colonies
Clonogenic assays of purified progenitors from PV patients have shown that their BFU-E are more sensitive than normal to a range of different growth factors including stem cell factor, IL-3, Epo and insulin-like growth factors.16 The culture of the mononuclear non-adherent fraction of peripheral blood cells from patients with PV in serum-containing medium without Epo leads generally to the growth of so-called endogenous erythroid colonies (EEC). This phenomenon, which is usually not found in SE, has been proposed as a reliable diagnostic marker of PV. However, the BFU-E grown in these conditions are rather pale and therefore difficult to visualize, and there is considerable variation in the number of BFU-E grown in different PV patients. An alternative approach, which is more scientifically sound but more time-consuming, is to produce a BFU-E/Epo dose-response curve. However, a similar response as that seen in PV can be found in patients with truncation of the Epo receptor, who also have low serum Epo values, as in PV. To provide a more robust assay, some authors have used serum-free media, but the finding of EEC in these circumstances is not specific for PV.14 In these media it is possible to use the growth modifiers, IL-3, Epo and α-interferon (IFN-α) to produce specific conditions, which only give growth of BFU-E in PV,17 but the assay then loses its sensitivity.
Culture techniques are laborious, poorly standardized, expensive and not generally available. They may have some value in a research setting. The linked diagnostic criterion of low serum Epo value can be more readily and cheaply demonstrated than the finding of EEC. Thus clonogenic assays are now rarely used in the routine diagnosis of PV.
Qualitative platelet changes and serum thrombopoietin levels
Various platelet changes including reduced platelet aggregation, increased platelet aggregation and altered nucleotide levels have been described in PV. These changes are not always present and the techniques involved are either poorly standardized and/or not generally available making them unattractive as routine diagnostic markers.
Reduced levels of platelet Mpl has been demonstrated by immuno-reactive and ligand-binding techniques in PV compared with SE.18,19 In addition, it has been shown that PV platelets express an incompletely glycosylated form of Mpl.20 Thus, it is possible that the reduced PV platelet Mpl expression may prove to be of diagnostic value.
Plasma thrombopoietin (Tpo) levels in PV are either normal or increased and there is no correlation with the platelet count.18,19 The reason for the increased Tpo levels despite increased platelet counts probably reflect the reduced removal of Tpo by the reduced level of functional platelet Mpl. Thus Tpo levels are of no diagnostic value in the erythrocytoses.
Oxygen dissociation curve (ODC)
Measurement of the p50 is important in those with an unexplained erythrocytosis to identify those with a high oxygen affinity Hb and the exceedingly rare patients with congenitally low 2:3 DPG levels.
Truncation of the erythropoietin receptor
The erythropoietic compartment is intrinsically abnormal in patients with one of these mutations in the erythropoietin receptor (EpoR) gene. However, since it is a constitutional defect, erythropoiesis is polyclonal. These patients have low serum Epo values and similar BFU-E findings to PV patients. Hence, the importance of not separating these criteria for PV. It is an exceedingly rare condition, but appropriate genetic screening should be undertaken in patients with an unexplained erythrocytosis and low serum Epo values.
Nature of Idiopathic Erythrocytosis
This heterogenous group of patients emerge from those patients with an absolute erythrocytosis but without a clear cause of primary or secondary erythrocytosis. Various mechanisms are involved.21 By definition, 1% of normal males and 0.5% of normal females will have a measured RCM above the reference range.4 In 5-10% of patients definite features of PV emerge over a few years. In a few patients a cause of SE either is not recognized initially, notably nocturnal arterial hypoxemia, or becomes apparent during follow-up. It is most important that an incorrect cause for an erythrocytosis is not ascribed to a patient. Where doubt remains, it is best to put the patient in the IE group and follow the patient's course. Further or repeat investigations can then be performed. Generally, follow-up of IE patients should be more protracted than those with apparent erythrocytosis, illustrating the importance of the RCM study.
Nature and Mechanisms Involved in Apparent Erythrocytosis
There are many alternative titles used for the AE group including relative, stress, spurious and pseudo-polycythemia and Geisbock's syndrome. About one-third have a reduced plasma volume (PV) below the normal range, but the majority have an increase in their RCM and reduction in PV within their normal ranges. The possible underlying mechanisms in these patients are listed in Table 5 and have been reviewed in detail.2 Many of the factors listed increase the RCM and reduce the PV independently and hence cause the rise in Hct. Notably, arterial hypoxemia is present in 15-25% of these patients.22
Possible underlying mechanisms and associated findings in patients with apparent erythrocytosis.
| Physiological variant |
| Hypertension |
| Renal disease |
| Fluid loss and diuretics |
| Alcohol |
| Obesity |
| Smoking |
| Arterial oxygen desaturation |
| Pheochromocytoma |
| Early stage of development of absolute erythrocytosis |
| Physiological variant |
| Hypertension |
| Renal disease |
| Fluid loss and diuretics |
| Alcohol |
| Obesity |
| Smoking |
| Arterial oxygen desaturation |
| Pheochromocytoma |
| Early stage of development of absolute erythrocytosis |
As far as investigation is concerned, it is particularly important to exclude renal disease and arterial hypoxemia. All investigations in Stage 1 of Table 4 should be performed. A sleep study should be performed if there are symptoms of nocturnal arterial oxygen desaturation. Iron B12 or folate deficiency may limit erythropoietic expansion. Previously, it had been found that Epo levels were normal in AE23 but a recent study has shown that some of these patients have serum Epo values below the normal reference range.11 AE comprise the majority of patients with modest elevations of Hct. Over a few months, in one-third of patients the Hct falls into the normal range, possibly through treatment of hypertension or reduction in smoking, alcohol or obesity. In a further third, the Hct is variably in the normal range or just slightly elevated. This leaves a third with permanently raised Hct22 and it is only these patients that warrant extended follow-up while recognizing that AE is not a diagnosis per se and a clear mechanism might evolve.
II. The Cellular and Genetic Pathology of Polycythemia Vera
Anthony J. Bench, Ph.D., Anthony R. Green, M.D, Ph.D., FRCP, FRCPath,* Brian J.P. Huntly, M.D, and Elizabeth P. Nacheva, M.D.
Department of Haematology, University of Cambridge, Cambridge Institute for Medical Research, Hills Road, Cambridge CB2 2XY, UK
PV is thought to result from clonal expansion of a transformed multipotent stem cell. Progenitors from patients with PV display abnormal responses to several growth factors, suggesting the presence of a defect in a signalling pathway common to different growth factors. A number of approaches are now focused on defining the molecular lesion or lesions.
Clonality
X-chromosome inactivation studies have played a pivotal role in establishing current concepts of the pathogenesis of many hematological malignancies. These assays provide a way of assessing clonality without any requirement for tumor-specific genetic or cytogenetic markers. X-inactivation occurs in females as a method of dosage compensation. During female embryogenesis one X-chromosome is inactivated at random in different cells (lyonization). This decision is then inherited by the progeny of each cell. As a result, an adult female is mosaic with respect to her X-inactivation pattern. Assessment of X-chromosome inactivation requires an ability to distinguish both X-chromosomes, and so the various assays are all based on polymorphic X-linked genes. It is also necessary to determine which X-chromosome is active. This can be achieved by monitoring expression of an appropriate X-linked gene at the RNA or protein level. Alternatively, DNA methylation can be used as a surrogate marker of gene inactivity, since for many genes methylation status correlates well with transcriptional activity.
The original studies of clonality in PV used a rare polymorphism in the glucose-6-phosphate dehydrogenase (G6PD) gene that gives rise to identifiably distinct protein products. Red blood cells, platelets, granulocytes and bone marrow buffy coat showed predominant expression of a single allele, whereas both alleles were expressed in skin or marrow fibroblasts.1 These data were felt to demonstrate that PV results from transformation and subsequent clonal expansion of a multipotent hemopoietic stem cell.
Early studies used fibroblasts or other non-hemopoietic tissue as a control to show that a skewed X-inactivation ratio was not merely a reflection of extreme lyonization. However, different tissues from the same individual show dissimilar X-inactivation patterns. T cells give the best concordance with granulocytes and have therefore been proposed as the most appropriate control.2 The finding of skewed X-inactivation in granulocytes in the presence of a balanced pattern in T cells is therefore felt to indicate a clonal disorder. The lack of T cell involvement presumably reflects the longevity of T cells or the origins of PV in a stem cell incapable of giving rise to T cells. The main drawback to this approach arises when both granulocytes and T cells are skewed, since it is then not possible to distinguish extreme lyonization from a clonal disorder capable of giving rise to both granulocytes and T cells.
Approximately 90% of patients with PV show a skewed pattern of X-inactivation in their white blood cell or granulocyte fraction (Table 6).3 Platelets, red cells and erythroid progenitors have all been shown to form part of the malignant clone. However, 108 of 117 EBV-transformed B cell lines derived from a single PV patient showed activity of the same X-chromosome, implying that some B cells were derived from the malignant clone. Interestingly, polyclonal granulocytes have been found in a small proportion of patients with well-characterized PV, while polyclonal granulocytes with partial skewing of the erythroid precursor population have also been described. These data suggest that there may be some heterogeneity of lineage involvement in PV.
Summary of X-inactivation studies in polycythemia vera.
| Gene(s) . | Method . | F . | L . | T . | WBC . | G . | PI . | Ret . | RBC . | NK . |
|---|---|---|---|---|---|---|---|---|---|---|
| G6PD | Protein | 0/2 | 0/2 | 2/2 | 2/2 | 2/2 | ||||
| PGK, HPRT | DNA | 5/6 | ||||||||
| PGK | DNA | 1/1 | ||||||||
| PGK | DNA | 0/3 | 16/17 | 3/3 | ||||||
| PGK | DNA | 0/1 | 2/3 | |||||||
| PGK, HPRT | DNA | 1/8 | 8/8 | |||||||
| AR | DNA | 4/25 | 21/25 | |||||||
| AR | DNA | 2/10 | 0/3 | 7/11 | ||||||
| AR | DNA | 14/22 | ||||||||
| P55, G6PD | RNA | 0/3 | 3/3 | 3/3 | 3/3 | 0/3 | ||||
| (Adapted from Hinschelwood S et al, 1997)3 | ||||||||||
| Key: For each cell type, numbers represent the number of patients with a skewed X-inactivation ratio over the total number of patients studied. Abbreviations: F, skin or marrow fibroblasts; L, lymphocytes; T, T lymphocytes; WBC, white blood cells; G, granulocytes; PI, platelets; Ret, reticulocytes; RBC, red blood cells; NK, natural killer cells; G6PD, glucose-6-phosphate dehydrogenase; PGK, phosphoglycerate kinase; HPRT, hypoxanthine phosphoryl ribosyl transferase; AR, androgen receptor | ||||||||||
| Gene(s) . | Method . | F . | L . | T . | WBC . | G . | PI . | Ret . | RBC . | NK . |
|---|---|---|---|---|---|---|---|---|---|---|
| G6PD | Protein | 0/2 | 0/2 | 2/2 | 2/2 | 2/2 | ||||
| PGK, HPRT | DNA | 5/6 | ||||||||
| PGK | DNA | 1/1 | ||||||||
| PGK | DNA | 0/3 | 16/17 | 3/3 | ||||||
| PGK | DNA | 0/1 | 2/3 | |||||||
| PGK, HPRT | DNA | 1/8 | 8/8 | |||||||
| AR | DNA | 4/25 | 21/25 | |||||||
| AR | DNA | 2/10 | 0/3 | 7/11 | ||||||
| AR | DNA | 14/22 | ||||||||
| P55, G6PD | RNA | 0/3 | 3/3 | 3/3 | 3/3 | 0/3 | ||||
| (Adapted from Hinschelwood S et al, 1997)3 | ||||||||||
| Key: For each cell type, numbers represent the number of patients with a skewed X-inactivation ratio over the total number of patients studied. Abbreviations: F, skin or marrow fibroblasts; L, lymphocytes; T, T lymphocytes; WBC, white blood cells; G, granulocytes; PI, platelets; Ret, reticulocytes; RBC, red blood cells; NK, natural killer cells; G6PD, glucose-6-phosphate dehydrogenase; PGK, phosphoglycerate kinase; HPRT, hypoxanthine phosphoryl ribosyl transferase; AR, androgen receptor | ||||||||||
The demonstration of clonal granulocytes and polyclonal T cells in most patients with PV has led to the suggestion that X-inactivation patterns could be used diagnostically. However, most MPD patients are elderly and studies of age-matched normal women using the HUMARA assay found that 25-50% of them displayed a clonal pattern in granulocytes with polyclonal T cells.4,5 A number of mechanisms could account for this finding. First, an acquired mutation could lead to clonal expansion as part of a neoplastic process. However, it is unlikely that so many otherwise normal elderly women would have a clonal hematological malignancy. Second, stem cell depletion may occur with increased age. Transplantation studies have demonstrated that a small number of stem cells can lead to clonal dominance by what appears to be a stochastic process. However, old mice have an increased number of stem cells arguing against this mechanism. A third possible explanation would be selection for allelic X-linked differences. Polymorphisms of X-linked loci may result in a subtle selective advantage for stem cells whose active X-chromosome is of one parental type. This concept is supported by studies of human twins and by the pattern of skewing observed in elderly cats. These data therefore imply the existence of one or more genes that regulate stem cell kinetics.
Whatever the mechanism underlying these results, they have important clinical and practical implications. First, determination of clonality status using the HUMARA assay is not a useful diagnostic tool in elderly women. Second, the data raise questions about the current dogma that clonal hemopoiesis in patients with PV necessarily reflects transformation of a multipotent stem cell.
Hematopoietic Progenitors and Signal Transduction
In 1974 the key observation was made that cultures of PV bone marrow cells yielded in vitro erythroid colonies even when no exogenous Epo was added to the culture media.6 These have been termed endogenous erythroid colonies or Epo-independent BFU-E. It was subsequently shown that these Epo-independent BFU-E from a given patient all expressed the same G6PD allele, and that this was the same allele that was expressed in granulocytes and platelets. By contrast, colonies grown in the presence of added Epo were mixed, with some colonies expressing one parental G6PD allele and the remainder expressing the other. In two patients the proportion of colonies expressing the dominant allele increased over 3 years. Several studies have shown that Epo-independent BFU-E provide a useful diagnostic tool and are found in the vast majority of PV patients.3,7 The mechanisms responsible for Epo-independent growth of BFU-E remain obscure. Growth of BFU-E in the absence of added Epo may reflect complete independence of this cytokine or hypersensitivity to trace amounts of Epo present in the culture reagents. In serum-free systems Epo-independent colonies are observed in normal individuals, raising the possibility that PV BFU-E have a reduced sensitivity to an inhibitory factor present in serum. In spite of this biological complexity two main conclusions have come from recent studies of PV progenitors. Firstly, erythroid progenitors have been shown to be hypersensitive to several different growth factors including stem cell factor (SCF), IL-3, GM-CSF and insulin-like growth factor-1 (IGF-1).8,9 Secondly, myeloid and megakaryocytic progenitors (CFU-GM and CFU-Meg) also show abnormal patterns of growth.10,11
These findings are consistent with a model in which the acquired genetic lesion in PV is not restricted to the Epo signalling pathway and affects progenitors committed to multiple lineages. However, a degree of caution is warranted when considering this model—abnormal patterns of colony growth may result from reactive changes in response to extrinsic factors as well as from intrinsic progenitor defects.
A number of signal transduction molecules have been studied as potential PV target genes. Initial attention focused on the EpoR. Experimentally introduced mutations in the EpoR result in hypersensitivity to, or independence from, exogenous Epo. Truncation of the cytoplasmic domain of the EpoR has been found in primary familial polycythemia9 and point mutations have been seen in several other cases. Several groups have therefore looked at the integrity of the EpoR gene in various cells from PV patients but none have detected mutations.12,13 Decreased expression of a truncated EpoR encoded by an alternate transcript has been implicated in PV, but similar alternate splicing events in solid tumours have not been shown to play a causal role in tumor pathogenesis. In addition, a number of other cytokine receptors have been studied. Examination of tyrosine phosphorylation of the IGF-1 receptor subunit in PV peripheral blood mononuclear cells showed a higher basal level of phosphorylation of this receptor and enhanced downstream signalling as judged by increased tyrosine phosphorylation following stimulation with IGF-1.14 The significance of this observation is unclear, particularly since T lymphocytes make up the majority of peripheral blood mononuclear cells and are not part of the PV clone. Reduced expression of the thrombopoietin receptor on PV platelets has been reported,15 but this is not specific for PV and its pathogenetic significance is unclear.
The reported hypersensitivity of PV progenitors to multiple cytokines suggested a defect in a downstream signalling pathway common to multiple different receptors. The tyrosine phosphatase SHP-1 (or HCP) was considered a prime candidate as it inhibits signalling from the EpoR and other cytokine receptors, and hemopoietic progenitors with a null mutation in SHP-1 are hypersensitive to cytokines. However, SHP-1 protein expression was normal in granulocytes from PV patients and complete nucleotide sequencing of the SHP-1 gene showed no mutation.16 These results do not exclude a role for other inhibitory phosphatases, and a novel protein with increased phosphatase activity has been reported in PV progenitors.17
Reduced apoptosis may play a part in PV since increased expression of Bcl-xL has been reported in erythroid cells.18 However, as with other reports of differentially expressed genes in normal and PV cells,19 the pathogenetic significance remains unclear. Distinguishing primary changes from secondary effects is a major challenge and requires a rigorous search for mutations in target genes.
Cytogenetics
A number of recurrent chromosomal abnormalities have been documented in PV but unlike chronic myeloid leukaemia there is no pathognomonic abnormality. The most common cytogenetic change is deletion of the long arm of chromosome 20 (Table 7). Trisomies of chromosome 8 and 9 are also common features and may be observed in the same clone. The incidence of chromosomal abnormalities is greater in patients receiving myelosuppressive therapy (45%) than in untreated patients (17%).20 This may reflect leukemogenic effects of treatment or indicate those with progressive disease (and therefore requiring treatment) are more likely to develop cytogenetic changes. An abnormal karyotype is also found more frequently in samples taken late in the course of the disease or following myelofibrotic or leukemic transformation.(20) Approximately 20% of patients have cytogenetic abnormalities at diagnosis, increasing to over 80% for those with more than 10 years follow-up. Although based on a small number of patients, the median survival in those with a chromosomal abnormality at diagnosis was significantly less than for those with a normal karyotype, suggesting that an abnormal karyotype is a poor prognostic indicator.20 Some changes such as deletions or monosomies of chromosomes 5 and 7 are almost invariably seen after myelosuppressive therapy and frequently as part of a complex karyotype. It is therefore unlikely that these chromosomes contain genes involved in the etiology of the MPDs. By contrast, deletions of part of the long arm of chromosomes 20 and 13, trisomies of chromosomes 8 and 9 and duplication of part of the long arm of chromosome 1 have all been seen in untreated patients, notably often as sole abnormalities. They are likely, therefore, to mark the site of genes that play an early role in the pathogenesis of PV.
Number and percentage of karyotypic abnormalities identified by G-banding in the myeloproliferative disorders.
| Abnormality . | Polycythemia Vera . | Idiopathic Myelofibrosis . | Essential Thrombocythemia . |
|---|---|---|---|
| Deletion of 20q | 45 (8.4%) | 28 (7.1%) | 1 (0.2%) |
| Deletion of 13q | 16 (3.0%) | 25 (6.3%) | 3 (0.7%) |
| Trisomy 8 | 37 (6.9%) | 20 (5.0%) | 4 (0.9%) |
| Trisomy 9 | 35 (6.6%) | 4 (1.0%) | 1 (0.2%) |
| Trisomy of 1q | 19 (3.6%) | 14 (3.5%) | 0 |
| Deletion of 7q or monosomy 7 | 5 (0.9%) | 15 (3.8%) | 0 |
| Deletion of 5q or monosomy 5 | 17 (3.2%) | 6 (1.5%) | 0 |
| Total number of patients with one or more abnormality | 180 (33.7%) | 157 (39.5%) | 23 (5.0%) |
| Total patients | 534 | 397 | 456 |
| Data taken from papers reviewed by Bench and colleagues.20 | |||
| Abnormality . | Polycythemia Vera . | Idiopathic Myelofibrosis . | Essential Thrombocythemia . |
|---|---|---|---|
| Deletion of 20q | 45 (8.4%) | 28 (7.1%) | 1 (0.2%) |
| Deletion of 13q | 16 (3.0%) | 25 (6.3%) | 3 (0.7%) |
| Trisomy 8 | 37 (6.9%) | 20 (5.0%) | 4 (0.9%) |
| Trisomy 9 | 35 (6.6%) | 4 (1.0%) | 1 (0.2%) |
| Trisomy of 1q | 19 (3.6%) | 14 (3.5%) | 0 |
| Deletion of 7q or monosomy 7 | 5 (0.9%) | 15 (3.8%) | 0 |
| Deletion of 5q or monosomy 5 | 17 (3.2%) | 6 (1.5%) | 0 |
| Total number of patients with one or more abnormality | 180 (33.7%) | 157 (39.5%) | 23 (5.0%) |
| Total patients | 534 | 397 | 456 |
| Data taken from papers reviewed by Bench and colleagues.20 | |||
Deletions of 20q
Del(20q) was the commonest sole structural chromosomal abnormality after t(9;22) in a series of almost 3,000 consecutive marrow karyotype analyses at the Mayo Clinic. In addition to PV, 20q deletions are also seen in approximately 4% of patients with myelodysplastic syndromes (MDS) and in 1-2 % with acute myeloid leukemia (AML), but are rarely seen in lymphoid malignancies. This pattern of disease association suggests that the deleted region of chromosome 20 marks the site of one or more genes, loss or inactivation of which perturbs the regulation of hemopoietic progenitors.(21) The finding of 20q deletions at diagnosis and as a sole abnormality suggests that, in at least some cases, it plays an early role in disease pathogenesis, although from studies of a small number of patients its presence does not appear to influence survival. Generally, the deletion can be detected in peripheral blood neutrophils, but in a subset of patients the 20q deletion is present in the majority of bone marrow metaphases but not detectable in peripheral blood granulocytes. This finding suggests that in some patients granulocytes carrying the deletion may be preferentially destroyed or retained within the bone marrow.
In any given patient characterization of the hematopoietic lineages carrying the 20q deletion provides important insights into the differentiation potential of the cell in which the deletion first arose. A patient with MDS has been described whose granulocytes and monocytes were clonal and contained the deletion, whereas their B cells and T cells were polyclonal and did not. However, EBV-transformed B-cell lines carrying the 20q deletion were derived from this patient. Similarly in another patient, a 20q deletion was reported in EBV-transformed B-cell lines as well as in CFU-GM, CFU-GEMM and BFU-E. Clearly, the 20q deletion can arise in a very early progenitor with both lymphoid and myeloid potential.
Deletions of 20q may identify a subset of myeloid disorders characterized by megakaryocytic and erythroid dysplasia. Analysis of bone marrow samples from 78 patients who possessed a 20q deletion as their only chromosomal abnormality showed that megakaryocytic abnormalities were seen in all MPD patients and in 46 of 47 MDS patients. In addition, 45% of the MPD patients and 87% of the MDS patients showed dyserythropoiesis. In contrast, none of the MPD patients and only 19% of the MDS patients showed granulocytic dysplasia. However, no comparison was made with any control group of patients lacking a 20q deletion. In addition, dyserythropoiesis has been observed in 8/8 MDS patients with a 20q deletion, but dysmegakaryopoiesis was only seen in one of them.
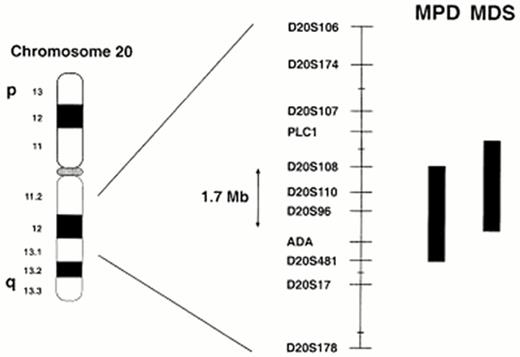
Molecular analysis of the 20q deletion has been undertaken to identify the gene or genes involved. FISH, microsatellite PCR (Figure 1) and Southern blotting have demonstrated heterogeneity of both the centromeric and telomeric breakpoints22,23 implying that the common deleted region (CDR) contains one or more tumor suppressor genes.
Physical maps of this region of chromosome 20, in the form of yeast artificial chromosome (YAC) contigs, have been constructed to facilitate gene identification. Given that MPD and MDS are overlapping but clinically different diseases, it remains possible that different or additional genes are involved in the two disorders. During subsequent analysis of further deletions two overlaping CDRs have therefore been defined.24,25 The most recent MDS AML CDR spans a distance of 2.6 megabase pairs (Mb), whereas the MPD CDR encompasses 2.7 Mb with an overlapping combined CDR of 1.7 Mb26 (Figure 1). Thirty-seven genes have been identified with the MPD CDR, 20 within the MDS CDR and 16 within the combined CDR. Of those within the combined CDR, five were expressed in both normal bone marrow and purified CD34-positive cells, and therefore represent positional and expression candidates for the target genes on 20q.
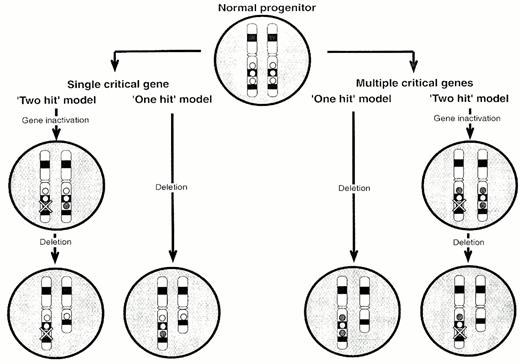
There are several potential mechanisms (Figure 2)21 to explain how loss/inactivation of a candidate gene on chromosome 20q might be responsible for the pathogenesis of PV.28 A `two-hit' model with a single target gene would involve inactivation of one copy of the gene by a subtle genetic alteration (e.g. a point mutation) followed by loss of the second copy by deletion. Alternatively, the `intact' copy may be transcriptionally silenced, for example by methylation as has been demonstrated for the VHL, p16 and p15 genes in other tumors and leukemias. However, loss of only a single gene copy may have a pathogenetic role in itself. There are an increasing number of examples in which haploinsufficiency influences development or tumorigenesis. However, loss of two or more genes may be required to produce the disease phenotype. Again, inactivation of one or both copies of critical genes may be necessary to perturb progenitor cell behavior. If there are multiple critical genes, the spectrum of genes lost in association with each deletion may determine the clinical syndrome.
Potential mechanism of the pathogenesis of 20q deletions.
Circles represent genes on 20q. If there is a single target gene on 20q (hatched circle), it may be necessary for both copies to be lost/inactivated (`two-hit' model). Alternatively, loss of a single copy (haplo-insufficiency) may be adequate to produce a phenotypic effect (`one-hit' model). If there is more than one target gene, the `one-hit' model would entail haplo-insufficiency for two or more genes, perhaps scattered over a large distance of the chromosome. In contrast, the `two-hit' model would involve bi-allelic inactivation of at least one of the target genes.
Potential mechanism of the pathogenesis of 20q deletions.
Circles represent genes on 20q. If there is a single target gene on 20q (hatched circle), it may be necessary for both copies to be lost/inactivated (`two-hit' model). Alternatively, loss of a single copy (haplo-insufficiency) may be adequate to produce a phenotypic effect (`one-hit' model). If there is more than one target gene, the `one-hit' model would entail haplo-insufficiency for two or more genes, perhaps scattered over a large distance of the chromosome. In contrast, the `two-hit' model would involve bi-allelic inactivation of at least one of the target genes.
III. The Basis of Current Management Strategies and Future Perspectives in Polycythemia Vera
Tiziano Barbui, M.D.,* and Guido Finazzi, M.D.
Department of Hematology, Ospedale Riuniti di Bergamo, Bergamo 24100, Italy
The clinical course in PV is marked by significant risk of thrombotic complications and a variable incidence of transformation into myeloid metaplasia with myelofibrosis (MMM) or acute myeloid leukemia (AML). Early studies in untreated patients found a high thrombotic incidence and a median survival of 18 months. Cytoreductive treatment by phlebotomy or chemotherapy has dramatically reduced the number of thrombotic complications and substantially improved survival. However, there is concern that certain myelosuppressive drugs accelerate the disease progression to AML. At present, there is no known treatment that eradicates the abnormal clone, apart from anecdotal cases of bone marrow transplantation. Thus, the objective of management is twofold: first, to minimize the risk of thrombotic complications; second, to prevent progression to myelofibrotic or leukemic transformation. This article provides updated estimates of thrombotic risk and disease progression and evaluates the various randomized and phase II studies in PV.
Natural History of Treated Disease
The natural history of 1,213 PV patients treated according to current practice at the time and followed for 20 years has been retrospectively analyzed in Italy.1 The cumulative median survival exceeded 15 years, with overall mortality of 2.94 deaths/100 patients per year. The age-and sex-standardized mortality rate was 1.7 times that of the general Italian population. This finding disagrees with a previous study suggesting that expected survival did not differ from a control sample.2 Thrombotic complications, particularly myocardial infarction, ischemic stroke and venous thromboembolism, were the most frequent causes of death (Table 8). The overall rate of fatal and non-fatal thrombotic events was 3.4/100 patients per year (Table 9). Age and thrombotic history were the most important clinical factors for vascular events. Age-adjusted estimates for the risk of thrombosis were as follows: 1.8; 2.8; 4.0; 5.1/100 patients years for patients younger than 40; 40-59; 60-69; 70 years and older, respectively (p < 0.001). The thrombosis-free survival in patients with and without a history of thrombosis was 24.6% and 17.3% respectively (p = 0.001). Major hemorrhage, usually gastrointestinal, was uncommon and the cause of death in less than 3% of patients.
Causes of death in 192 patients with polycythemia vera.1
| . | Patients . | ||
|---|---|---|---|
| Cause of Death . | no. (%) . | no. (%) . | no. (%) . |
| Cardiovascular | 70 (36.4) | ||
| Arterial thrombosis | 46 (24.0) | ||
| Myocardial infarction | 14 (7.3) | ||
| Sudden death | 15 (7.8) | ||
| Ischemic stroke | 17 (8.8) | ||
| Venous thromboembolism | 10 (5.2) | ||
| Cerebral sinus thrombosis | 2 (1.0) | ||
| Pulmonary embolism | 6 (3.1) | ||
| Splanchnic thrombosis | 2 (1.0) | ||
| Other cardiovascular | 14 (7.3) | ||
| Hemorrhagic | 6 (3.1) | ||
| Gastrointestinal bleeding | 5 (2.6) | ||
| Cerebral hemorrhage | 1 (0.5) | ||
| Cancer | 57 (30.0) | ||
| Acute myeloid leukemia | 28 (14.6) | ||
| Breast cancer | 5 (2.6) | ||
| Colorectal cancer | 6 (3.1) | ||
| Lung cancer | 4 (2.1) | ||
| Other cancers | 14 (7.3) | ||
| Complications of polycythemia | 5 (2.6) | ||
| Myelodysplasia | 1 (0.5) | ||
| Spent phase | 4 (2.1) | ||
| Other causes | 54 (28.1) | ||
| Respiratory failure | 14 (7.3) | ||
| Hepatic failure | 4 (2.1) | ||
| Other | 36 (18.9) | ||
| Total | 192 (100) | ||
| . | Patients . | ||
|---|---|---|---|
| Cause of Death . | no. (%) . | no. (%) . | no. (%) . |
| Cardiovascular | 70 (36.4) | ||
| Arterial thrombosis | 46 (24.0) | ||
| Myocardial infarction | 14 (7.3) | ||
| Sudden death | 15 (7.8) | ||
| Ischemic stroke | 17 (8.8) | ||
| Venous thromboembolism | 10 (5.2) | ||
| Cerebral sinus thrombosis | 2 (1.0) | ||
| Pulmonary embolism | 6 (3.1) | ||
| Splanchnic thrombosis | 2 (1.0) | ||
| Other cardiovascular | 14 (7.3) | ||
| Hemorrhagic | 6 (3.1) | ||
| Gastrointestinal bleeding | 5 (2.6) | ||
| Cerebral hemorrhage | 1 (0.5) | ||
| Cancer | 57 (30.0) | ||
| Acute myeloid leukemia | 28 (14.6) | ||
| Breast cancer | 5 (2.6) | ||
| Colorectal cancer | 6 (3.1) | ||
| Lung cancer | 4 (2.1) | ||
| Other cancers | 14 (7.3) | ||
| Complications of polycythemia | 5 (2.6) | ||
| Myelodysplasia | 1 (0.5) | ||
| Spent phase | 4 (2.1) | ||
| Other causes | 54 (28.1) | ||
| Respiratory failure | 14 (7.3) | ||
| Hepatic failure | 4 (2.1) | ||
| Other | 36 (18.9) | ||
| Total | 192 (100) | ||
Fatal and non-fatal thrombotic events (n=254) during follow-up in 1,213 patients with polycythemia vera.1
| . | Non-Fatal Event . | Fatal Event . | ||
|---|---|---|---|---|
| Type of Complication . | no. (%) . | no. (%) . | no. (%) . | no. (%) . |
| Arterial thrombosis | 101 (50.5) | 44 (81.5) | ||
| Myocardial infarction | 28 (14.0) | 27 (50.0) | ||
| Ischemic stroke | 19 (9.5) | 17 (31.5) | ||
| Transient ischemic attack | 39 (19.5) | - | ||
| Peripheral arterial thrombosis | 15 (7.5) | - | ||
| Venous thromboembolism | 77 (38.5) | 10 (18.5) | ||
| Deep venous thrombosis | 35 (17.5) | - | ||
| Superficial thrombophlebitis | 37 (18.5) | - | ||
| Unknown | 5 (2.5) | - | ||
| Unknown | 22 (11.0) | |||
| Total | 200 (100) | 54 (100) | ||
| . | Non-Fatal Event . | Fatal Event . | ||
|---|---|---|---|---|
| Type of Complication . | no. (%) . | no. (%) . | no. (%) . | no. (%) . |
| Arterial thrombosis | 101 (50.5) | 44 (81.5) | ||
| Myocardial infarction | 28 (14.0) | 27 (50.0) | ||
| Ischemic stroke | 19 (9.5) | 17 (31.5) | ||
| Transient ischemic attack | 39 (19.5) | - | ||
| Peripheral arterial thrombosis | 15 (7.5) | - | ||
| Venous thromboembolism | 77 (38.5) | 10 (18.5) | ||
| Deep venous thrombosis | 35 (17.5) | - | ||
| Superficial thrombophlebitis | 37 (18.5) | - | ||
| Unknown | 5 (2.5) | - | ||
| Unknown | 22 (11.0) | |||
| Total | 200 (100) | 54 (100) | ||
The natural history of PV involves progression to MMM, also called the “spent phase” of the disease. Approximately 10-15% of patients will develop postpolycythemic MMM, after an average of ten years from diagnosis. This incidence rises to over 30% in patients surviving to the 20th year.3 The features of the spent phase include increasing splenomegaly, a leukoerythroblastic peripheral blood picture and extensive bone marrow fibrosis. Current therapy does not modify the course of this phase and most patients die in less than three years, frequently due to AML. In the Gruppo Italiano Studio Policitemia (GISP) study,1 myelofibrosis/myelodysplasia and AML accounted for 2.6% and 14.6% of total causes of death, respectively (Table 8).
Management Strategies
Therapeutic recommendations in PV are based on a limited number of randomized clinical trials (summarized in Table 10) and prospective and retrospective studies that described the natural history of PV patients and indirectly evaluated the role of different treatments. Thus, a sound scientific basis for therapeutic decisions is still lacking, and recommendations should be based on a careful assessement of the advantages and disadvantages of each therapeutic option.
Randomized clinical trials in polycythemia vera.
| Study . | Patents and Follow-Up . | Treatments and Main Results . | P . | |||
|---|---|---|---|---|---|---|
| PVSG-014 | 431 pts; 18 yrs (max) | 32P | Phlebotomy | Chlorambucil | ||
| Median survival | 11.8 yrs | 13.9 yrs | 8.9 yrs | 0.02 | ||
| Thrombosis | 30% | 34% | 25% | 0.08 | ||
| Acute Leukemia | 10% | 1.5% | 13% | < 0.0012 | ||
| EORTC11 | 293 pts.; 8 yrs (median) | 32P | Busulfan | |||
| 10-year survival | 55% | 70% | 0.02 | |||
| Vascular deaths | 18% | 5% | n.r. | |||
| Najean et al.12 | 461 pts; 16 yrs (max) | 32P | 32P+Hydroxyurea | |||
| Age > 65 yrs | Median survival | 10.9 yrs | 9.3 yrs. | n.s. | ||
| Najean et al.14 | 292 pts; 16 yrs (max) | Hydroxyurea | Pipobroman | |||
| Age <65 yrs | 14-year survival | 70% | 70% | n.s. | ||
| Myelofibrosis | 17% | 2.1% | 0.03 | |||
| PVSG-057 | 166 pts; 1.2 yrs (median) | ASA (900 mg/d)* | 32P | |||
| Thrombosis | 8% | 2% | n.r. | |||
| Hemorrhage | 7% | 0% | 0.02 | |||
| GISP21 | 112 pts; 1.4 yrs (median) | ASA (40 mg/d) | Placebo | |||
| Thrombosis | 5% | 7.7% | n.s. | |||
| Hemorrhage | 1.7% | 1.9% | n.s. | |||
| Abbrevations: n.r, not reported; n.s., not significant; max, maximum follow-up; pts, patients | ||||||
| Study . | Patents and Follow-Up . | Treatments and Main Results . | P . | |||
|---|---|---|---|---|---|---|
| PVSG-014 | 431 pts; 18 yrs (max) | 32P | Phlebotomy | Chlorambucil | ||
| Median survival | 11.8 yrs | 13.9 yrs | 8.9 yrs | 0.02 | ||
| Thrombosis | 30% | 34% | 25% | 0.08 | ||
| Acute Leukemia | 10% | 1.5% | 13% | < 0.0012 | ||
| EORTC11 | 293 pts.; 8 yrs (median) | 32P | Busulfan | |||
| 10-year survival | 55% | 70% | 0.02 | |||
| Vascular deaths | 18% | 5% | n.r. | |||
| Najean et al.12 | 461 pts; 16 yrs (max) | 32P | 32P+Hydroxyurea | |||
| Age > 65 yrs | Median survival | 10.9 yrs | 9.3 yrs. | n.s. | ||
| Najean et al.14 | 292 pts; 16 yrs (max) | Hydroxyurea | Pipobroman | |||
| Age <65 yrs | 14-year survival | 70% | 70% | n.s. | ||
| Myelofibrosis | 17% | 2.1% | 0.03 | |||
| PVSG-057 | 166 pts; 1.2 yrs (median) | ASA (900 mg/d)* | 32P | |||
| Thrombosis | 8% | 2% | n.r. | |||
| Hemorrhage | 7% | 0% | 0.02 | |||
| GISP21 | 112 pts; 1.4 yrs (median) | ASA (40 mg/d) | Placebo | |||
| Thrombosis | 5% | 7.7% | n.s. | |||
| Hemorrhage | 1.7% | 1.9% | n.s. | |||
| Abbrevations: n.r, not reported; n.s., not significant; max, maximum follow-up; pts, patients | ||||||
plus phlebotomy and dypiridamole
Phlebotomy
The only randomized study comparing phlebotomy with myelosuppressive therapy was done by the PVSG more than twenty years ago (PVSG-01 trial).4 Four hundred thirty-one patients were randomized to phlebotomy alone; radiophosphorus (32P) plus phlebotomy; or chlorambucil plus phlebotomy. The results of this trial and subsequent analysis of the long-term outcome of the patients originally enrolled5 provided useful information on the value of phlebotomy and of `old' myelosuppressive agents in the management of PV. Patients randomized to the phlebotomy arm showed a higher incidence of thrombosis in the first three years of treatment, which was most common in those with previous thrombotic events or advanced age. A number of factors might have played a role in the high thrombosis rate. These are the uncontrolled thrombocythemia and the fact that over the first few years of the study the target hematocrit marking adequate treatment was below 0.52. The target hematocrit was subsequently reduced to less than 0.45 when further evidence of the influence of hematocrit on blood flow and thrombosis was produced.6 After the first three years, the rate of thrombosis in all three arms was the same.
After 3-5 years of study, patients treated with 32P or chlorambucil began to develop an excess incidence of acute leukemia and other malignancies, lymphoma and carcinomas of the gastrointestinal tract and skin, compared to those treated with phlebotomy alone. Patients treated in the phlebotomy arm of the PVSG-01 trial therefore had a better overall median survival at 13.9 years than the other two arms (chlorambucil, 8.9 years; 32P, 11.8 years). However, this must be interpreted with caution in the light of long-term follow-up studies. Najean et al5 reported that of the 104 patients entered by their group into the phlebotomy arm of PVSG-01 and the later PVSG-05 study,7 more than 50% were excluded from this treatment arm by the 5th year and 90% by the 10th year. Most of the early treatment changes were due to age, the risk of vascular events and excessive thrombocytosis. Subsequently, reasons for giving myelosuppressive agents were poor patient compliance with phlebotomy and the development of splenomegaly with signs of spent phase or myelofibrosis.
The large-scale withdrawal of patients from the phlebotomy arm is considered to play a role in the incidence of acute leukemic transformation in this group of patients.8 This was reported to be 1.5% and to reflect the natural incidence of leukemia in PV in patients not receiving any cytoreductive therapy. However, this figure relates to a very selected group of patients with a less proliferative form of PV, which probably has a lower incidence of leukemic transformation. If the analysis includes events in patients originally randomized to the phlebotomy-only arm and then removed from the study for the above reasons (`intention to treat analysis') this figure rises to 3.7%.9
Long-term observation also raised concern about an increased incidence of myelofibrosis in PV patients treated with phlebotomy alone. In one study,5 the actuarial risk of developing the spent phase of the disease or MMM was much higher in patients who were treated by phlebotomy alone compared with those treated with 32P. In another analysis,10 progression to myelofibrosis in phlebotomized patients was not accelerated provided that the platelet count was satisfactorily controlled by low-dose chemotherapy.
In summary, phlebotomy remains the cornerstone of therapy of PV, but additional myelosuppressive treatments are required in most patients.
Radiophosphorus and alkylating agents
Besides the PVSG-01 trial, two randomized clinical trials have evaluated 32P therapy. The EORTC randomized 293 PV patients to 32P or busulfan.11 Median follow-up was eight years. Ten-year survival was significantly better in the busulfan group (70% vs. 55%, p=0.02), due to a lower incidence of vascular deaths (25/140 vs. 8/145). There was no difference in the occurrence of acute leukemia, `myeloid splenomegaly' and cancer. The French Polycythemia Study Group (FPSG) then carried out a study in 461 patients over 65 years randomized to receive 32P alone or 32P plus hydroxyurea.12 After a maximum follow-up of 16 years, overall survival was slightly, not significantly, better in the 32P-only arm (10.9 vs. 9.3 years). Vascular events or progression to myelofibrosis were similar, whereas significantly more hematological and non-hematological malignancies were seen in patients treated with combination chemotherapy.
These studies indicated that both radiophosphorus and busulfan are effective in managing PV, reducing vascular occlusive events, and delaying myelofibrosis. However, their mutagenic potential suggests they should be reserved for elderly patients. Chlorambucil and combination chemotherapy should be avoided because of their excessive leukemogenic risk.
Hydroxyurea
The search for a nonmutagenic myelosuppressive agent led the PVSG to investigate hydroxyurea (HU). This antimetabolite prevents DNA synthesis by inhibiting the enzyme ribonucleoside reductase. Because of its non-alkylating mechanism of action, it has been assumed that it would not be leukemogenic or carcinogenic in patients with PV.
The PVSG experience with HU in PV has recently been updated (PVSG-08).9 Fifty-one patients were followed for median and maximum of 8.6 and 15.3 years respectively. The incidence of acute leukemia, myelofibrosis and death were compared with the incidence in 134 patients treated only with phlebotomy in the PVSG-01 protocol. There were no statistically significant differences in any of the three parameters, although the HU group showed a tendency to more acute leukemia (9.8% vs. 3.7%), less myelofibrosis (7.8% vs. 12.7%) and fewer total deaths (39.2% vs. 55.2%). In another study13 of HU therapy in 71 PV patients with a median and maximum treatment duration of 7.3 and 15 years, respectively, a lower incidence of acute leukemia (5.6%) and thrombotic incidence (5.6% vs. 21.6%) was shown compared with the PVSG-08 study. The lower thrombotic incidence probably reflects the better hematocrit (< 0.45) and platelet (< 400 × 109/1) control that was achieved.
The efficacy and safety of HU have also been analyzed in two randomized clinical trials in PV and essential thrombocythemia (ET). In a French study, 292 PV patients less than 65 years old were randomized to treatment with HU or pipobroman.14 Pipobroman is a bromide derivative of piperazine with a chemical formula similar to the alkylating agents but with a mechanism of action also involving metabolic competition of pyrimidine bases. No significant differences between the two groups were observed in overall survival, rate of thrombotic complications and actuarial incidence of secondary leukemia (about 5% at the 10th and 10% at the 13th year). A significant increase in risk of progression to myelofibrosis was seen in the patients treated with HU (26 cases) compared to those treated with pipobroman (three cases). However, three-quarters of the patients developing myelofibrosis had permanently raised platelet counts (> 400 × 109/1) despite therapy, particularly in the HU arm. Uncontrolled thrombocytosis, therefore, appeared to increase the incidence of myelofibrotic transformation. One hundred fourteen patients with ET at a high risk of thrombosis were randomly assigned to receive HU or no myelosuppressive therapy.15 HU significantly reduced the incidence of thrombosis (3.6% versus 24% over a median follow-up of 27 months), supporting the concept that controlling platelet number, at least in ET, is useful for lowering the thrombotic risk.
Another important issue relates to patients who become resistant or develop significant side effects while taking HU. Various studies, mainly in ET, have shown that substituting either alkylating agents or 32P for HU greatly increases the incidence of leukemic transformation. It is possible that failure of HU to control thrombocytosis identifies a subset of patients who have a poor prognosis and increased likelihood of developing leukemia. Alternatively, HU may act as a sensitizer, so that exposure to it and then to an alkylating agent or 32P is highly leukemogenic. Whatever the explanation, newer therapies that may be less leukemogenic should be considered for patients who do poorly with HU. Overall, some anxiety remains about the leukemogenic and myelodysplastic16 potential of HU, which has prompted trials with other agents thought to be less toxic such as interferon-α or anagrelide.
Interferon-α
Clinical and experimental data supported the introduction of IFN-α in the management of PV. This drug prolongs survival in chronic granulocytic leukemia and controls thrombocytosis in the Philadelphia-negative chronic myeloproliferative disorders. From a biological point of view, it suppresses the proliferation of hematopoietic progenitors, has a direct inhibiting effect on bone marrow fibroblast progenitor cells, and antagonizes the action of platelet-derived growth factor (PDGF), a product of megakaryocytes, TGF-β and other cytokines, which may be involved in the development of myelofibrosis. Most importantly, IFN-α is not known to be leukemogenic or teratogenic.
To date, no controlled clinical trials have been published on the efficacy and safety of IFN-α in PV. The cumulative experience with IFN-α therapy in 100 patients from 11 studies has been reviewed.17 Overall responses were 60% for reduction of hematocrit to less than 0.45, 75% for reduction in spleen size and 75% for control of pruritus. Results from single-institution studies with long-term follow-up were similar. The major problem with IFN-α therapy, apart from its cost and parental route of administration, is the incidence of well-known side effects leading to cessation of treatment in about one-third of patients.
The role of IFN-α in PV therapy is still uncertain, although it seems particularly promising for the management of younger patients and those with intractable pruritus. Controlled clinical studies evaluating long-term clinical end-points are required. The recent development of pegylated-IFN may be one step forward because of its slower clearance, permitting once-weekly dosing.18
Anagrelide
Anagrelide is an oral imidazoquinazoline agent with anticyclic AMP phosphodiesterase activity, which inhibits platelet aggregation in both humans and animals. In human it has species-specific platelet-lowering activity at doses lower than those required to inhibit platelet aggregation. At therapeutic concentrations, anagrelide has a profound effect on the maturation of megakaryocytes, resulting in a reduction of platelet production. Because of this effect and the lack of mutagenic activity, the drug has been tested in patients with clonal thrombocytosis and found to effectively control thrombocytosis in PV.
As for IFN-α, only non-randomized phase II trials have analyzed the hematological effects and toxicity. No comparative data are available on survival, incidence of thrombosis and hematological progression. Clinical studies of anagrelide treatment of chronic myeloproliferative diseases started in the mid-1980s. In a recent update of this experience, 942 patients, including 113 with PV, were treated for a minimum of 4 years.19 Reduction in platelet counts to less than 600 × 109/1 or less than 50% of the pretreatment level were achieved in 76 (66%) patients with PV. Time to response generally ranged between 17 and 25 days and no appreciable effect was noted in other hematological parameters. Most of the side effects of anagrelide are cardiovascular, due to its peripheral vasodilatory effect and positive inotropic activity. They include headache, observed in more than one-third of the patients, forceful heartbeat (27%), fluid retention (24%), dizziness, and less frequently, arrhythmias (less than 10%) and congestive heart failure (2%). Non-cardiovascular side effects are less common and include diarrhea, abdominal pain, nausea, fatigue and transient rash.
The long-term toxicity of the drug has been analyzed in a cohort of 37 young patients with ET followed for a median of 10.7 years.20 Overall response rate was approximately 90% and the reduction of platelet count was sustained in more than 80% of patients. The side effect profile was no different from that observed in the short-term, but seven patients (19%) had thrombotic complications, in some cases due to inadequate platelet control, and six (16%) had major hemorrhage.
Thus, anagrelide appears useful for the control of thrombocytosis in PV and other myeloproliferative disorders but there is no evidence that it controls the splenomegaly and the overall myeloproliferative activity of PV. Its therapeutic role needs to be verified in a randomized setting.
Antithrombotic therapy
The use of aspirin or other antiplatelet agents in patients with PV and other myeloproliferative disorders remains controversial. Laboratory tests of platelet function (e.g. bleeding time, platelet aggregation studies) have been generally unreliable in predicting the risk of bleeding and thrombosis, so the therapeutic decision rests primarily on clinical judgement.
Two small, randomized clinical trials evaluated the effect of different doses of aspirin in PV patients.7,21 The PVSG randomized 163 subjects to be treated by phlebotomy associated with aspirin 900 mg/day plus dipyridamole or by 32P.7 After a median follow-up of 1.6 years, six hemorrhagic episodes, mostly gastrointestinal, and seven thrombotic events were observed in the group assigned to antiplatelet therapy, many of whom had particularly raised platelet counts, while two thrombotic episodes but no bleeding were observed in the group that received cytoreductive therapy. The study was therefore discontinued, and the authors concluded that antiplatelet agents were both ineffective and potentially dangerous in PV patients. However, the excess of thrombosis was not reported to be statistically significant and the excess of gastrointestinal bleeding was most likely related to the high dose of aspirin.
Since then, large clinical trials in patients with vascular disease have shown that lower aspirin doses (30-250 mg/day) are at least as effective as higher doses and are better tolerated. Thus, in 1997 the GISP started a pilot study on the safety of low-dose aspirin (40 mg/day): 112 PV patients, with no clear indications for or contraindications to aspirin, were randomized to low-dose aspirin or placebo and followed for more than 12 months.21 No differences in side effects or adverse events were apparent, confirming the safety of that dosage. The question of the efficacy of asprin remained unsettled, however, because of the limited size and length of the pilot study. A large-scale, randomized, placebo-controlled clinical trial is now under way in twelve European countries (European Collaboration on Low-Dose Aspirin; ECLAP). The project will admit 3,500 patients with uncertain risk/benefit ratio to aspirin treatment. A similar sample, expected to be judged by their physicians as having a clear indication or contra-indication to aspirin treatment, will be entered in an observational arm.
Until the trial is completed, it seems reasonable to recommend low-dose aspirin for patients who have cardiovascular disease, such as myocardial infarction or ischemic stroke, for which antiaggregating therapy would be prescribed in any event irrespective of the presence of PV. In addition, aspirin has an elective indication for the treatment of erythromelalgia and other microvascular, neurological and ocular disturbances. Aspirin is best avoided in patients with prior hemorrhage, particularly of the gastrointestinal tract.
Summary and Conclusions
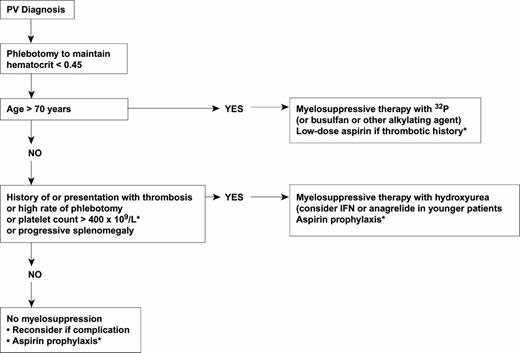
The following recommendations can be given for the management of patients with PV (Figure 3). All patients should undergo phlebotomy with the goal of keeping the hematocrit value below 0.45. No additional therapy may be needed in stable patients who are at low risk for thrombosis (age below 60 years, no history of thrombosis). In patients at high risk of thrombosis or who develop progressive thrombocytosis or splenomegaly, the choice of myelosuppressive agent depends on the patient's age. Older patients can be managed with 32P, low-dose busulfan or pipobroman. HU has a role in the management of patients at all ages despite concerns about its potential leukemogenicity. IFN-α or anagrelide should be considered in younger patients, but controlled clinical studies are urgently needed to assess their efficacy and safety on hard clinical end-points, such as survival, major thrombosis and hematological progression. In all patients with cerebrovascular, coronary or peripheral arterial ischemia, the use of low-dose aspirin is recommended.
Flow chart of recommended treatment for patients with polycythemia vera, depending on risk factors.
Areas of uncertainty are marked with an asterisk.
Abbrevations: IFN, interferon.
Flow chart of recommended treatment for patients with polycythemia vera, depending on risk factors.
Areas of uncertainty are marked with an asterisk.
Abbrevations: IFN, interferon.
Randomized clinical trials aimed at producing scientific evidence on the benefit/risk profile of therapeutic modalities are mandatory but difficult to arrange in a rare disease such as PV. The end-points of such studies should be clinically relevant and patients should be followed at least for 3-5 years if the main outcome is the reduction of thrombotic events and 5-10 years to assess the rate of malignancies. Only international collaboration can recruit rapidly and follow an adequate number of cases for these purposes. The European network set up for the low-dose aspirin clinical trial represents an important step towards this goal.
The authors wish to acknowledge the clinicians/scientists who have made significant contributions to the MPD literature and whose work is alluded to but no citation given. Unfortunately, due to lack of space it was necessary to restrict the number of references provided.