Abstract
Drs. Hartmut Döhner, Michael J. Keating, Kanti R. Rai and Emili Montserrat form the panel to review chronic lymphocytic leukemia (CLL) while focusing on the clinical features of a particular patient. The pace of progress in CLL has accelerated in the past decade. The pathophysiological nature of this disease, as had been known in the past, was based largely on the intuitive and empiric notions of two leaders in hematology, William Dameshek and David Galton. Now the works of a new generation of leaders are providing us with the scientific explanations of why CLL is a heterogeneous disease, perhaps consisting of at least two separate entities. In one form of CLL, the leukemic lymphocytes have a surface immunoglobulin (Ig) variable region gene that has undergone somatic mutations, with tell-tale markers suggesting that these cells had previously traversed the germinal centers. Such patients have a distinctly superior prognosis than their counterparts whose leukemic lymphocytes IgV genes have no mutations (these are indeed immunologically naive cells), who have a worse prognosis. The introduction of fluorescence in situ hybridization (FISH) technique has provided us with new insights into the diverse chromosomal abnormalities that can occur in CLL, and which have significant impact on the clinical behavior and prognosis of patients with this disease.
Major advances in therapeutics of CLL also have occurred during the past decade. Two monoclonal antibodies, Campath-1H (anti-CD52) and rituximab (anti-CD20), and one nucleoside analogue, fludarabine, have emerged as three agents of most promise in the front-line treatment of this disease. Studies currently in progress reflect our attempts to find the most effective manner of combining these agents to improve the overall survival statistics for CLL patients. As in many other hematological malignancies, high dose chemotherapy followed by autologous or HLA-compatible allogeneic stem cells rescue strategies are under study as a salvage treatment for a relatively younger age group of CLL patients with poor prognosis characteristics.
Case Presentation
Kanti R. Rai, MD*
Division of Hematology-Oncology, Long Island Jewish Medical Center, New Hyde Park NY 11040 Dr. Rai received research support from Berlex 1992-1996.
In December 1998, while the patient remained asymptomatic, lymphadenopathy was noted in both laterocervical, axillary, and inguinal areas. Spleen and liver were not found to be clinically enlarged. The WBC count was 50,000/μL with 75% small mature-appearing lymphocytes and 15% larger lymphocytes; the Hb level was 130 g/L, and the platelet count 110,000/μL. Biochemical parameters were again within normal limits. Cytogenetic study, this time using fluorescence in situ hybridization, revealed the presence of a 13q and 11q deletion. Treatment with fludarabine (25 mg/m2 i.v./day x 5 days, every 4 weeks) was started. After 6 cycles of fludarabine, the WBC count was 9,000 (35% lymphocytes), the Hb level 142 g/L, and the platelet count 140,000/μL; no lymphadenopathy was detected. A bone marrow trephine biopsy was normal except for the presence of some lymphoid aggregates. Cytofluorometric studies of peripheral blood lymphocytes demonstrated a clonal excess. The patient was considered as in nodular-PR and no further treatment was considered necessary.
In December 2001, the patient presents with fever and night sweats, generalized lymphadenopathy, a WBC count of 35,000/μL, a Hb level of 90 g/L, platelet count of 90,000/μL Biochemical parameters are essentially normal but serum LDH is 520 U/L (normal values < 400) and serum β2-microglobulin is 3.8 mg/L (normal values < 2).
Questions for the panel:
.Decision after initial diagnosis
.Decision upon disease progression
.Clinical workup and treatment possibilities at this time (December 2001).
I. Prognostic Implications of Findings from Cytogenetics and Molecular Genetics
Hartmut Döhner, MD*
Department of Internal Medicine III, University of Ulm, Robert-Koch-Str 8, D-89081 Ulm, Germany
Methodological Aspects of Genetic Analysis in CLL
In our case, initial diagnostic work-up in March 1997 included conventional chromosome banding analysis which showed a normal karyotype. The technique of conventional chromosome banding has been hampered in CLL by the low in vitro mitotic activity of the clonal B-cells. With this method, clonal chromosome abnormalities are detected in only 40% to 50% of cases.1 In many cases only normal metaphase spreads are obtained, mostly due to the fact that despite the use of B-cell mitogens, the mitotic cells originate from non-leukemic T lymphocytes contained in the specimens. This was shown by the study of Autio et al. using the technique of sequential immunophenotyping and karyotype analysis.2
The development of fluorescence in situ hybridization (FISH) using genomic DNA probes has greatly enhanced our ability to detect chromosome aberrations in tumor cells. One major advantage of this technique is that aberrations can be detected in both metaphase and interphase cells, an approach commonly referred to as interphase cytogenetics. Given the methodological problems associated with conventional chromosome banding in CLL, it was not surprising that the spectrum and frequency of aberrations reported in the various FISH studies differed considerably from the results obtained in banding studies.3 Using molecular cytogenetics, genomic aberrations can now be identified in approximately 80% of CLL cases.
In our case, molecular cytogenetic work-up at the time of disease progression in December 1998 revealed the presence of a 13q and an 11q deletion. It is very likely that these two abnormalities were already present at the time of diagnosis but were missed by the conventional cytogenetic technique. Conventional chromosome banding studies can no longer be recommended in the routine diagnostic work-up of a CLL patient. The novel molecular cytogenetic techniques are now recognized to provide the most reliable data.
Prognostic Impact of Genomic Aberrations
Based on conventional chromosome banding analysis, trisomy 12 was the first abnormality that in univariate analysis was associated with both shorter treatment-free interval and shorter survival.1 Other abnormalities associated with inferior survival were 11q and 17p deletion.4,5 In contrast, patients with structural aberrations of chromosome 13, mostly 13q deletions, and patients with a normal karyotype seemed to have a favorable outcome.1
Owing to the methodological problems of conventional chromosome banding it became necessary to reassess the prognostic value of the genetic markers based on the novel techniques. We recently reported data from a molecular cytogenetic study in CLL evaluating the incidence and prognostic significance of the most important disease-associated genomic aberrations.6 Samples from 325 CLL patients were analyzed by FISH using a comprehensive set of diagnostic DNA probes for deletions in chromosome bands 6q21, 11q22-q23, 13q14, 17p13, for trisomies of bands 3q26, 8q24, 12q13, and for translocations involving the immunoglobulin heavy chain locus in band 14q32. Genomic aberrations were detected in 268 of 325 cases (82%). The most frequent aberration was 13q deletion (55%), followed by 11q deletion (18%), 12q trisomy (16%) and 17p deletion (7%). On the basis of regression analysis we proposed a hierarchical model of genomic aberrations, in which each patient was allocated to a single category. This model comprised five major categories, i.e. patients with a 17p deletion; patients with an 11q deletion but not a 17p deletion; patients with 12q trisomy but not a 17p or 11q deletion, patients with a normal karyotype, and patients with a 13q deletion as sole aberration (Table 1 ). The estimated median survival time of the entire study group was 108 months; and the median survival times for patients of the five major categories were 32, 79, 114, 111, and 133 months, respectively. Furthermore, the cytogenetic categories were associated with distinct presenting clinical features. Patients with 17p or 11q deletion had more advanced disease stage compared to the other 3 categories, they were more likely to have splenomegaly, mediastinal and abdominal lymphadenopathy, and they had more extensive peripheral lymphadenopathy; furthermore, these patients had B-symptoms more frequently. Finally, there were statistically significant differences in disease progression among the five categories as measured by the treatment-free interval: the median treatment-free intervals for the groups with 17p deletion, 11q deletion, 12q trisomy, normal karyotype, and 13q deletion as sole aberration were 9, 13, 33, 49, and 92 months, respectively. Multivariate analysis identified six significant prognostic factors: 17p deletion, 11q deletion, age, Binet stage, serum lactate dehydrogenase level, and white cell count.
The data from this single center study indicate that genomic aberrations in CLL are important independent predictors of disease progression and survival. It is now important to investigate the impact of these genomic aberrations prospectively in clinical trials of the large cooperative groups. Table 1 shows preliminary results from the prospective genetic study within the CLL1 (Binet A patients) and the CLL3 (high-dose therapy followed by autologous transplantation for patients with stage Binet B and C disease) treatment trials of the German CLL Study Group (GCLLSG). These data are consistent with our single center data with respect to the overall incidence of genomic aberrations. In the CLL1 trial for patients with stage Binet A disease there is a higher incidence of the 13q deletion as sole abnormality and a lower incidence of the high-risk groups 17p deletion and 11q deletion, whereas in the CLL3 trial there is a higher incidence of the 11q deletion group likely reflecting the higher disease burden and the younger median age of these patients.
As shown by the data from the CLL1 trial, high-risk genomic aberrations are detected in approximately 15% of stage Binet A patients (Table 1). The case under discussion belongs to this subgroup of patients. Not unexpectedly, he developed rapid disease progression with generalized lymphadenopathy, hematopoietic insufficiency and B-symptoms. I wonder whether the disease in December 2001 had undergone clonal evolution when the patient relapsed with marked disease activity following treatment with fludarabine. Few data so far address this question by using molecular cytogenetic techniques. We applied FISH for sequential interphase cytogenetic studies on 55 patients over a median observation time period of 42 months (range 24-81 months).7 Clonal evolution was found in 9 of the 55 (16%) patients. The most frequent acquired changes were 17p deletion (4 cases), 6q deletion (3 cases), 11q deletion (1 case), and evolution from mono- to bi-allelic 13q deletion in 3 cases. Two thirds of the patients exhibiting clonal evolution have died, compared to only 20% in the group of patients without genetic evolution.
Prognostic Impact of the IgV Genes Mutational Status
I also wonder whether the mutational status of the immunoglobulin variable (IgV) genes was assessed in our patient.
One important issue of biological risk classification in CLL relates to the stage of differentiation of the malignant B cells. The process of differentiation can be divided into a pregerminal, germinal and postgerminal center phase. Selection and recombination of variable (V), diversity (D) and joining (J) genes as well as the insertion of nontemplated nucleotides at the V-D and D-J junction are early events in the pregerminal phase. In the later stage of differentiation, the germinal center phase, the B cells undergo somatic hypermutation. In the microenvironment of the germinal center, somatic mutations are introduced in the V(D)J-rearrangement. This process occurs in part with and without antigen stimulation.8 Although the B cells involved in CLL were originally considered to be naïve, pregerminal lymphocytes, more recent data indicate that somatic IgV gene mutations are present in approximately 50 percent of CLL cases.9,10 These data indicate that there are two variants of the disease “CLL,” a pregerminal variant which originates from naïve B lymphocytes showing no IgV gene mutation, and a postgerminal variant which originates from memory B lymphocytes exhibiting IgV gene somatic hypermutation. Correlation of the IgV mutational status with clinical data revealed that the presence of unmutated IgV predicts for inferior prognosis.11,12 Based on this observation, other differentiation markers such as the CD38 expression level have been studied in CLL. In one study, CD38 expression was shown to correlate with the presence of unmutated IgV genes and an unfavorable clinical outcome.12 The correlation of CD38 with unmutated IgV genes or survival probability is currently a matter of discussion.13,14 Ibrahim et al have recently confirmed previous results on the prognostic significance of CD38 expression in multivariate analysis.15 However, their study did not include IgV mutation status and genomic aberrations.
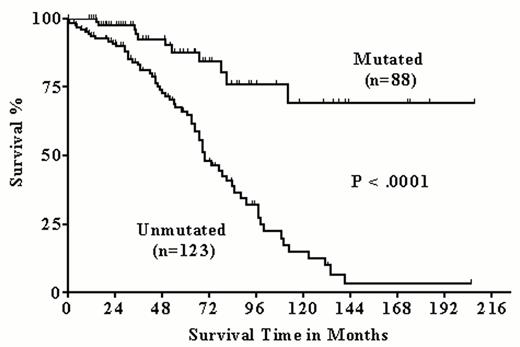
In our single center study, we analyzed 211 CLL samples for the IgV mutation status.16 Eighty-eight patients had their IgV genes mutated. Analogous to the pivotal studies of Hamblin et al11 and Damle et al12, the patients with mutated IgV genes had significantly higher survival probabilities compared to the patients with unmutated IgV genes. This could be shown for the entire group of patients as well as for the subgroup of patients (n = 131) with stage Binet A disease (Figures 1 and 2Figure 1,Figure 2). In agreement with Damle et al we also observed an inverse correlation between CD38 expression and IgV gene mutation status. However, in about a third of cases CD38 expression failed to predict the IgV gene mutation status, and CD38 expression level > 30% was not significantly associated with lower survival probability.
Correlation of genomic aberrations with the mutational status of the IgV genes:
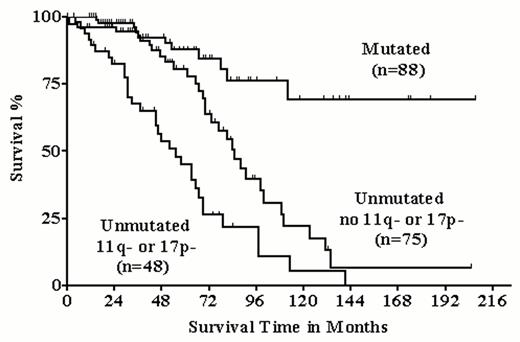
In our single center study, we correlated the genomic aberrations with the mutational status of the IgV genes in 211 cases (Table 2).16 The overall incidence of aberrations was not different between the two groups. The prevalence of 13q deletion was significantly more frequent in the mutated group, but still almost half of the unmutated cases exhibited this abnormality, which suggests that the inactivation of an as yet unknown tumor suppressor in band 13q14 may be important in both unmutated and mutated CLL cases. However, there were striking differences in the incidence of the prognostically important categories between the two groups: 13q deletions as sole abnormality predicting a favorable prognosis were significantly more frequent in cases with mutated IgV genes; in contrast, the prognostic unfavorable abnormalities 17p and 11q deletion were almost exclusively found in cases with unmutated IgV genes. Interestingly, within the IgV unmutated group those cases with high-risk genomic aberrations, as defined by 17p and/or 11q deletion, had a significantly inferior survival probability (Figure 3 ). Thus, IgV mutational status and genomic aberrations likely give complementary prognostic information.
Genomic Aberrations and Choice of Treatment
The impact of specific prognostic factors may depend on the treatment given to the patients. Whether the resistance to specific therapeutic agents may be overcome by the use of alternative drugs is an important issue that has to be addressed in future treatment trials. To answer this question it is mandatory to perform a state-of the-art profiling for the biological parameters that are now available.
11q deletion identifies patients at high risk for molecular disease persistence after high-dose therapy and autologous stem cell transplantation:
The CLL3 trial of the GCLLSG investigates the role of myeloablative radiochemotherapy with autografting after cytoreduction (CHOP, fludarabine, or fludarabine/cyclophosphamide) and autologous stem cell mobilization (Dexa-BEAM) in younger CLL patients. Within the trial all cases are analyzed for the CLL-associated genomic aberrations, for the IgV mutation status, and for the molecular remission status by genescan analysis of CDR3 PCR amplification products.17 Among 46 patients with molecular follow-up data available, a clonal CDR3 PCR as molecular evidence of disease persistence was present in 5 of 13 (38%) patients with 11q deletion at 1 to 3 months post transplantation, but only in 2 of 33 (6%) patients without 11q deletion (p = .014). This difference was still significant at 12 months post transplantation. If in our case autologous transplantation would be envisaged as next therapeutic step, one should consider that 11q deletion may be a factor predicting for post-transplant failure.
p53 gene mutations predict for non-response to treatment with alkylating agents and with purine analogs:
Evidence for the prognostic impact of 17p/p53 abnormalities in CLL initially came from a study applying single strand conformation polymorphism (SSCP) analysis.18 In this study p53 mutation was associated with in vitro resistance to chlorambucil and with poor clinical response to therapy with chlorambucil or other alkylating agents. In our initial FISH study, 17 of 100 patients with chronic B-cell leukemias exhibited a monoallelic p53 deletion and the presence of such deletion had a strong impact on the clinical course of the disease.19 Patients with a p53 deletion had a significantly shorter survival time than patients without a deletion. In addition, a significant association between a p53 deletion and treatment failure was identified: while 56% of patients without p53 deletion responded to treatment with purine analogs, none of the patients with the deletion did. From these studies it is clear that p53 deletion/mutation confers a phenotype of high resistance to standard treatment with alkylating agents and purine analogs. Alternative drugs need to be investigated whose mode of action do not involve the p53 signalling cascade. In our case, a sequential genetic analysis at the time of relapse following treatment with fludarabine would have identified or ruled out a p53 abnormality and would have given important information for the responsible physician.
In summary, in CLL patients state-of-the-art risk assessment at the time of diagnosis should ideally include profiling of genomic aberrations by molecular cytogenetics as well as evaluation of the IgV mutational status and CD38 expression level. Sequential analysis of the p53 mutational status by FISH and/or DNA sequencing may be indicated at the time of marked disease progression. We recognize that at this time (December 2001) the above cited exciting new developments in prognostic markers might not be readily available as routine tests for the practicing clinician. We do expect that, because of the power these tests offer in predicting prognosis and in guiding us to a more rational therapeutic approach for individual patients, in the not-too-distant future we will be able to offer some of these tests to many of our patients.
As new therapeutic strategies have become available—combination therapies with purine analogs, monoclonal antibodies (rituximab, Campath-1H), and potentially curative therapeutic modalities such as autologous and allogeneic SCT—it is important to have prognostic markers at hand that allow the more accurate prediction of the clinical course for an individual patient. In future treatment trials, these biological markers may become important to refine disease management.
II. New Developments in Therapy
Michael J. Keating, MB, BS*
MD Anderson Cancer Center, 1515 Holcombe Blvd., Box 61, Room B8-4442, Houston TX 77030
Initial Evaluation
The patient under review, when first evaluated, had early stage disease with a classical immunophenotype for B-cell CLL. He had no indication for any therapeutic intervention using the NCI Working Group criteria.2 There were no symptoms, marrow compromise, bulky lymphadenopathy or hepatosplenomegaly. The bone marrow evaluation which demonstrated a non-diffuse pattern was in keeping with CLL early stage and the normal LDH and β2-microglobulin were all elements that predicted this patient would have a long period of time without progressive disease. Conventional cytogenetics were also consistent with an indolent course. Thus it was a perfectly sound decision to offer this patient no therapeutic intervention and to re-evaluate him at 6 month intervals. A number of clinical trials have been conducted by the French Cooperative Group demonstrating that early intervention in Binet stage A disease with chlorambucil with or without corticosteroids has not had an impact on the survival of patients.3,4 There was a delay in development of progressive disease in the patients randomized to early treatment, but survival after progression was correspondingly decreased leading to no change in overall survival. There is also a suggestion of an increase in cancer in the early chlorambucil group and in a more complete analysis, an increase in the number of patients with acute myelogenous leukemia (AML).4
Categorization of patients as having “smoldering leukemia” has been established by a number of groups. The French and Spanish groups both define patients with relatively normal hemoglobin, platelet count, with a white cell count of less than 25,000-30,000/μL, minimal lymphadenopathy or splenomegaly to be classified as smoldering CLL if the doubling time of their blood lymphocytes is > 12 months.5,6 The only values provided in the protocol of the blood lymphocyte counts in this patient were at presentation in March 1997 and again 21 months later at the time of the development of progressive disease in December 1998. These values were 14,400/μL and 37,500/μL, respectively. However calculations of the doubling time from these data suggest that this patient had a doubling time of more than one year. A number of studies have demonstrated that doubling times of < 12 months are predictive of a shorter survival when compared with patients with longer doubling times after adjusting for stage of disease.7,8
Disease Progression
The patient demonstrated that despite the good general prognosis which would have been offered to him at initial presentation, he was in the subset of patients who did develop progressive disease less than two years later. Although still asymptomatic, the patient developed progressive lymphadenopathy and an increase in the white cell count. He also developed a subpopulation of larger lymphocytes, a poor prognostic factor in some series.9 The hemoglobin level and platelet count fell slightly. All of these parameters suggest that the patient is developing a more proliferative population of cells with the ability to suppress production of normal marrow elements. The fluorescence in situ hybridization (FISH) study revealed the presence of a 13q del and also 11q del. As mentioned by Dr. Döhner, 13q deletion is a common abnormality in CLL and 13q is likely to be the site of a tumor suppressor gene.10 Döhner et al have shown that the 11q del is associated with a poor prognosis and usually associated with bulky lymphadenopathy.10 The presence of the larger cells and the del 11q do suggest a shift in prognosis in this patient to a more aggressive subvariety of CLL. Information on the prognostic significance of subsets of the CLL cells which co-express CD3811,12 or immunoglobulin gene mutations11,13 were not available at the time of the patient's initial diagnosis or at the time of progression but it is likely that the patient was more likely to have naïve B-CLL cells without hypermutation of the immunoglobulin gene sequences and would have had a proportion of CLL cells (> 20– 30%) that were CD38+.
Initial Therapy
The cornerstone of the treatment of CLL for some decades has been the use of alkylating agent regimens such as chlorambucil either alone or combined with corticosteroids or cyclophosphamide. Other investigators have used regimens more commonly prescribed for low grade lymphoma mainly the cyclophosphamide, vincristine, prednisone (CVP) regimen or the CHOP regimen which adds doxorubicin to CVP. At the time of first evidence of disease progression in December 1998, this patient would be classified as Rai stage I or Binet stage B. French Cooperative Group studies have demonstrated that there is no advantage of CVP over chlorambucil in stage B patients,14 but in Binet stage C CHOP with reduced dose of doxorubicin (mini-CHOP) is associated with a superior outcome.15 Single agent studies with fludarabine have demonstrated a higher complete response rate and overall response rate compared with historical experience with alkylator based regimens.16 These results have now been confirmed by comparative clinical trials.17,18
The initial comparative clinical trial of fludarabine versus CAP (CHOP without vincristine) demonstrated a higher complete response rate and overall response rate in previously untreated patients who were recipients of fludarabine as initial therapy.17 There was a significant prolongation of time-to-progression and a suggestion (P = .08) of a survival advantage with fludarabine. Another major clinical trial conducted by the North American Intergroup has recently been published comparing fludarabine to chlorambucil as initial therapy.18 In the intergroup study,18 the overall response rates (63% vs. 37%) and complete response rates (20% vs. 4%) were significantly higher in the fludarabine arm with a significant prolongation of time-to-progression. The study had a crossover design with patients who failed to achieve a response or relapsed after responding to their initial therapy being crossed over to the alternate arm. The salvage rate for patients failing fludarabine and crossing over to chlorambucil was only 7% versus 46% for patients initially randomized to chlorambucil and being retreated with fludarabine.
A French Cooperative Group study comparing fludarabine to CAP and mini-CHOP has led to discontinuation of the CAP arm because of inferior results and a higher mortality rate.19 There is not significant difference in survival in the other two arms but the response rate to fludarabine is slightly higher than the mini-CHOP with a higher complete response rate and a slightly prolonged survival. Thus I believe that in the case under discussion, the treatment decision to use fludarabine initially was based on positive evidence. There is a trend to use fludarabine as initial therapy particularly in younger patients because of the higher response rate than for alkylator based regimens.
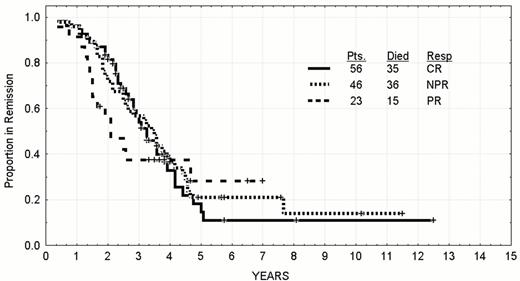
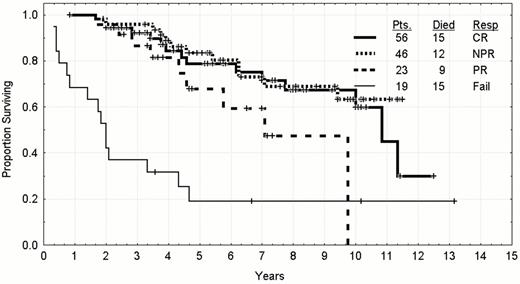
The aim of trying to improve the CR rate is justified by the longer time-to-relapse of patients receiving fludarabine initially according to response.17 The median time-to-progression of patients of any age achieving a CR after fludarabine as a single agent or combined with prednisone is 42 months with approximately 20% of patients expected to still be in remission at eight to ten years. The results for younger patients are updated in Figure 4 and Figure 5 . The patient under discussion achieved a nodular partial remission (PR) in which there is a normal bone marrow apart from persistence of lymphoid aggregates in the marrow biopsy. The median remission duration of these patients is 30 months with only 10% expected to be in long term clinical remission. Patients who only achieve a partial response have a shorter median remission duration and lower prospects for long term control. The persistence of a clonal excess in the peripheral blood after achieving a nodular PR also is suggestive that this patient would have a shorter remission duration than average.21 Patients who have more than 10% CD5 and CD19 co-expressing B-cells or have a kappa or lambda excess in the marrow have a shorter remission duration than patients without evidence of persistence clones of CLL cells (Figure 6 ). This patient would be classified a nodular PR and likely to relapse.
Post Remission Therapy
The question which could now be raised is whether this patient should have some post-remission therapy for residual disease; however, at the time of his treatment in 1998 this issue was seldom addressed. Studies have been conducted with α-interferon as a post-remission strategy for this was not associated with prolongation of time-to-treatment failure or any significant clinical benefit.22 Investigators at the Dana-Farber Cancer Institute have taken patients with persistent disease after induction therapy to autologous stem cell transplantation as intensification of initial remission,23 and a substantial number of patients have become PCR negative for the immunoglobulin heavy chain gene. This is considered to be a reasonable surrogate based on the findings of the benefits of survival in low grade lymphoma patients who are PCR negative after autologous bone marrow transplantation. Some investigators would propose allogeneic bone marrow transplantation in this setting but there are no data in single arm or comparative clinical trials to suggest that this is of benefit. Current studies are exploring the use of monoclonal antibodies such as rituximab, Campath-1H or radioconjugates of CD20 antibodies (Zevalin, Bexxar) for treatment of residual disease to achieve PCR negativity in this setting.
Relapse
The patient now presents with a very serious clinical situation. He has fever and night sweats associated with the generalized lymph node enlargement, a rapidly rising white cell count, and suppression of hemoglobin and platelet production, all of which are adverse prognostic parameters. The LDH is elevated and the beta-2-microglobulin is approaching twice normal. Such an aggressive relapse after first remission is uncommon. Most patients who relapse after achieving a remission do so in an indolent fashion. The abrupt onset of these B-symptoms suggest that it is important to look for alternative explanations other than CLL. Opportunistic infections such as tuberculosis, cytomegalovirus, reactivation of Epstein-Barr virus (EBV) or toxoplasmosis should be investigated in this context. Other alternatives are that this patient has developed a transformation of his disease into a prolymphocytic form, a lymphoplasmacytoid morphology with a high proliferative index, or a large cell lymphomatous transformation (Richter's syndrome). A full restaging of the patient is mandatory at this point. A biopsy of the lymph node would be optimal for evaluating the histopathology. However, deciding which lymph node(s) to biopsy is often difficult. If there is not a single lymph node which is rapidly growing, the decision as to which one to biopsy is often made on the basis of ease of access for the surgeon. However there is evidence that gallium scanning will identify areas that are more likely to have transformation events.24 Thus a gallium scan would assist in identifying for the surgeon the appropriate node to biopsy. It is quite probable that CAT or PET scan would also be useful in identifying areas likely to have transformation. The biopsied node should not only be submitted for histopathologic sections but also sent for molecular studies and culture for viruses, TB and fungi.
Treatment of Relapsed CLL
If the patient is found to have relapse of his CLL and it is not a Richter's transformation, the question arises as to what treatment will be recommended. Most experience with salvage therapy has been obtained in patients who have previously received alkylating agents. In such patients, purine analogs such as fludarabine and 2-chlorodeoxyadenosine are most likely to be effective with response rates of 30-55%, with higher response rates in patients who are still sensitive to alkylators.25,26 Retreatment of patients who have been previously treated only with purine analogs is less clearly defined. In the North American Intergroup Study, patients who either failed to respond to fludarabine or relapsed after treatment with fludarabine were crossed over to chlorambucil with a response rate of only 7%.18 There is less experience with other regimens for the management of patients previously treated with fludarabine. In one series, patients who had failed or relapsed after receiving fludarabine as a single agent as initial therapy received salvage therapy.27 The response rate correlated with their initial response: CR patients were more likely to attain a second CR than nodular PR patients, who were more likely to have a second nodular PR (Table 3 ). A combination of fludarabine and cyclophosphamide (FC) has been evaluated in relapsed patients. The response rate to FC was 39% in 28 patients refractory to fludarabine and 80% in 40 patients who were considered sensitive to fludarabine. Relatively few patients obtained a complete remission. On review of our institutional data, 8 of 9 (89%) young patients (< 60 years) who responded to initial therapy with fludarabine with a nodular PR and then relapsed, had a second remission with single agent fludarabine.
Monoclonal antibodies have been evaluated as salvage therapy. Rituximab has been effective in high doses28,29 and the response rate correlates with whether the patients are considered fludarabine sensitive or refractory.28 Campath-1H has been evaluated in fludarabine refractory patients and has a response rate of 33%.30 Fewer data are available for fludarabine sensitive patients. More recently combinations of rituximab with chemotherapy have been explored as initial therapy, fludarabine alone31 or combined with cyclophosphamide.32 In the fludarabine, cytoxan, and rituximab (FCR) combination in previously treated patients the response rate is high and is highest in patients who are still considered sensitive to fludarabine.33 Remissions which occur following salvage therapy are significantly shorter than initial remissions. Thus, this patient even if he gets a response is likely to have recurrence of his disease within a period significantly shorter than his first remission. Therefore consideration should be given to intensifying any remission with treatment of residual disease. Although this could be accomplished by the use of monoclonal antibodies such as rituximab, Zevalin, or Campath-1H, no data are available to support this approach.
Autologous bone marrow transplants have been used as intensification of second remissions with no long term control obtained with this approach.34,35 Allogeneic bone marrow transplant has been applied with conventional or non-myeloablative conditioning. In view of the fact that this patient is young, consideration should be given to one of these strategies. If he has no HLA compatible sibling, a matched unrelated donor transplant should be considered. Initial reports suggests that the outcome with non-ablative transplants in the older patient population is equivalent to ablative transplants at one center.36
If the patient has Richter's transformation, prognosis is grave. A number of approaches have been taken to the management of Richter's transformation with CHOP-type regimens and ara-C/platinum regimens.37,38 The overall response rate is 25–30%, the remissions are short and the median survival after Richter's transformation is approximately 5 months. Combinations of agents with Rituximab have also been used without any evidence of clinical improvement (personal communication; F. Giles). In patients with relapsed CLL, all modalities need to be integrated to give the best chance for prolongation of survival.
III. Hematopoietic Stem Cell Transplants
Emili Montserrat, MD*
Hematology Department, Hospital Clinic, C/Villarroel, 170, 08036, Barcelona, Spain Dr. Montserrat receives research support from Schering AG, Roche, and Amgen.
The clinical workup in this now 55-year-old patient with advanced stage (Binet's stage C; Rai's stage IV) CLL should include-besides a complete clinical history, physical examination and routine analyses-other tests which are listed below along with their specific purpose:
Review of a peripheral blood smear. This is aimed at determining whether the number of prolymphocytes or other atypical cells (15% in December 1998 when he progressed to stage B(I) disease) has increased. A high proportion of atypical cells is observed in disease transformation and indicates poor prognosis. Of note, atypical morphology is frequently associated with trisomy 12, a cytogenetic abnormality apparently not detected in this case.
Bone marrow aspirate and biopsy. To rule out causes of pancytopenia other than the infiltration of the bone marrow by lymphocytes (e.g., myelodysplasia). In the case under consideration, the bone marrow is most likely to be packed by lymphocytes with megakaryocytes and erythroid precursors being scarce. Bone marrow examination would also be useful as a baseline parameter for assessing response to therapy.
Coombs and other tests for hemolysis. Although the most likely mechanism for anemia in this patient is massive lymphocytic infiltration of the bone marrow, an important proportion of patients with CLL (10-30%) may present with autoimmune hemolytic anemia; it is important to rule out this possibility.
Cytogenetic study (FISH). This will establish whether new cytogenetic abnormalities indicating clonal evolution are present. Complex karyotype, as well as abnormalities in 11q (existing in this patient) and in 17p, is associated with a poor outcome.
Lymph node biopsy. Disease transformation into large-cell lymphoma occurs in approximately 10% of CLL patients. Patients with disease transformation usually present with general symptoms and increased serum LDH levels. These features are present in this case, although they could also be easily explained by active CLL. However, it is important to rule out disease transformation into large cell lymphoma (Richter's syndrome) because disease transformation implies a very poor prognosis (median survival after transformation of less than one year), and in such a case the treatment should be that of the lymphoma.
For the rest of my discussion, I will assume that our patient has active CLL in advanced stage (Binet's stage C; Rai's stage IV) due to a high tumor burden and that he has good performance status and no serious comorbidity. I will also suppose that he has an HLA-compatible sibling donor. The factors to be considered when recommending treatment in this patient are discussed below.
This patient was 51 years old when he was first diagnosed with CLL in 1997. Although the median age of patients with CLL at diagnosis is close to 70 years, the number of patients diagnosed at a younger age is increasing as a result of the practice of routine CBC analysis. In our own series at the Postgraduate School of Hematology, Hospital Clínic, Barcelona, the proportion of patients under the age of 60 at diagnosis is 33% and has been increasing over the past few years.
The patient's age is important for designing therapy, particularly when intensive treatments such as hematopoietic stem cell transplants are considered. Currently, there is a tendency to increase the age limit for transplantation. Thus, allotransplants from unrelated donors are carried out in patients up to 50 years; allotransplants from a sibling donor in patients up to 60, and allotransplants with reduced intensity conditioning regimens (also known as non-myeloablative or “mini” transplants) are considered to be feasible in individuals up to 70; for autologous transplantation the upper age limit is 70 (European Bone Marrow Transplantation Group: Current practice of stem-cell transplants in Europe 2000. submitted). These age cut-offs are, however, somewhat arbitrary and the absence of important comorbidity and the presence of good performance status are as important as age. In our 55-year-old patient all types of transplantation could be a priori considered.
CLL has a highly variable clinical course, ranging from a very long to an extremely short survival. To decide therapy, therefore, prognosis has to be considered. Intensive treatments are justified in individuals in whom a short survival can be anticipated if conventionally treated.
What about the prognosis of this patient? Although at diagnosis he had early stage disease, which can run an indolent course for many years with no need for therapy, he progressed to a more advanced clinical stage 21 months after the diagnosis. Disease progression eventually occurs in about 50% of the patients in early stage and can be reasonably predicted by a number of prognostic parameters, both classical (e.g., Hb level ≤ 13 g/L; lymphocyte count > 30,000 per microliter; massive bone marrow infiltration; rapid doubling time) and new (e.g., serum levels of thymidine kinase, CD23 or β2-microglobulin, cytogenetics, IgV gene mutational status) (Montserrat E. Hematology Journal, in press). These tests were either not performed or not available when this patient was diagnosed in 1997.
What is the life expectancy of this patient at this point? In a recent update of the series from Barcelona (F. Bosch and J. Esteve. unpublished results), the overall survival of 210 individuals with CLL under the age of 60 conventionally treated is 12 years. However, the median survival of patients with intermediate-risk (Binet's stage B; Rai's stage I+II) is approximately 5 years, with only 30% of them projected to be alive at 10 years after diagnosis; patients with high-risk disease (Binet's stage C; Rai's stage III+IV) have a median survival inferior to 3 years and only 10% can expect to be alive at 10 years from diagnosis. Moreover, poor cytogenetic findings such as those present in this patient [i.e., del(11q)] and disease transformation (i.e., increasing number of prolymphocytes in peripheral blood) also imply poor prognosis. The life expectancy of the patient asking for our advice is, unfortunately, short: unless he achieves a complete and durable response his median median survival is inferior to 3 years. This information is important for treatment decisions and for weighing potential risks and benefits of treatment at this time.
With respect to treatment, the first step for this patient is to achieve the best possible response. To obtain this, the patient should be offered the option to enter one of the many trials in which combined therapies consisting of fludarabine and other cytotoxic agents or monoclonal antibodies are being investigated. Subsequently, either intensification therapy in the form of an autologous transplant or an allogeneic transplant should be considered. These procedures are discussed in the following sections. The interpretation of transplant results, however, requires caution because of the heterogeneity of the patients and treatments administered, differences in transplantation strategies and the retrospective nature of most studies.
Autologous stem-cell transplants1–12 are feasible until the age of 70 and are increasingly being performed in patients with CLL. Transplant-related mortality (TRM) varies from 4 to 19% in different series. With current standards, the TRM rate should be less than 10%. Careful selection of patients and appropriate management of the complications to which CLL patients are prone, particularly opportunistic infections, are critical.
An important problem with autologous transplants is that, in most instances, harvested stem cells are contaminated with residual CLL cells. This has prompted the investigation of different in vitro purging techniques in order to diminish the risk of relapse due to the reinfusion of tumor cells. Several methods, such as B-cell negative selection, CD34+ positive selection, or double selection (i.e., positive selection of CD34 cells followed by B-cell negative selection), are available. A benefit of purging has been observed in single center series13 but not in other studies.6,10,12 Purging may retard hematologic and immune recovery, which is of concern when considering the already depressed immune status in CLL patients. The potential advantages of purging should be investigated in randomized trials, with the theoretically ideal method to be investigated being negative B cell selection.
Campath-1H is an anti-CD52 monoclonal antibody that is highly effective in CLL. Of note, Campath-1H may improve the response achieved with chemotherapy, particularly in peripheral blood. Because of this, it has been successfully used as an in vivo purging agent.14 Rituximab, an anti-CD20 monoclonal antibody, has been reported to also be useful for the same purpose.15
Sensitivity of the disease to treatment is, as discussed later, essential for the success of the transplant. Intensive treatment may be necessary to achieve response. In heavily treated patients, collecting enough stem cells for transplantation can be a difficult, if not impossible, task. From the practical point of view, an interval of no less than three months should be left between the last treatment and stem cells harvesting, since a shorter interval has consistently been associated with a poor collection of hematopoietic stem cells, particularly in heavily pre-treated patients receiving fludarabine. On the other hand, in prospective studies from Kiel and the German CLL Study Group, advanced clinical stage (Binet's stage B/C) was found to be associated with poor response to rescue treatment and the impossibility of collecting enough progenitor cells for transplantation.8
Not surprisingly, patients with sensitive disease transplanted in CR have a much better outcome than the other patients.6,7,10–,12 The proportion of CR's after autologous transplantation is approximately 80% and the overall survival is between 50-80% at 4 years after transplantation. However, in most series a constant relapse pattern is observed, reaching 50% at 4 years post-transplant, with no plateau in survival curves.1,2,6,9–,12,16 The detection of MRD predicts clinical relapse.1,2,11,13 The use of sensitive, specific, and quantitative techniques to measure MRD should allow the investigation of the treatments (e.g., monoclonal antibodies) aimed at achieving MRD-negative status and its impact on the outcome of transplantation.17,18
Taken together, the high relapse rate and the absence of a plateau in disease-free survival curves suggest that autotransplants do not cure CLL. However, survival is likely to be improved in some subsets of patients, particularly those transplanted in CR without having received more than one therapeutic regimen before transplantation.1,2,6–,8,10,12
If the patient under discussion achieves response—particularly CR—with rescue treatment, an autologous transplantation should be considered an option. However, this patient has a number of features that have been correlated with poor results after autotransplantation. These parameters include advanced stage, prior therapy, elapsed time from diagnosis greater than 36 months, and adverse cytogenetic findings.1,2,6–,8,10,12 Although the patient's survival could be improved, the probability of a sustained control of the disease seems extremely unlikely. Furthermore, the toxicity of an autologous transplant could jeopardize the success of subsequent treatments that this patient would most likely require.
Given these facts, should this patient undergo an allogeneic transplantation?
Allogeneic stem-cell transplants1–,5,11 in CLL patients result in a TRM ranging from 25 to 50% which is mostly due to GVHD and other complications. The response rate is approximately 80%, although it may vary depending on the status of the disease at transplantation. In contrast with autologous transplants, in allotransplant series there is a survival plateau of 40-60%, the relapse rate being 10-25%. This suggests that a fraction of patients is cured. Moreover, allogeneic transplants can induce sustained complete responses in patients refractory to treatment. The reason is that, while the efficacy of autotransplantation relies entirely on the cytotoxic agents administered, the antileukemic effect in allogeneic transplantation is not only the result of the intensification therapy but also of a graft-versus-CLL effect. Facts supporting a graft-versus-CLL effect include the clearance of leukemic cells upon discontinuation of immunosuppressive drugs, when GVHD develops, and also when lymphocytes from the donor are infused to the patient.19–,21 Moreover, while in autologous transplantation the detection of MRD usually heralds clinical relapse, in allogeneic transplantation the detection of MRD is not incompatible with a sustained remission.11,13 In an update of our series, six of seven patients with detectable MRD after autologous transplantation have relapsed as compared to one of 11 patients MRD-negative. By contrast, none of the four patients MRD-positive after allogeneic transplantation has clinically progressed (Esteve J et al. ASH Annual Meeting, 2001). Parameters correlating with the outcome of allotransplantation in CLL have not been extensively studied. Nevertheless, the sensitivity of the disease to treatment, disease status before transplantation, younger age, performance status, use of peripheral blood as source of stem cells, normal cytogenetics, and prior therapy with fludarabine have been associated in some retrospective studies with a better outcome.1–,5,11
In most centers, TBI and cyclophosphamide are used as conditioning regimen. However, since the graft-versus-CLL effect seems to be crucial to eradicate the disease, the intensity of the conditioning regimen may not be as important as in other diseases, particularly in patients transplanted when in remission or very good partial remission. In this regard, allogeneic transplants using reduced intensity (non-myeloablative) conditioning regimens are being investigated in this form of leukemia.22– 24 In this modality of allogeneic transplantation, the preparative regimen (including in most cases fludarabine along with melphalan, low dose TBI, ATG or Campath-1H) is aimed not at eradicating the disease, but at providing sufficient immunosuppression to allow engraftment of allogeneic stem cells and development of a graft-versus-tumor effect. The hope is that this strategy will decrease the TRM and that the graft-versus-tumor effect will be sufficient to eliminate the leukemic cells, thus allowing safer transplantation and an increase in the upper age limit of transplantable patients.
Given the advanced age of most CLL patients, the high TRM associated with standard allotransplants, and the important role that the graft versus tumor effect plays in eradicating the disease, the use of non-myeloablative allotransplants is appealing. Preliminary results are encouraging although extremely immature. A meaningful analysis of the series reported is hampered by the heterogeneity of the patients and conditioning regimens used, as well as by a short follow-up. The TRM at one year is quite low (< 10 to 20%), yet the long-term mortality is not known because of the short follow up. For the same reason, the long term event-free survival and overall survival can not be determined. However, about 60-80% of the patients are reported to be alive and disease-free one year after transplantation. Not surprisingly, results are better in younger patients with chemosensitive disease. Of interest, Khouri et al found similar outcomes when comparing survival of CLL patients undergoing regular or non-myeloablative allotransplants, with survivals of 48% and 53%, respectively.23 In a retrospective analysis of 63 patients reported to the EBMT, the TRM at 1 year was 19%. With a median follow-up of 8 (1-30) months, the event-free survival and the overall survival at 1 year were 69% and 80%, respectively; unrelated donor, less than PR at transplant, and TBI as part of the conditioning regimen correlated with poor outcome (Dreger P et al. ASH Annual Meeting, 2001). Allotransplants with non-myeloablative regimens are conceptually interesting and studies in which carefully selected patients are treated according to strict rules are warranted. Randomized studies comparing regular with non-myeloablative allotransplants might be eventually warranted.
As far as our patient is concerned, an allogeneic transplant from his sibling donor is the procedure that would offer the only probability not only of a sustained control, but also cure, of his disease. The considerable risk of TRM would be more than outweighed by the potential benefits of the procedure The transplant should be performed in a highly experienced center and within the setting a controlled clinical trial. Whether the conditioning regimen should be standard or non-myeloablative is impossible to answer. Thus, while the risks and the benefits of regular allogeneic transplants are reasonably well known, the long term outcome of patients undergoing non-myeloablative allotransplants has yet to be established. To make a decision, the lower TRM with non-myeloablative allotransplants (<10 to 20% at one year) as compared with the 25 to 50% TRM with regular allotransplants, should be weighed against the still immature and uncertain long-term results with non-myeloablative transplants and the much more solid information regarding the outcome of standard allotransplants. In Table 4 some key-characteristics and results of different transplant modalities that can be considered in our patient are shown.
In summary, this 55-year-old patient, having been diagnosed with CLL almost 5 years earlier, presents to us with advanced disease as well as other poor risk features. The prognosis of this patient is dismal, and unless he achieves a complete and durable response, his life expectancy is probably inferior to 3 years. Therefore, an experimental treatment within a clinical trial setting is justified. As far as the different modalities of transplantation, the prospects for this patient of gaining a clear benefit from an autologous transplant are limited. On the other hand, if a standard allogeneic transplantation is envisaged, the risk of this patient dying as a result of procedure-related complications is extremely high, probably in the order of 40%, although he would have a definite probability of cure. On the other hand, an allogeneic transplantation with a non-myeloablative regimen, particularly if the disease is sensitive to treatment, could offer to this patient the possibility of a long-term control and even the cure of his disease with a more acceptable immediate mortality associated with the procedure. Unfortunately, there is not enough evidence to firmly recommend one modality of allotransplantation over the other.
The final treatment decision should be made following a careful discussion with the patient of the potential advantages and problems related to all possible therapies, not only transplants but also novel forms of chemotherapy and gene therapy, and taking into account all of the concerns and expectations of the patient.
Summary
Kanti R. Rai, MD
The case presented here and discussed by three consultants, each reviewing the pathobiology, clinical prognostic features and recommendations for therapy from the perspective of his own areas of expertise, provides us with an opportunity to critically examine the recent progress in CLL.
Prognostic Features
The first lesson we learn from this case is that, no matter how good the outlook may appear, we should use considerable restraint in making long-term prognostications when a patient has just been diagnosed to have CLL. The patient under discussion had, initially when the diagnosis was made, all the criteria that would be consistent with a long survival time and a relatively uneventful clinical course. He was free of symptoms, was in the low-risk category or Binet Stage A, with a relatively low absolute lymphocyte count of 14,400, normal hemoglobin and platelet counts of 142 g/L% and 210,000 per microliter, respectively, non-diffuse pattern of lymphocytic infiltration in bone marrow biopsy specimen, normal karyotype by conventional banding method and normal serum levels of β2-microglobulin and LDH. He even fits the definition of smoldering CLL. However, good prognostic features indicate a good prognosis for most patients and not for each and every patient. Therefore, after reassuring a patient that there does not seem to be any evidence that his life is in imminent danger, it is prudent to add that a more reliable statement concerning his long-term prognosis can only be made after the physician has had the opportunity to watch the clinical course of this particular patient over a period of time- usually lasting about a year. We learn of ominous developments rather quickly. Less than two years from the time of initial diagnosis, there is evidence of rapid progression of the disease. Even though the patient continues to remain free of disease-related symptoms, there is generalized enlargement of lymph nodes. Even though the rate of increase in blood lymphocyte count is not very rapid (it increased from 14,400 to 45,000 in 21 months; the estimated doubling time was about one year), there is a marked decrease in platelet count and a slight decrease in hemoglobin. The patient has progressed to Binet's B stage or into the intermediate-risk category. Even though LDH and β2-microglobulin levels continued to remain normal, cytogenetic studies have now been performed by the more reliable FISH technique and reveal complex chromosomal abnormalities that indicate a poor prognosis. The second lesson, therefore, is that one cannot rely on any single prognostic marker but should use several of them and only in conjunction with the patient's clinical picture. Finally, the third lesson is that exciting progress has been made recently in the laboratory that might translate in the foreseeable future to additional clinical tools for making accurate long-term prognostications in CLL patients at the time of initial diagnosis. Included in this category are cytogenetic studies using FISH techniques, studies of mutation status of IgV genes in the CLL lymphocytes and evaluation of the expression of CD38 on the leukemic monoclonal B-lymphocytes.
Therapeutics in CLL
The first lesson in therapeutics of CLL from this case is that although fludarabine is an effective drug for the front-line treatment of CLL patients with active disease, the chance of achieving a CR remains relatively low. The patient under review had eradication of all abnormal physical findings and total normalization of blood counts, but the bone marrow showed persistence of lymphoid nodules and flow cytometry showed persistence of clonal excess. Unless we are able to attain a CR in a large proportion of patients, we are unlikely to make an impact on the overall survival statistics of this disease. Therefore, future clinical trials ought to be directed towards increasing the incidence of CR in CLL. The second lesson is that even though the duration of remission after initial fludarabine therapy in this case was quite long, 30 months, we still lack an effective and proven therapy when the disease relapses. Campath-1H has been demonstrated to be an effective salvage therapy in nearly a third of patients relapsing after an initial response with fludarabine. A combination of fludarabine, Campath-1H and rituximab might be considered as a part of a clinical investigational study. A relatively young patient, such as the one under discussion who had a durable and good quality response following initial therapy with fludarabine, and who is not previously heavily treated, might also be an excellent candidate for high dose chemotherapy followed by in vivo purging with Camapth-1H or rituximab and followed thereafter with stem cells rescue. The source of stem cells could be autologous or an HLA compatible sibling. However, as has been already mentioned, none of these proposed therapeutic strategies should be considered as standard and clinically proven to be effective and ought to be undertaken only in a setting of prospectively planned investigational studies.
In conclusion, the panel is unanimous in its response to each of the three questions posed at the end of the case presentation. All available prognostic criteria strongly suggested an excellent overall outlook at the time of initial diagnosis and therefore a wait and watch policy without any therapeutic intervention was quite appropriate. If the same patient were to present himself today, the panel, however, recommends that cytogenetic studies be performed by FISH technique, status of CD38 expression on leukemic B-lymphocytes be evaluated and if a research lab collaboration is accessible, the status of IgV gene mutation also be tested. The panel seems to be in complete agreement on the choice of fludarabine for treatment of the patient when he showed evidence of disease progression. Finally, treatment possibilities at this time when the patient presents with disease-related symptoms, with the disease having progressed to an advanced stage, and all clinical and laboratory features indicate poor prognosis, the panel again agrees that no single well tested treatment is available, that the patient should be encouraged to enter an ongoing clinical trial either using Campath-1H either alone or with fludarabine and rituximab combination, or using high dose chemotherapy and stem cell rescue strategies.
Incidence of the major cytogenetic risk groups* in various studies.
| Karyotype . | Döhner et al. 2000 n=325 . | CLL1** n=230 . | CLL3*** n=113 . |
|---|---|---|---|
| * According to the hierarchical model of chromosome aberrations in CLL as proposed by Döhner et al. (2000)6 | |||
| ** CLL1 trial of the German CLL Study Group (GCLLSG) for Binet A patients | |||
| *** CLL3 trial of the GCLLSG evaluating high-dose therapy followed by autologous hematopoietic stem cell transplantation | |||
| 17p deletion | 23 (7%) | 14 (6%) | 4 (4%) |
| 11q deletion | 56 (17%) | 20 (9%) | 25 (22%) |
| trisomy 12 | 47 (14%) | 22 (10%) | 13 (11%) |
| normal karyotype | 57 (18%) | 52 (22%) | 21 (19%) |
| 13q deletion single | 117 (36%) | 99 (43%) | 35 (31%) |
| various aberrations | 25 (8%) | 23 (10%) | 15 (13%) |
| Karyotype . | Döhner et al. 2000 n=325 . | CLL1** n=230 . | CLL3*** n=113 . |
|---|---|---|---|
| * According to the hierarchical model of chromosome aberrations in CLL as proposed by Döhner et al. (2000)6 | |||
| ** CLL1 trial of the German CLL Study Group (GCLLSG) for Binet A patients | |||
| *** CLL3 trial of the GCLLSG evaluating high-dose therapy followed by autologous hematopoietic stem cell transplantation | |||
| 17p deletion | 23 (7%) | 14 (6%) | 4 (4%) |
| 11q deletion | 56 (17%) | 20 (9%) | 25 (22%) |
| trisomy 12 | 47 (14%) | 22 (10%) | 13 (11%) |
| normal karyotype | 57 (18%) | 52 (22%) | 21 (19%) |
| 13q deletion single | 117 (36%) | 99 (43%) | 35 (31%) |
| various aberrations | 25 (8%) | 23 (10%) | 15 (13%) |
Correlation of the IgV mutation status with genomic aberrations in 211 chronic lymphocytic leukemia (CLL) patients.
| Karyotype . | IgV mutated n=88 . | IgV unmutated n=123 . | P-value . |
|---|---|---|---|
| aberrant karyotype | 78% | 83% | n.s. |
| normal karyotype | 22% | 27% | n.s. |
| 13q deletion | 63% | 46% | =.017 |
| 13q deletion single | 49% | 21% | <.0001 |
| trisomy 12 | 13% | 17% | n.s. |
| 11q deletion | 2% | 29% | <.0001 |
| 17p deletion | 2% | 13% | <.01 |
| Karyotype . | IgV mutated n=88 . | IgV unmutated n=123 . | P-value . |
|---|---|---|---|
| aberrant karyotype | 78% | 83% | n.s. |
| normal karyotype | 22% | 27% | n.s. |
| 13q deletion | 63% | 46% | =.017 |
| 13q deletion single | 49% | 21% | <.0001 |
| trisomy 12 | 13% | 17% | n.s. |
| 11q deletion | 2% | 29% | <.0001 |
| 17p deletion | 2% | 13% | <.01 |
Response to salvage therapy in patients retreated after failing or relapsing from fludarabine regimens (data from 27).
| . | Fludarabine Retreatment . | Other Treatment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Response . | CR . | PR . | Total Pts . | % . | CR . | PR . | Total Pts . | % . |
| Overall response | 13 | 28 | 63 | 67 | 4 | 3 | 28 | 25 |
| Complete remission | 10 | 8 | 25 | 72 | 1 | 0 | 4 | 25 |
| Nodular partial remission | 3 | 13 | 23 | 64 | 1 | 1 | 7 | 28 |
| Partial remission | 0 | 7 | 11 | 70 | 0 | 0 | 5 | — |
| Fail 0 | 0 | 4 | — | 2 | 2 | 12 | 33 | |
| . | Fludarabine Retreatment . | Other Treatment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Response . | CR . | PR . | Total Pts . | % . | CR . | PR . | Total Pts . | % . |
| Overall response | 13 | 28 | 63 | 67 | 4 | 3 | 28 | 25 |
| Complete remission | 10 | 8 | 25 | 72 | 1 | 0 | 4 | 25 |
| Nodular partial remission | 3 | 13 | 23 | 64 | 1 | 1 | 7 | 28 |
| Partial remission | 0 | 7 | 11 | 70 | 0 | 0 | 5 | — |
| Fail 0 | 0 | 4 | — | 2 | 2 | 12 | 33 | |
| Regimen . | CR . | PR . | Total Pts . | % . |
|---|---|---|---|---|
| Abbreviations: CR, complete response; PR, partial response | ||||
| Fludarabine (3 or 5 days) | 8 | 18 | 35 | 74 |
| Fludarabine + Mitoxantrone | 0 | 3 | 9 | 33 |
| Fludarabine + Doxorubicin + Prednisone (FLAP) | 1 | 0 | 4 | 25 |
| Fludarabine + Cytosine Arabinoside + Cis-platinum | 0 | 0 | 2 | 0 |
| Fludarabine + Cyclophosphamide | 4 | 7 | 13 | 85 |
| 2-CDA | 0 | 1 | 2 | 50 |
| Cyclophosphamide + Doxorubicin + Vincristine + Prednisone (CHOP) | 2 | 2 | 7 | 57 |
| Chlorambucil | 0 | 2 | — | |
| Allogeneic bone marrow transplant | 2 | 0 | 4 | 50 |
| VP-16 | 0 | 3 | — | |
| Taxol | 0 | 2 | — | |
| Adriamycin + Cytosine Arabinoside + Cis-platinum (ASHAP) | 0 | 1 | — | |
| Topetecan | 0 | 7 | — | |
| Regimen . | CR . | PR . | Total Pts . | % . |
|---|---|---|---|---|
| Abbreviations: CR, complete response; PR, partial response | ||||
| Fludarabine (3 or 5 days) | 8 | 18 | 35 | 74 |
| Fludarabine + Mitoxantrone | 0 | 3 | 9 | 33 |
| Fludarabine + Doxorubicin + Prednisone (FLAP) | 1 | 0 | 4 | 25 |
| Fludarabine + Cytosine Arabinoside + Cis-platinum | 0 | 0 | 2 | 0 |
| Fludarabine + Cyclophosphamide | 4 | 7 | 13 | 85 |
| 2-CDA | 0 | 1 | 2 | 50 |
| Cyclophosphamide + Doxorubicin + Vincristine + Prednisone (CHOP) | 2 | 2 | 7 | 57 |
| Chlorambucil | 0 | 2 | — | |
| Allogeneic bone marrow transplant | 2 | 0 | 4 | 50 |
| VP-16 | 0 | 3 | — | |
| Taxol | 0 | 2 | — | |
| Adriamycin + Cytosine Arabinoside + Cis-platinum (ASHAP) | 0 | 1 | — | |
| Topetecan | 0 | 7 | — | |
Hematopoietic stem-cell transplants in chronic lymphocytic leukemia.
| . | Autologous . | Allogeneic . | NMA-Allogeneic . |
|---|---|---|---|
| (1)Indicates that chemosensitivity of the disease is a necessary condition for the success of the procedure | |||
| Abbreviations: NMA-Allogeneic, Non-myeloablative allogeneic transplants; GVL, graft-versus-leukemia; TRM, transplant related mortality; RR, relapse rate; OS, overall survival | |||
| Age limit (yrs) | 70 | 50-60 | 60-70 |
| Need for response (1) | yes | no | no? |
| Antitumor effect | cytotoxic agents | cytotoxic + GVL | GVL |
| TRM | 10% | 25-50% | <10-20% (at 1 yr) |
| RR (4 yrs) | 50% | 10-25% | 10-20% (at 1 yr) |
| OS (4 yrs) | 40-70% | 40-60% | ? (60-80% at 1 yr) |
| Plateau | no | yes | ? |
| . | Autologous . | Allogeneic . | NMA-Allogeneic . |
|---|---|---|---|
| (1)Indicates that chemosensitivity of the disease is a necessary condition for the success of the procedure | |||
| Abbreviations: NMA-Allogeneic, Non-myeloablative allogeneic transplants; GVL, graft-versus-leukemia; TRM, transplant related mortality; RR, relapse rate; OS, overall survival | |||
| Age limit (yrs) | 70 | 50-60 | 60-70 |
| Need for response (1) | yes | no | no? |
| Antitumor effect | cytotoxic agents | cytotoxic + GVL | GVL |
| TRM | 10% | 25-50% | <10-20% (at 1 yr) |
| RR (4 yrs) | 50% | 10-25% | 10-20% (at 1 yr) |
| OS (4 yrs) | 40-70% | 40-60% | ? (60-80% at 1 yr) |
| Plateau | no | yes | ? |
Prognostic significance of the IgV mutation status in 211 chronic lymphocytic leukemia (CLL) patients.
Prognostic significance of the IgV mutation status in 211 chronic lymphocytic leukemia (CLL) patients.
Prognostic significance of the IgV mutation status in 131 chronic lymphocytic leukemia (CLL) patients with Binet stage A disease.
Prognostic significance of the IgV mutation status in 131 chronic lymphocytic leukemia (CLL) patients with Binet stage A disease.
Prognostic significance of the IgV mutation status and of the high-risk genomic aberrations 11q and 17p deletion in 211 chronic lymphocytic leukemia (CLL) patients. Among the 123 patients with unmutated IgV genes, those patients with high-risk genomic aberrations (n = 48) had a significantly inferior survival compared to the patients not exhibiting 11q- or 17p- (n = 75).
Prognostic significance of the IgV mutation status and of the high-risk genomic aberrations 11q and 17p deletion in 211 chronic lymphocytic leukemia (CLL) patients. Among the 123 patients with unmutated IgV genes, those patients with high-risk genomic aberrations (n = 48) had a significantly inferior survival compared to the patients not exhibiting 11q- or 17p- (n = 75).
Time to progression by response to treatment with fludarabine +/- prednisone in previously untreated CLL patients whose age was less than 56 who were treated between 1986 and 1999.
Time to progression by response to treatment with fludarabine +/- prednisone in previously untreated CLL patients whose age was less than 56 who were treated between 1986 and 1999.
Survival by response to treatment in previously untreated CLL patients whose age was less than 56 who were treated between 1986 and 1999.
Survival by response to treatment in previously untreated CLL patients whose age was less than 56 who were treated between 1986 and 1999.
Time to progression by residual bone marrow CD19+5% in previously untreated CLL patients whose age was less than 56 who were treated between 1986 and 1999.
Time to progression by residual bone marrow CD19+5% in previously untreated CLL patients whose age was less than 56 who were treated between 1986 and 1999.