Abstract
This review covers the diagnosis and management of natural killer and peripheral T-cell lymphomas (PTCL). Problems with PTCL include their rarity, representing usually 10-15% of non-Hodgkin's lymphomas in the Western Hemisphere, morphologic heterogeneity, and lack of immunophenotypic markers for clonality. Additionally, their clinical behavior is variable and may not correlate with morphology.
Dr. Kinney gives a general overview of the diagnosis of PTCL and NK cell neoplasms. Emphasis will be placed on extranodal T cell and natural killer (NK) cell lymphomas such as hepatosplenic lymphoma, subcutaneous panniculitis-like lymphoma and nasal/nasal type T/NK-cell lymphoma. The use of ALK gene regulation in the classification of anaplastic large cell lymphoma is also reviewed.
Dr. Loughran describes current understanding of the pathogenesis of large granular lymphocyte (LGL) leukemia. The discussion focuses on LGL leukemia as an instructive model of dysregulated apoptosis causing both malignant and autoimmune disease. Current management options and mechanisms of therapeutic response are also described.
Dr. Greer addresses whether PTCL should be treated differently from the more common diffuse large B cell lymphomas. He discusses the therapeutic options for anaplastic large cell lymphoma (ALCL), from a conservative approach for primary cutaneous ALCL to combination chemotherapy for the highly chemosensitive ALCL expressing anaplastic lymphoma kinase. He reviews therapy options for the extranodal subtypes of PTCL by drawing from series in adults, pediatrics, dermatology, and the Far East.
I. Extranodal T-Cell and NK-Cell Neoplasms—Diagnostic Challenges
Marsha C. Kinney, MD**
Vanderbilt University, Department of Pathology, 1301 22nd Ave S, Room 4605 TVC, Nashville TN 37232-5310
Similar to earlier functional classification systems (Lukes-Collins and Kiel), the Revised European American Lymphoma (REAL) and new World Health Organization (WHO) classifications have divided lymphomas into B and T cell types and whether they are composed of precursor (thymic or lymphoblastic) or peripheral (mature or post thymic) lymphocytes.1,2 More importantly, these new classification systems have aimed to recognize specific clinicopathologic entities and not just be biologically correct. Nowhere is this more evident than in the recognition of specific extranodal lymphomas. The only extranodal lymphoma listed in early lymphoma classifications was mycosis fungoides/Sézary syndrome. Most extranodal lymphomas were lumped together with nodal non-Hodgkin's lymphomas. The REAL and WHO classifications have emphasized that extranodal lymphomas are intrinsically different from their nodal counterparts and have integrated clinical features (particularly site of disease) with more traditional pathologic criteria in defining these tumors. In addition natural killer (NK)-cell neoplasms have been recognized and are now formally included into the classification of lymphoid neoplasms. Post thymic T-cell and NK-cell neoplasms included in the REAL and WHO classifications are listed in Table 1 .
One third of T-cell lymphomas, and virtually all of NK-cell tumors, arise at extranodal sites where they are more difficult to diagnose and can be confused with reactive processes. Despite these difficulties extranodal T-cell and NK-cell lymphomas share common features that help in their recognition.3 Clinically they spread to or relapse at extranodal sites, and nodal disease is uncommon. The site of disease is important in disease definition. Pathologically there is a broad cytologic spectrum with variability from pleomorphic mixed small, medium, or large cells to large cell predominant. The immunophenotype in most cases is not specific but most have a cytotoxic T-cell or NK-cell phenotype with frequent apoptosis and/or necrosis.
After a brief basic review of T-cell and NK-cell biology, this discussion will focus on the salient pathologic features of hepatosplenic T cell lymphoma and subcutaneous panniculitis-like T cell lymphoma, discuss the use of ALK gene expression in defining anaplastic large cell lymphoma (ALCL) and the diagnosis of primary cutaneous ALCL, and finally review nasal and nasal type NK/T-cell lymphoma and current concepts of NK-cell tumor classification.
T Cells and NK Cells: Biology, Immunophenotype, and Distinguishing Features
T cells and NK cells are distinguished by immunophenotype and molecular genetic studies although there is overlap in T-cell and NK-cell antigen expression, function, and patterns of disease. The ontogeny from naive to effector T-cell is largely obscure. Most T cells express the αβ TCR (T cell receptor) protein and have a helper (CD4+) or suppressor/cytotoxic (CD8+) phenotype. A small population of T cells expresses the γδ TCR and usually has a double negative (CD4-CD8-) phenotype, although some may express CD8 or more rarely, CD4. Normal γδ T cells are preferentially located at extranodal sites such as the splenic red pulp, gastrointestinal tract, and skin.
NK-cells arise from a pluripotential stem cell and are related to T-cells, but at some point branch off to form a separate lineage.4 NK-cells are distinguished immunologically by the absence of TCR gene rearrangements and TCR protein and lack of surface CD3 (Leu 4) and usually CD5 (Table 2 ). NK-cells and T-cells can express CD2, CD7, and in paraffin sections cytoplasmic CD3 (the epsilon chain), CD45RO and CD43. NK-cells usually express one or more “NK-associated” antigens (CD16, CD56, CD57), but some populations of NK-cells do not express these antigens and are identified by functional studies. A subset of cytotoxic T-cells also expresses NK associated antigens and have been called “NK-like” T-cells (Table 2).5
Most extranodal lymphomas arise from cytotoxic cells which contain cytotoxic granule proteins such as TIA-1, granzyme B and perforin. Three categories of cytotoxic cells can be identified by immunologic and molecular studies (Table 2).
Although not specific, most extranodal T-cell and NK-cell neoplasms have a characteristic immunophenotype with a propensity to involve certain cell populations such that one phenotype predominates but is not exclusive (Table 3 ).
Specific Neoplasms and Diagnostic Issues
Hepatosplenic T-cell lymphoma
Hepatosplenic lymphoma is an uncommon but distinct T-cell lymphoma with a characteristic growth pattern involving the hepatic and splenic red pulp sinuses.7–,13 The tumor cells are homogeneous, medium-sized lymphocytes with partially dispersed chromatin, inconspicuous nucleoli and generally little cytologic atypia (Figure 1; see color page 547). Circulating tumor cells are present in the blood in 25-50% or more of patients during the course of disease. On Wright's stain the cells have partially condensed chromatin and pale blue cytoplasm. The presence of cytoplasmic granules is variable, but most report the cells are agranular. Marrow involvement is present in approximately two-thirds of cases but is usually subtle involving the sinuses. Immunoperoxidase staining for T-cell antigens is important to aid in recognition.9,12 Most tumors have a γδ T-cell phenotype; tumors arising from αβ T-cells are clinically and pathologically similar.13 γδ T-cells are normally the first line of defense at epidermal and epithelial surfaces and they represent 10-12% of lymphocytes in the spleen.14,15 Hepatosplenic lymphoma arises from an CD4 CD8 T cell or, in 15%, a CD4 CD8+ T-cell. The NK-cell associated antigen CD56 is expressed in 60-70% of cases, so many of these cases have an NK-like T-cell phenotype. TIA-1 is present in virtually all cases, but approximately 50% lack granzyme B and or perforin, indicating a non-activated cytolytic T-cell phenotype. Hepatosplenic lymphoma has a recurrent chromosome abnormality, isochromosome 7q, that appears to be the primary genetic event in its pathogenesis.10,12,16–,17 Approximately 20% arise in immunosuppressed, predominantly posttransplant patients.5,18– 19
The differential diagnosis of hepatosplenic lymphoma includes large granular lymphocyte (LGL) leukemia which usually occurs in older patients often with a history of rheumatologic disease, is indolent, and most often arises from CD57+ rather than CD56+ T-cells.20,21 Aggressive NK-cell leukemia may present with hepatosplenomegaly but morphologically the tumor has a more blastic cytology and expresses all cytolytic proteins whereas hepatosplenic lymphoma usually expresses TIA-1 only and lacks granzyme B and perforin.22–,27 CD8+ T-cell CLL is a rare disease that has more marrow involvement than hepatosplenic lymphoma and it lacks cytolytic granules and NK-cell associated antigens.28
Subcutaneous panniculitis-like T-cell lymphoma
Subcutaneous panniculitis-like (SCPTCL) lymphoma is an uncommon T-cell lymphoma localized to the subcutaneous tissue.29– 31 The median age of patients is 43 years with a broad range (1-84 years). Females are affected more often than males. Multiple, or less often solitary, erythematous to violaceous, deep-seated 0.5-12 cm nodules or plaques are present on the legs>arms>trunk>face. Ulceration and extracutaneous disease are uncommon.
SCPTCL produces a lobular panniculitis with little involvement of the reticular dermis and sparing of the papillary dermis (Figure 2a, 2b; see color page 547). The lymphocytes are pleomorphic varying in size from small to medium and large with dysplastic folded nuclei. The lymphocytes rim the fat spaces producing a lacelike pattern of infiltration and infiltrate small venules, but angiodestructive lesions are not present. Necrosis of fat and/or connective tissue septae is seen in all cases but may be focal. Variable numbers of benign histiocytes are present and exhibit phagocytosis of red blood cells or nuclear debris. Some patients have a systemic hemophagocytic syndrome (HPS) and erythrophagocytosis is seen in the bone marrow, liver or spleen, but tumor is generally not present. Most cases express the αβ T-cell receptor protein but approximately 25% have a γδ phenotype.30,32– 33 The T cells are CD8+>CD4CD8 (seen in the γδ phenotype) >CD4+ and are TIA-1+. Most SCPTCL are CD56, although SCPTCL with a γδ phenotype is more likely to be CD56+ and have a more aggressive clinical course.
The differential diagnosis of SCPTCL includes lupus profundus, eythema nodosum, erythema induratum and a controversial entity—cytophagic histiocytic panniculitis (CHP).34–,38 CHP is thought to represent a reactive proliferation of cytophagic histiocytes as a result of an abnormal or exaggerated T-cell immune response. The relationship of CHP to SCPTCL is not clear. SCPTCL with a protracted CHP-like phase has been described.39 SCPTCL and CHP may represent a continuum with CHP being a “smoldering” lymphoid neoplasm rather than an inflammatory disease,37,38 or CHP and SCPTCL may be different diseases.40 Distinguishing SCPTCL and CHP is difficult. If cytophagocytosis is present with some necrosis and lack of definite cytologic dysplasia in the lymphocytes, CHP should be considered. If systemic symptoms are present, a bone marrow should be performed and examined for erythrophagocytosis. If present this likely has a bad prognosis despite lack of evidence of dysplasia.
Anaplastic Large Cell Lymphoma
ALCL was described in 1985 by Stein et al as a pleomorphic large cell lymphoma with strong membrane and Golgi associated CD30 expression in virtually every cell and prominent involvement of nodal sinuses.41 Prior to that time many cases of ALCL were misdiagnosed as metastatic carcinoma, melanoma, or malignant histiocytosis due to the anaplastic appearance and sinus pattern of infiltration.
In the years that followed the initial description of ALCL it became apparent that the clinical and pathologic features of ALCL were heterogeneous.42,43 The age distribution was bimodal with young and old patients. 43 The various clinical presentations included a systemic form with nodal and/or extranodal involvement, primary cutaneous disease, HIV-related ALCL, and ALCL occurring as a secondary event in patients with lymphomatoid papulosis, mycosis fungoides, and rarely Hodgkin's disease (HD). The pathologic characteristics of ALCL were no less variable than the clinical. The histology varied from pleomorphic to monomorphic;44,45 small cell predominant,46 lymphohistiocytic;47 Hodgkin's-like (HD-related)48 or rarely sarcomatoid49 or neutrophil-rich.50 Approximately 70-80% of cases had a T or null cell phenotype and 60-70% were epithelial membrane antigen (EMA)+, but approximately 20% of CD30+ lymphomas were B cell. This heterogeneity in ALCL and the nonspecificity of CD30 expression led to controversy as to whether ALCL was a specific entity.
In the late 1980s the association of ALCL with a characteristic translocation, t(2;5)(p23;q35), was recognized.51–,52 In 1994 the t(2;5) was cloned by Morris et al, and a new gene ALK (anaplastic lymphoma kinase), important in the pathogenesis of most ALCL, was cloned.53–,54 As a result of the t(2;5) ALK on 2p23 is fused to the strong nucleophosmin (NPM) promoter on 5q35 and the ALK protein, not normally expressed in lymphoid tissue, is present. The NPM-ALK fusion protein can be detected immunohistochemically using antibodies (ALK1 and p80NPM/ALK) against the ALK protein.55– 56
A large number of the various clinical and pathologic types of ALCL were subsequently examined, and ALK expression was present in 30-60% of cases overall (range13-92%).42,57– 58 The ALK+ cases formed a distinct clinicopathologic entity (Table 4 ) characterized by young patient age, systemic disease, a morphologic spectrum varying from small (small cell and lymphohistiocytic variants) to large cell predominant (pleomorphic and monomophic) (approximately 80% of monomorphic, 75-100% of small cell, and 30% of pleomorphic histologies are ALK+), and a T or null cell immunophenotype with frequent expression of EMA and cytolytic proteins. The ALK-negative group was heterogeneous and included primary cutaneous ALCL, HD related ALCL, large CD30+ B cell lymphomas with anaplastic or non-anaplastic morphology, CD30+ large cell lymphoma in HIV+ patients, secondary ALCL arising in patients with lymphomatoid papulosis (LyP), mycosis fungoides, and HD.
Primary cutaneous anaplastic large cell lymphoma
Primary cutaneous ALCL is an indolent CD30+ lymphoma usually treated with local therapy. It must be distinguished from systemic ALCL as the latter is an aggressive disease which requires multiagent systemic chemotherapy and from LyP which spontaneously regresses. The definition of “primary” cutaneous disease is somewhat controversial but generally is defined as skin disease without evidence of systemic involvement and no previous history of lymphomatoid papulosis, mycosis fungoides, or HD. Cases with cutaneous disease and nodal involvement limited to a regional draining node are problematic, and it is uncertain if they have a different prognosis or should be treated as primary cutaneous disease.59
The histologic features of primary cutaneous ALCL include sheets or large clusters of tumor cells in the dermis that usually extend into the subcutaneous tissue. The tumor cells in most cases are anaplastic rather than monomorphic. Vascular invasion, but not destruction, is often present.60 Neutrophils and/or eosinophils may be prominent and one group has suggested that large numbers of eosinophils may be predictive of subsequent lymph node involvement.50,59,61 Cases previously called regressing atypical histiocytosis are now thought to represent ALCL.61
The diagnosis of primary cutaneous ALCL may be difficult as there can be overlap with lymphomatoid papulosis, a regressing lymphoproliferative disorder.59,62,63 LyP has three histologic appearances.59 Type A is composed of lymphocytes, other inflammatory cells and large, atypical cells that are CD30+ and oftentimes Reed-Sternberg-like. LyP type B has a band-like infiltrate with epidermotropism and atypical small to medium sized cerebriform lymphocytes simulating classical plaque stage mycosis fungoides. LyP type C has a monotonous population or large clusters of CD30+ T cells with few admixed inflammatory cells and a clinical history of regression. LyP types A and C can be distinguished from ALCL, in most cases by clinical and pathologic features as summarized in Tables 5 and 6 .
ALCL produces nodules or tumors that are single, but may be multiple and grouped, and are often ulcerated. (Table 5; Figure 3a, see color page 548). LyP typically presents with multiple small papular lesions, usually < 1 cm, on the trunk or extremities that usually ulcerate and heal with a scar in 4-6 weeks (Figure 3b). Histologically the infiltrate of LyP is wedge-shaped and perivascular and is composed of small lymphocytes and scattered single and small clusters of large atypical cells often resembling Reed-Sternberg cells. Invasion of the subcutaneous tissue is uncommon. As the lesions progress acute inflammatory cells including eosinophils become more prominent. The large atypical cells mark as T-cells and EMA may be present in up to approximately one-third of LyP. Borderline lesions (sometimes called LyP type C) typically are 1-2 cm in size and have larger clusters of CD30+ cells than typically seen in LyP.64 Invasion of the subcutaneous tissue is minimal or absent. If a history of similar regressing lesions is present LyP should be favored.
Finally, the importance of ruling out systemic disease with cutaneous spread cannot be overemphasized as systemic ALCL with cutaneous spread has a 5-year survival of 29-44% as opposed to 90-100% in primary cutaneous ALCL.65 This poor prognosis in systemic disease with skin involvement may correlate with ALK negativity but has not been studied in detail. Due to the poor prognosis, careful staging is imperative. Immunostaining for ALK should be performed and if positive the lesion should be considered systemic ALCL.66 Monomorphic histology also suggests systemic disease. A recent study by Wellman et al has recently shown that all systemic ALCL expressed the adhesion molecule clusterin, and none of the primary cutaneous ALCL, but few cases were tested.67 EMA expression is not useful as it has been reported in 54% of simultaneous cutaneous and systemic ALCL and 100% of secondary skin involvement and up to one-third of primary cutaneous ALCL.68 Even after a diagnosis of primary cutaneous ALCL is made, careful followup is indicated as 25% later develop nodal involvement.61
NK Cell Neoplasms—An Overview
Similar to T-cell neoplasms, NK-cell neoplasms can be subclassified according to their state of maturation and clinical characteristics of the tumor (Table 7 ).69– 70 Common characteristics that distinguish immature and mature NK cell neoplasms are listed in Table 8 .
NK-cell neoplasms overall are rare, the most common and well characterized being the nasal and nasal type NK-cell neoplasms.69–,80 The characteristic histologic feature is an angiocentric/angiodestructive growth pattern with zonal necrosis. These tumors have a predilection for the nasal cavity and paranasal sinuses and were formerly called lethal midline granuloma or polymorphic reticulosis. “Nasal-type” lymphomas show the same histologic features but arise from extranasal sites such as the skin, gastrointestinal tract, testis, kidney and upper respiratory tract, and rarely the eye/orbit.81–,86 Many nasal type NK/T-cell lymphomas arise in the skin. The cutaneous nasal type NK/T-cell lymphomas occur more often in females, have a higher frequency of a T-cell rather than a NK-cell phenotype and less association with EBV.82–,83 Approximately 10-20% of nasal lymphomas also have skin involvement. Although the cell of origin is often a NK cell, particularly in patients in the Far East, central and South America and in native Americans, some cases arise from cytolytic T cells (NK-like T cells that express CD56 and TIA-1). Hence, the designation nasal and nasal type NK/T-cell lymphoma. It should be noted that 35-55% of nasal tumors overall have a B cell phenotype.78,80 Lymphomas involving the sinuses without nasal lymphoma are predominantly diffuse large B cell lymphomas, whereas lymphomas involving the nasal cavity alone are predominantly NK/T-cell lymphomas.78
Tumor cells in nasal/nasal type NK/T-cell lymphoma can be a mixture of small, medium or large cells, but most are large dysplastic cells with hyperchromatic or vesicular chromatin (Figure 4a, 4b; see color page 548). Admixed inflammatory cells may be numerous. Zonal necrosis is prominent. Angiodestructive lesions are present in most (> 60%) but not all cases. Approximately 80% of cases arise from true NK cells; 10-30% are T cell with γδ > αβ type. 75–,77 EBV is present in the majority (∼80-100%) of nasal NK/T-cell lymphomas and less often in nasal type NK/T-cell lymphomas (15-40% or more in some series).87– 90
The other less common mature NK cell neoplasms include the indolent large granular lymphocyte (LGL) leukemias in which a small percentage arise from true NK cells21,71–,72 and aggressive NK-cell leukemia. The former will be discussed in Section II. Aggressive NK-cell leukemia occurs in young to middle age adults and rarely children.22–,27 Patients have systemic disease with generalized adenopathy, hepatosplenomegaly, and atypical LGLs (some having a blastic appearance) in the blood. The extent of marrow involvement tends to be patchy or minimal compared to other leukemias. The tumor cells vary from mature-appearing LGLs to cells with fine chromatin and more prominent nucleoli. These are very aggressive tumors with median survival of less than 2 months. There is variable association with EBV.25,70
Immature NK-cell neoplasms are very rare and include myeloid/NK cell precursor acute leukemia and blastic NK-cell lymphoma.70,91 Myeloid /NK-cell precursor acute leukemias are poorly defined and likely arise from an early multipotential myeloid/NK cell. Extramedullary disease is more prominent than usually seen in AML with nodal disease in 87%, mediastinal involvement in 20% and skin involvement in approximately 10%. The tumor cells have a lymphoblastic appearance (L2 morphology) and are agranular. The immunophenotype is CD2+, surface CD3-, CD4+/-, CD5-/+, CD7+, CD11b+, CDllc+, CD13+ and/or CD33+, myeloperoxidase-/+, CD56+, CD16-, CD57-, CD34+.70 EBV has not been detected. Care should be taken in making a diagnosis of myeloid/NK-cell precursor acute leukemia as 13-41% of acute myeloid leukemias can express CD56.92– 93
Blastic NK-cell lymphoma occurs in older patients (median 52 yrs) who present with multiple pigmented or erythematous plaques (60-70%).70,94– 95 Marrow involvement is frequent (50-75%) and some progress to overt leukemia. Tumor cells have a blastic appearance and involve the superficial and deep dermis. Subcutaneous infiltration is also present but has a diffuse pattern rather than the lobular pattern seen in subcutaneous panniculitis-like T-cell lymphoma. There is no evidence of angioinvasion/angiodestruction and necrosis. Cytoplasmic granules are absent or inconspicuous in 75%. The immunophenotype is CD2+/-, surface CD3-, CD4+, CD5-/+, CD7-/+, CD33-/+ (weak), CD56+, CD57, TdT+/-, TIA-1-/+, CD34-/+. EBV is negative. The clinical course is aggressive.
In summary, NK-cell neoplasms are uncommon and difficult to diagnose. Blastic NK-cell lymphoma, aggressive NK-cell leukemia, and myeloid/NK-cell precursor leukemia in particular are rare tumors with considerable overlap. Table 9 summarizes some of the characteristic clinical and pathologic features of these neoplasms that are useful in distinguishing them.
II. Pathogenesis of Large Granular Lymphocyte Leukemia
Thomas P. Loughran, Jr., MD*
H. Lee Moffitt Cancer Center, 12902 Magnolia Dr., Suite 3157, Tampa FL 33612
Large granular lymphocytes (LGL) comprise 10-15% of normal peripheral blood mononuclear cells.1 They are characterized morphologically by a size bigger than normal lymphocytes and azurophilic granules in the cytoplasm (Figure 5; see color548). LGL can be divided into two major lineages: CD3-negative and CD3+. Most LGL in the peripheral blood of normal individuals represent NK cells. These LGL are CD3-negative, therefore lacking the CD3/TCR complex, and mediate non-MHC-restricted killing.2 In contrast, CD3+ LGL are T cells that do express the CD3/TCR complex and rearrange TCR genes.3 These cells mediate non-MHC-restricted cytotoxicity in vitro and are thought to represent in vivo activated cytotoxic T cells (CTL).4
Clonal diseases of LGL can arise from either of their normal counterparts and thus be of NK or T cell lineage.5 A syndrome of increased numbers of circulating LGL associated with chronic neutropenia was described in 1977.6 A major question in this disorder was whether the lymphocytosis was reactive or neoplastic in nature. In 1985 we first detected clonal chromosomal abnormalities in such patients.7 We termed this disease LGL leukemia based on this observation of clonality and demonstration of tissue invasion by LGL of marrow, spleen, and liver. In 1993 we proposed the classification of LGL leukemia into NK- and T-LGL leukemia, for clonal LGL diseases of NK cell and T cell origin, respectively.5 The REAL classification recommended that LGL leukemia be a distinct entity classified in the peripheral T cell and NK cell neoplasms.8 This review will discuss our current understanding of the T-cell form of LGL leukemia.
Clinical Features
The diagnosis of LGL leukemia is established by documenting increased numbers of circulating, clonal CD3+ LGL. We have defined increased LGL counts as being more than 3 standard deviations above the number of T cells coexpressing CD3 and CD57, an LGL marker. In our laboratory, the normal values for CD3+, CD57+ cells are 22 ± 99/μl,9 so we consider values above approximately 520/μl as being abnormal. Clonality is best determined by utilization of T cell receptor gene rearrangement studies, either using Southern blotting or PCR. It has been recognized that patients may have a clonal T cell population with even normal LGL counts, and such cases would be compatible with the diagnosis of LGL leukemia.10 Since the total lymphocyte count may not be elevated, it is recommended that the peripheral blood smear be carefully evaluated for LGL in patients with hematologic and/or autoimmune abnormalities suggestive of LGL leukemia (see below). Indeed we also recommend flow cytometry determination for LGL phenotype in such cases.
The clinical features of this illness are hematologic and autoimmune in nature. The median age of onset is about 55 years of age. Pediatric cases have been recognized. A variety of hematologic abnormalities may occur including: chronic neutropenia or anemia, cyclic neutropenia, pure red cell aplasia, autoimmune hemolytic anemia, ITP, aplastic anemia, and MDS.5,10–,12 The usual clinical problem is either neutropenia or anemia. Mechanisms of cytopenia are not clearly defined. Inhibition of erythropoiesis in patients with pure red cell aplasia appears to be mediated directly by leukemic LGL. In one patient with LGL of γδ phenotype, it was demonstrated that leukemic LGL expressing killer receptors were cytotoxic for red cell progenitors lacking HLA class I.13 As there may be clinical overlap with LGL leukemia, MDS, and aplastic anemia, it is conceivable that hematologic suppression in these diseases occurs through a similar pathway involving activated CD8+ T cells producing inhibitors belonging to the TNF family. For example, we have shown that Fas ligand plays a role in mediating neutropenia in LGL leukemia.14
Autoimmune features are prominent in LGL leukemia. Frequent serologic abnormalities include positive tests for rheumatoid factor and/or ANA, high levels of circulating immune complexes, polyclonal hypergammaglobulinemia, and high levels of β2-microglobulin.15 Autoimmune diseases are also a characteristic finding in LGL leukemia. In our opinion, clinical, immunologic, molecular, and genetic data indicate that patients with LGL leukemia and rheumatoid arthritis (RA) and patients with Felty's syndrome are part of the spectrum of the same disorder. Up to one third of neutropenic patients with RA have clonal LGL expansions.16,17 In addition, oligoclonal CD3+ CD8+ CD57+ T cells have been demonstrated in RA patients.18 Increased numbers of cells with a phenotype similar to leukemic LGL have been observed in blood or synovial fluid of RA patients.19 The identical major histocompatibility locus association with HLA-DRβ *0401 is found in patients with LGL/RA as well as those with Felty's syndrome.20 It has been recently recognized that clonal expansion of CD28-negative T cells with many phenotypical and functional characteristics of LGL, including CD57 and perforin expression as well as T cell receptor-mediated cytotoxicity, is a characteristic finding in RA.21 These data suggest a common pathogenetic link between LGL leukemia and RA.
Distinctive pathologic findings in LGL leukemia are found in bone marrow, spleen, and liver (Table 10 ).22 Unlike other T cell malignancies, skin and lymph node involvement is uncommon. It is important to emphasize that it is difficult to recognize LGL in tissue sections; consequently morphologic findings may be indistinguishable from other indolent lymphoproliferative disorders. Correlation with immunophenotyping studies are therefore very helpful for diagnostic evaluation. In contrast to our initial description of frequent lymphoid aggregates in marrow sections, an interstitial diffuse infiltration appears more common. This finding is particularly highlighted by use of immunostaining for T cells in marrow sections. Typical findings in the spleen include red pulp infiltration by leukemic cells and often reactive follicular hyperplasia of the white pulp. This pattern of red pulp infiltration needs to be distinguished from hairy cell leukemia, in which red pulp infiltration is especially characteristic. Liver biopsies from LGL leukemia patients show prominent intrasinusoidal infiltration. In cases where infiltration is marked, portal areas are sometimes involved.
Pathogenesis
Our central hypothesis is that leukemic LGL represent antigen-driven cytotoxic T lymphocytes (CTL). Data supporting this contention include 1. the unique subset of normal CD3+ LGL may represent in vivo primed CTL directed against viral targets;4 2. the CD3+, CD8+, CD57+, DR+ phenotype of leukemic LGL and the rapid triggering of non-MHC-restricted killing via CD3/CD16 pathway;23,24 and 3. structural analysis of rearranged TCR genes as well as TCR Vβ repertoire analyses in some LGL cases have provided evidence for antigenic selection.25–,26 Perhaps the most compelling data come from analyses of molecules involved in the cytotoxic process, perforin and Fas ligand. These proteins are only expressed in CTL after activation. Leukemic LGL constitutively express high levels of both perforin and Fas ligand.27,28 We have found high expression of other cytotoxic molecules, such as granzyme and calpain, in leukemic LGL compared to normal CD8+ cells, using microarray analyses (unpublished observations). Constitutive expression of such proteins suggests that leukemic LGL are constantly exposed to some antigen in vivo.
Although these data suggest that leukemic LGL are antigen driven CTL, the antigen specificity of these leukemic clones is not known. It is interesting to note that increased numbers of LGL can be seen after infection with viruses such as CMV and HIV.29,30 CTL responding to these infections have been characterized as memory or effector CD8+ populations based on expression pattern of antigens such as CD28, CD45, CD62L, and perforin. Using Vβ antibodies to identify the leukemic clone, we have determined that leukemic LGL are CD3+, CD8+, CD57+, CD45RA+, CD45RO-, CD25-, CD62L-, and CD28- (unpublished observation). These data show that leukemic LGL are effector CTL. Others have detected the clonal abnormality in both memory and effector CD8+ cells.31 This effector cytotoxic phenotype of the leukemic clone is similar to that of CTL generated after HIV infection.32 Retroviral infection is also characterized by production of pro-inflammatory chemokines such as RANTES, MIP1-α, and MIP1-β.33 A similar pattern of chemokine expression is observed in patients with LGL leukemia (unpublished observations). Taken together, these findings suggest that retroviral infection may be a stimulus for LGL activation. LGL leukemia patients are HIV negative; a few patients have been infected with HTLV-I or HTLV-II.34,35 Although most patients are not infected with prototypical HTLV, sera from these patients do show reactivity against gag p24 and env p21e of HTLV-I.36 Epitope mapping studies have shown that reactivity against env p21e is directed at the BA21 epitope of HTLV-I.37 We hypothesize that a cellular or retroviral protein having homology to BA21 may be important in the pathogenesis of LGL leukemia.
Dysregulated apoptosis is a characteristic feature of LGL leukemia. Normal CTL recognizing viral peptide in the correct MHC context upregulate Fas ligand. Virally infected target cells expressing Fas are then eliminated through apoptosis. Deletion of such antigen-activated T cells occur through the same process of Fas-mediated apoptosis.38,39 Leukemic LGL express high levels of both Fas and Fas ligand.40 We hypothesize that illness in LGL leukemia such as neutropenia, anemia, or RA may be caused in part by constitutive production of Fas ligand. We also propose that accumulation of the leukemic clone is due to inhibition of the Fas apoptotic pathway in the leukemic LGL (Figure 6; see color page 549).
The mechanism causing neutropenia in LGL leukemia has not been defined. Normal neutrophils undergo apoptosis through Fas triggering.41 We found high levels of circulating Fas ligand in sera from 39 of 44 patients with LGL leukemia. Serum from patients caused apoptosis of normal neutrophils that depended partly on the Fas pathway. Resolution of neutropenia was associated with disappearance or marked reduction in Fas ligand levels in 10 of 11 treated patients.14 These data suggest that neutropenia in LGL leukemia is mediated by Fas ligand. Decreased levels of Fas ligand have also been observed in a few LGL leukemia patients who had correction of anemia on therapy.14,42
Leukemic LGL are resistant to Fas-mediated death in vitro, despite expressing high levels of both Fas and Fas ligand.40 Fas resistance can not be explained on the basis of Fas mutations,28 unlike children with autoimmune lymphoproliferative syndrome.43 Resistance to Fas mediated death can be overcome in vitro.40,44 These data suggest that resistance is due to inhibition of Fas signaling pathway in leukemic LGL. We found that leukemic LGL displayed high levels of activated STAT3. Inhibition of STAT signaling with either AG-490, a JAK-selective tyrosine kinase inhibitor, or STAT3 antisense reversed apoptotic resistance in leukemic LGL. AG-490-induced apoptosis was independent of Bcl-XL or Bcl-2 expression. In contrast, we found that levels of Mcl-1 expression decreased in leukemic LGL after AG-490 treatment and that Mcl-1 is a STAT-3 regulated gene. We concluded that STAT3 activation contributes to the accumulation of the leukemic LGL clones, possibly through upregulation of the anti-apoptotic protein Mcl-1.45 These results identify the STAT3 signaling pathway as molecular targets for drug discovery in LGL leukemia.
Treatment
LGL leukemia is a chronic disease. In the largest series of 68 patients reported from a single institution, the median survival was superior to ten years.10 In an earlier smaller series we had reported that actuarial survival at 5 years was 67%.46 However, the majority of patients in both series eventually needed treatment for symptoms resulting from neutropenia or anemia (69% and 89%, respectively). Active agents for treatment of this disease are drugs which are categorized as immunosuppressants and include oral low-dose methotrexate (10 mg/m2 po once weekly), cyclosporine (2 mg/kg po q12 hours), oral Cytoxan (100 mg po daily), and prednisone (1 mg/kg po daily).10,47–,49 Prednisone alone is not recommended as cytopenias almost always recur as the dose of prednisone is tapered.10 Low dose methotrexate has been used by us primarily for treatment of severe neutropenia. In a small series we documented a complete remission in 50% of cases.47 This favorable response was also observed in a group of patients who had both LGL leukemia and rheumatoid arthritis.48 Cyclosporine has also been used effectively for correcting neutropenia in some patients.49 LGL leukemia was identified as the most common cause of pure red cell aplasia in two large single institution studies.50,51 Immunosuppressive therapy typically given to patients with pure red cell aplasia was effective in the cases associated with LGL leukemia.51 A small proportion of patients with LGL leukemia will present with a more aggressive clinical course.52,53 In such cases, lymphoma type regimens do not appear particularly effective although reported experience is quite limited.52,53 This resistance might be explained on the basis of leukemic LGL expressing high levels of multidrug resistant genes such as P-glycoprotein and lung resistant protein.54 Some of these patients failing combination chemotherapy have had sustained clinical responses to low dose methotrexate and prednisone.53
The mechanisms of therapeutic response in LGL leukemia are not well understood. Retrospective analyses of patients treated primarily with methotrexate did show that responses were associated with decreased levels of Fas ligand. It is likely that a similar mechanism occurs in patients treated with cyclosporine, as resolution of neutropenia occurs despite persistence of the leukemic clone.49 Indeed reduction of Fas ligand levels on cyclosporine treatment have been observed in a case report.42 It is likely that methotrexate has additional mechanisms of action. Prolonged methotrexate therapy of 1-2 years duration does result in complete remission and disappearance of the leukemic clone in some patients, in contrast to cyclosporine therapy.47 We hypothesize that methotrexate treatment leads to reversal of apoptotic resistance seen in leukemic LGL. Preliminary studies from our lab indicate that methotrexate induces apoptosis of activated T cells through a mitochondrial-dependent pathway (unpublished observation). We are currently investigating whether regulation of Mcl-1 is involved in the mitochondrial pathway triggered by methotrexate.
Clinical Trials
Enrollment into clinical trials should be encouraged as there are only limited treatment data available, as noted above. Two trials are currently being conducted in the cooperative group setting. Both of these trials involve correlative laboratory studies designed to examine mechanisms of treatment response. ECOG is studying initial treatment of LGL leukemia with methotrexate (TP Loughran, PI) whereas CALGB is investigating cyclosporine (Maria Baer, PI, Roswell Park). Indications for treatment in both studies include either neutropenia (ANC < 500 or neutropenia with recurrent infections) or anemia (ECOG: symptomatic or transfusion-dependent anemia: CALGB: hemoglobin < 9g/dL). A national registry for LGL leukemia is established at Moffitt Cancer Center (http://www.moffitt.usf.edu/lgl-leukemia/lgl.htm) in order to define the natural history and prognosis of this disease. Clinicians with patients suspected to have this disorder are encouraged to contact us (phone 813-903-6841 or email loughrat@moffitt.usf.edu).
III. Should Peripheral T Cell Lymphoma Be Treated Differently from Large B Cell Lymphoma?
John P. Greer, MD*
Vanderbilt University Medical Center, 2617 The Vanderbilt Clinic, Nashville, TN 37232-5505
The classification of T/NK neoplasms as described by the REAL and the WHO is based upon principles outlined by Lukes and Collins in the United States and Lennert in Europe in the 1970s.1 The Working Formulation (WF), devised in the 1980s, did not recognize T/NK lymphomas so that many diseases of different biology were “lumped” together. The present day classification is an evolving one, which identifies diseases as distinct entities according to clinical, morphologic, immunophenotypic, and genotypic data when available (Table 1).
Peripheral T cell and NK-cell neoplasms, have been difficult to classify and to treat for multiple reasons.2,3 Most importantly, these diseases are rare, representing less than 15% of non-Hodgkin's lymphomas (NHL) in the Western Hemisphere. Within this minority of diseases, there are multiple subtypes so that some of the neoplasms represent less than 1% of NHL. Besides their scarcity, there are numerous problems in the pathologic diagnosis of T/NK lymphomas: 1) there is no immunophenotypic marker of clonality, 2) there is morphologic heterogeneity, and 3) there is poor correlation between cytomorphology and prognosis.2,3
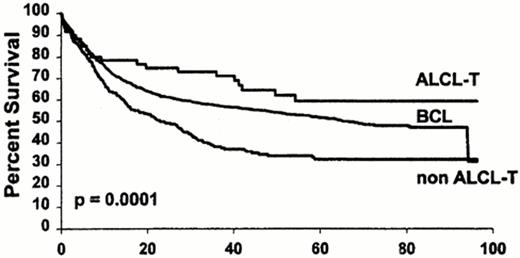
All of the above problems in diagnosis have contributed to the lack of consensus on therapy. Early series of peripheral T cell lymphoma (PTCL) disagreed upon the importance of distinguishing PTCL from the more common counterpart of diffuse large B cell lymphoma (DLBCL).4,5 Although small series suggested no difference in prognosis between PTCL and DLBCL, large, prospective series did indicate a higher relapse rate and a poorer survival for PTCL than DLBCL.6–,8 Differences among series could be due to the small number of PTCL, the heterogeneity within PTCL, and the inclusion of inaccurate diagnoses due to lack of a clonal immunophenotypic marker for T cells. For example, T cell rich B cell lymphoma, which is characterized by a large number of T cells surrounding the minority malignant B cell, may have been included in the diagnosis of PTCL and is more accurately considered a subset of DLBCL. Similarly, there are PTCL with increased CD20+ large B cells that could be mistakenly diagnosed as a DLBCL. Gene rearrangement studies may be useful in identifying the malignant cell as either a B cell or T cell.9,11 Additionally, anaplastic large cell lymphoma (ALCL) of T origin has a prognosis that is equivalent or superior to DLBCL (Figure 7 ).8
The optimal therapy for T/NK neoplasms is an area of controversy due to the rarity of the diseases, their variable clinical course, and the lack of randomized trials. Although the intergroup trial comparing CHOP to three newer regimens established CHOP as the standard for WF intermediate grade lymphoma, there were few data available regarding the impact of immunophenotype.12 The assumption is that PTCL is a subset of the WF groups in the trial, and no regimen would be superior to CHOP. Despite this assumption, there are numerous reports of small series of rare subsets of T/NK neoplasms, such as hepatosplenic and nasal types, which have had a poor prognosis despite anthracycline-based therapy. Alternatively, ALCL associated with the t(2;5) has been highly chemosensitive, while primary cutaneous ALCL, which lacks the t(2;5), has often had an indolent course for which combination chemotherapy may not be warranted. In the subsequent paragraphs, we will review the clinical spectrum and therapy of ALCL, nodal PTCL, subsets of extranodal T/NK neoplasms, and potential novel therapeutic strategies, including therapy for mycosis fungoides (MF) and adult T cell leukemia/ lymphoma (ATLL) and the role of transplantation.
Anaplastic Large Cell Lymphoma
Since its original description by Stein et al in 1985, ALCL has become a paradigm in the classification for NHL.13 In the past two decades, the clinical, morphologic, immunophenotypic, and genetic spectrum has been defined (Table 4). The first clinical description was in a series by Kadin et al in six children, all of whom had skin lesions.14 Subsequent studies including adults recognized a disease with a wide morphologic spectrum, young median age, frequent extranodal involvement, and a good prognosis.15,16 In 1989, a proportion of ALCLs were associated with a 2;5 chromosomal translocation.17,18 Morris et al in 1994 identified the genes involved in the t(2;5), a nucleophosmin (NPM) gene at 5q35 fuses with a gene at 2p23 encoding the receptor tyrosine kinase anaplastic lymphoma kinase (ALK).19
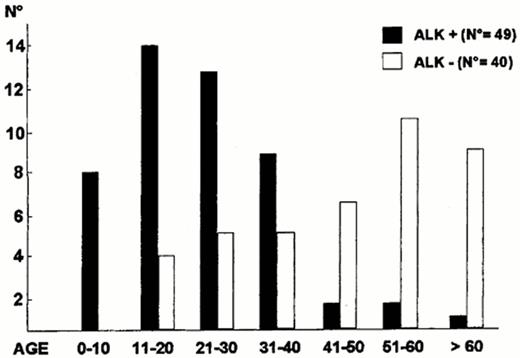
ALK expression correlates with the t(2;5), can be identified by monoclonal antibodies, and further subdivides ALCL into at least three clinical subtypes of ALCL: 1) ALK+ systemic ALCL, 2) ALK- systemic ALCL, 3) primary cutaneous ALCL (also, ALK-). ALK+ ALCL occurs at a younger median age than ALK- ALCL (Figure 8 ), has a male predominance, and often has advanced stage disease with frequent B symptoms (75%) and extranodal involvement (62%).13 Skin (21%) and bone (17%) are common extranodal sites with disease rarely occurring in the gastrointestinal tract and central nervous system.13 Bone marrow involvement is identified in 10-15% with hematoxylin and eosin stains but up to 30% if immunohistochemistry stains are used to identify isolated ALCL cells.13,20
Cytogenetic variants of ALCL include t(1;2)(q25;p23), inv(2)(p23;q35), and t(2;3)(p23;q21).13,21 Immunocytochemical labeling for the ALK protein in patients with t(2;5)(p23;q35) is usually present in both cytoplasm and nuclei while it tends to be only in the cytoplasm of the variants.13,21 Because the variants are of T or null cell origin and occur in a similar age group as the t(2;5), Falini et al. have proposed the term “ALKoma” for all patients expressing the ALK protein.21
Systemic anaplastic large cell lymphoma
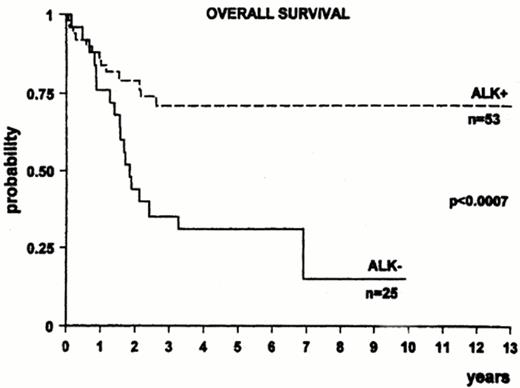
ALK+ ALCL tends to respond better to chemotherapy than ALK- systemic ALCL. There has been approximately a two fold or higher increase in survival in ALK+ ALCL compared to ALK- ALCL in three series (Figure 9 ).22–,24 Other series, however, have not confirmed an improved survival for ALK+ ALCL.25 Differences among studies could be due to variable percentages in adverse prognostic factors between groups and due to the younger age of ALK+ ALCL; however, an improved survival has been reported for ALK+ ALCL over ALK- ALCL in patients less than 30 years of age.22
Unlike most NHL, no significant differences were observed between ALCL patients with a low or high International Prognostic Index (IPI) in a report of the Non-Hodgkin's Lymphoma Classification Project.1 These data were based on a small number of patients and did not have information on ALK expression. Subsequent studies have identified a worse prognosis for ALCL patients with an age-adjusted intermediate/high IPI (> 2) compared to the low/intermediate risk group (IPI 0-1).23,24
While therapy for adults has not been consistently stratified according to prognostic features, pediatric groups have based therapy according to risk factors. Therapy for pediatric ALCL has varied from prolonged therapy for acute lymphoblastic leukemia to short course combination chemotherapy. Both German and French groups have studies supporting the use of short course therapy developed for B NHL in pediatric ALCL.26,27 After a brief cytoreduction phase, the German group stratified therapy according to stage: three 5-day courses for St. Jude's stage I and II resected, six courses for stage II non-resected and stage III, and six intensified courses, including high dose methotrexate, cytarabine, and etoposide, for stage IV or multifocal bone disease. The 5-year event free survival (EFS) was 76% + 5% for all patients and 100%, 73% + 6%, and 79% + 11% for the three groups, respectively.26 The French reported a similar EFS of 66% + 12%, but identified visceral involvement, mediastinal disease, and an elevated lactate dehydrogense as adverse prognostic factors.27
Primary cutaneous ALCL
Primary cutaneous ALCL should be distinguished from systemic ALCL because it often does not need to be treated with combination chemotherapy, and it may spontaneously regress. Unlike systemic ALCL, it is negative for epithelial membrane antigen and it does not express ALK. Primary cutaneous ALCL is part of the spectrum of CD30+ cutaneous lymphoproliferative disease and clinically overlaps with lymphomatoid papulosis (see Tables 5 and 6Table 5,Table 6).28,29 It usually arises in the skin in an older patient (median age ∼60 years) as an isolated reddish violet tumor, which may be ulcerated.13 Less commonly, the disease may present as multiple nodules in a circumscribed area, or rarely with widespread skin lesions.
Treatment of localized lesions may be watchful waiting with approximately one quarter of patients regressing, local excision with or without radiation, or radiation alone.13 Patients with disseminated skin disease or localized skin disease with nodal extension may benefit from combination chemotherapy, but these patients tend to relapse.30 Lymphomatoid papulosis (LyP) usually occurs in small crops of papules, spontaneously regresses, and rarely develop into a lymphoma. Low dose weekly methotrexate can control the lesions of LyP.31
Nodal Peripheral T Cell Lymphoma
The distribution of PTCL, which can be subdivided into nodal and extranodal diseases, varies according to geography and referral patterns (Table 11 ).8,32–,37 In the Western hemisphere, the nodal diseases tend to predominate with PTCL, not otherwise specified, and ALCL representing the largest groups, while in the East, there are increased numbers of patients with extranodal disease, particularly of the nasal type.36 In areas where HTLV-1 is endemic, such as Kyushu, Japan, approximately one-half of the T/NK neoplasms will be ATLL.37 ALCL has the best prognosis in the different series of PTCL.
Problems in management of PTCL include the fact that, unlike B cell lymphomas, cell size does not correlate well with prognosis, and there is sparse data regarding the impact of cytogenetics on diagnosis other than the t(2;5) of ALCL. Utilizing the Kiel classification of PTCL, which subdivided PTCL into low grade and high grade groups, Schlegelberger et al identified patterns of abnormalities with the grade of PTCL.38 Trisomy 3 and a higher proportion of normal metaphases were found in the low grade PTCL, which included T zone lymphoma and Lennert's lymphoma, rare subtypes within the not otherwise specified group of PTCL, and angioimmunoblastic (AILD) type PTCL. Deletions in 6q, total or partial trisomy of 7q, and monosomy 13 or changes in 13q14 were more common in the high grade PTCL, or the pleomorphic medium to large cell types.38 The genes involved in these abnormalities have not been identified, and again, the so-called low grade PTCL frequently have an aggressive course. Additional data, such as patterns of expression of chemokine receptors and their chemokines may subdivide PTCL and explain their biologic differences.39 Genomic profiling which has been utilized to identify prognostic groups of DLBCL is likely to clarify different profiles and prognosis among PTCL.40
Angioimmunoblastic PTCL
AILD PTCL is particularly difficult to diagnose and manage because there may be the presence of oligoclonal proliferations, including both T call and B cell gene rearrangements, EBV positivity, and a variable clinical course.41,42 There may be rare spontaneous regressions and it can respond to single agents, primarily steroids, but also alkylating agents, interferon, and cyclosporine.42 The disease primarily occurs in the elderly with distinctive clinical features, B symptoms, skin rash, generalized lymphadenopathy, polyclonal gammopathy, autoimmunity, and an increased risk of infection.42 While AILD PTCL can be responsive to single agents, combination chemotherapy is warranted in many cases once a diagnosis is confirmed. The CR rate with anthracycline-based regimens is in the 50-70% range with frequent relapses or deaths due to infection and median survivals between 11-30 months.42,43
Extranodal NK/T Lymphomas
Although less common than the nodal PTCL, these entities tend to be better described because of their unique sites of origin, associations with clinical syndromes, and poor prognosis.
Hepatosplenic γ/δ T cell Lymphoma
Hepatosplenic γ/δ T cell lymphoma is an extranodal lymphoma that probably arises from the γ/δ T cells of the sinusoidal area, or red pulp, of the spleen.44,45 Most cases occur in young adult males (median age, 29 yrs) with B symptoms, splenomegaly (98%), hepatomegaly (80%), no lymphadenopathy, anemia, and severe thrombocytopenia (80-85%). Isochrome 7q with trisomy 8 is a common cytogenetic abnormality. Most patients are refractory or have brief responses to anthracycline therapy with death occurring less than a year after diagnosis.45,46
Subcutaneous panniculitis-like T cell lymphoma
Subcutaneous panniculitis-like T cell lymphoma (SCPTCL) presents with subcutaneous nodules, especially in the extremities, and is often associated with hemophagocytosis.47–,49 Median age is 43 years with females affected more than males. The unique pathologic appearance includes sparing of the epidermis and dermis with rimming of fat spaces by CD56-negative, cytotoxic T cells. There can be nodal dissemination but the poor prognosis is usually due to fulminant hemophagocytosis.47–,49 Median survival is usually less than 2 years, but occasional patients without hemophagocytosis have had an indolent course and some patients have had prolonged remissions after chemotherapy.48,49
Enteropathy-associated intestinal T cell lymphoma
Enteropathy-associated intestinal T cell lymphoma (EATL) may or may not follow celiac disease. Most patients are middle-aged to elderly with a median age of 50 years.50 Abdominal pain and weight loss occur in over four-fifths of patients at presentation, followed by diarrhea or vomiting in approximately one-third of patients.50 Small bowel perforation or obstruction is common and the diagnosis of enteropathy-type intestinal T cell lymphoma is usually made at laparotomy. Prognosis is poor with median survival of 7.5 months and less than 20% failure-free survival at one year.50 Nutritional support is critical in the management of these patients.
Nasal and nasal-type NK cell lymphomas
Nasal and nasal-type NK cell lymphomas are characterized by extranodal presentation, angiocentric and angiodestructive proliferation, large granular lymphocyte morphology, CD2+, CD3-, CD16-/+, CD56+, CD57- phenotype, and an aggressive clinical course. Nasal NK lymphoma is associated with EBV and more commonly occurs in Asia and Latin America. Nasal obstruction, nasal discharge, and epistaxis are common symptoms. The disease occurs more commonly in males, and the median age is 50–55 years.3,51,52 The disease may present with facial swelling or midfacial/destructive disease and was formerly called lethal midline granuloma. Nasal NK/T lymphoma is localized stage I/II in 80% of patients at diagnosis but can disseminate early to skin, gastrointestinal tract, testis, orbit and central nervous system (CNS). Although radiation alone can achieve complete remission in approximately two-thirds of localized disease, local relapse occurs in half the patients and systemic progression develops in one quarter of patients.52 Combined modality therapy is recommended, and CNS prophylaxis should be considered. Prognosis is variable with long-term survival of 20-80%, primarily in stage I, non-bulky disease. Systemic progression is usually fatal.52,53
Nasal-type NK/T cell lymphoma has a worse prognosis that those originating in the nasal region due to disseminated extranodal disease, including skin, gastrointestinal tract, soft tissue, and testis.54 Only one-fifth of patients have stage I disease.54 Despite anthracycline therapy, median survival is less than one year.54 Patients with cutaneous only involvement have a better survival.55
Novel Therapies in T/NK Neoplasms
While CHOP is considered standard therapy for diseases within the previously described intermediate grade NHL of the WF, the prognosis in many of the T/NK neoplasms has been so poor that investigations into new therapy are warranted. Mycosis fungoides (MF) and adult T cell leukemia/lymphoma (ATLL) represent the extremes of the clinical spectrum of T/NK neoplasms, from the indolent MF to the aggressive leukemia or lymphoma of ATLL. Both diseases, MF and ATLL, have novel therapies which could be applied to other NK/T neoplasms.
Mycosis fungoides
In its earliest stage, IA (patches and/or plaques involving less than 10% of body surface area), MF has a survival that does not impact the expected normal life span.56,57 Alternatively, patients with extracutaneous disease, or stage IV, have median survivals less than two years.58 Treatment is usually skin directed for limited stage disease and includes ultraviolet light therapy with or without psoralen, topical chemotherapy, and radiation therapy.57 Systemic therapy is usually reserved for more advanced disease and includes photopheresis, interferon, steroids, and chemotherapy. New agents that have been developed for progressive and/or stage IV disease include bexarotene (Targretin, LGD 1069) and denileukin difitox (DAB339 IL-2, Ontak).
Bexarotene
Bexarotene is an oral agent, a rexinoid or RXR-selective retinoid agonist, approved in 2000 in the US for patients with MF who are refractory to at least one prior systemic therapy. In patients with relapsed MF who were heavily pretreated, response rates of 45% and 55% were observed among patients receiving 300 mg/m2/d or higher doses, respectively.59 The median time to response was 180 days (range, 14 to 197) for patients receiving 300 mg/m2/d and 59 days (range, 22 to 169) for the higher doses.59 Approximately one-third of patients progressed at a median time of one year. The most common adverse events were hypertriglyceridemia (82%), hypercholesterolemia (30%), hypothyroidism (29%), elevated liver functions (20%), headache (20%), asthenia (16%), pruritus (13%), leukopenia (11%), and rash (11%).59 Lipid lowering agents and thyroid replacement for central hypothyroidism are recommended.
Denileukin difitox
Denileukin difitox, also approved in the US in 2000, is a recombinant fusion protein consisting of peptide sequences for the enzymatically active and membrane translocation domains of diphtheria toxin with recombinant interleukin (IL)-2. CD25, an IL-2 receptor marker, has been utilized for determining eligibility for denileukin difitox; however, studies are investigating whether the marker is required for response. In a trial of two dose levels of denileukin difitox, 9 or 18 μg/kg/d x 5 days every 3 weeks, 30% of 71 patients with MF had an objective response (20% PR, 10% CR).60 The median time to first response was 6 weeks (range 3-27), and 95% of patients who had a response had at least a 25% decrease in disease by week 9.60 The median duration of response was 6.9 months (range, 2.7-46.1+). Adverse events included acute infusion-related events in 60% of patients [dyspnea (20%), back pain (17%), hypotension (17%), and chest pain/tightness (13%)], flu-like symptoms in 85% of patients, and a vascular leak syndrome (hypoalbuminemia, edema and/or hypotensin) in 25% of patients. Hypoalbuminemia occurred in 79% (15% grade 3 or 4) and elevated hepatic transaminases were observed in 61% (17% grade 3 or 4) of patients.60
Nucleoside analogs
Nucleoside analogs, 2′-deoxycoformycin (dCF; pentostatin), 2 chlorodeoxyadenosine (CdA), and fludarabine (F-ara-A AMP) are purine antimetabolites that inhibit adensosine deaminase, an enzyme with high concentrations in lymphoid cells, particularly T cells. The phosphorylated derivatives of the analogs induce apoptosis through down regulation of ribonucleotide reductase and inhibition of DNA replication and repair. Most of the clinical trials of these agents in T cell lymphomas have been in patients with MF, but some of the studies have included PTCL, usually with cutaneous involvement.61–,67 Gemcitabine (2′,2-difluorodeoxy-cytidine) is a pyrimidine antimetabolite that has also shown activity in MF and PTCL.66
Table 12 outlines the results of some of the largest series of patients primarily with advanced stage MF who received single agent nucleoside analogs. The response rates vary from a low of 19% for fludarabine to 70% and 71% for gemcitabine and dCF, respectively; the latter two series included patients with PTCL whose response was similar to MF. The median duration of response is usually less than six months. The addition of interferon to dCF in MF did not appear to increase the response rate (41%) but did improve the progression-free survival in responders to over a year (13.1 mo).67
Adult T Cell Leukemia/Lymphoma
ATLL was first described in Japan in 1977, and the causative agent, HTLV-1, was identified by Gallo's group in 1980.68 ATLL has a variable clinical course with a leukemic presentation in four-fifths of patients as either acute, chronic, or smoldering subytypes with lymphoma in the remaining fifth of patients.68 There is no consensus on the optimal therapy of ATLL. Survival is poor with median survivals of 6.2 months for acute leukemia and 10.2 months for lymphoma.69 Poor performance status, high LDH, age above 40 years, tumor bulk, and hypercalcemia are adverse prognostic factors.69 CHOP-like regimens have had CR rates of 17-22%.69 Newer or more intensive regimens may be associated with higher CR rates but have not improved prognosis.70 Supportive care to decrease opportunistic infections and CNS prophylaxis are recommended for the aggressive ATLL.68
Recent studies using α-interferon and zidovudine (AZT) have reported significant responses (66%) in patients, including those who had failed chemotherapy.71 Nucleoside analogs have had modest response rates of 10-20% in ATLL.72 Topoisomerase inhibitors. have had 40% response rates in small phase II trials.68,73,74 Conjugated and unconjugated monoclonal antibodies directed at the IL-2 receptor have activity in ATLL, but how they and other agents should be incorporated into therapy are areas of investigation.75
Understanding genetic mechanisms in ATLL could lead to earlier and more effective therapy. Aneuploidy and multiple chromosomal breaks are associated with an aggressive course.76 Ongoing genetic abnormalities, including defective HTLV-1 integration and deletion of tumor suppressor genes p15INK4B and p16INK4A, are associated with a worse prognosis.77 Similar to Epstein-Barr virus in Burkitt's lymphoma, HTLV-1 may not have direct oncogenic activity but contributes to a multistep process of worsening genetic instability by interfering with mitotic checkpoints or preventing DNA repair.78
Dose Intensive Therapy Including Transplantation
The Intergroup trial comparing CHOP to newer regimens did not address the role of dose intensity since the doses of cyclophosphamide and doxorubicin were either equivalent or smaller in the newer regimens when compared to CHOP. Phase II trials have developed regimens with higher doses of drugs than CHOP, but the numbers of patients with T/NK neoplasms are too small to assess the impact of dose intensity.79,80 Small series have reported that new regimens with higher doses of adriamycin than are in CHOP along with the addition of etoposide have equivalent responses and survivals between PTCL and aggressive B cell lymphomas. However, the numbers of PTCL are small; the PTCL group includes chemosensitive ALCL patients, and the studies are not randomized.79,80 Despite the chemosensitivity of ALCL, pediatric studies have indicated a role for more intensive regimens in ALCL patients with poor prognostic factors.26,27
The role of transplantation in first relapse is accepted as standard of care after the findings of the phase III PARMA study which favored autologous stem cell transplant (ASCT) over additional platinum-based chemotherapy;81 however, its use in first remission is an area of controversy because randomized trials have yielded mixed results.82 European investigators have advocated a role of ASCT in first CR for ALCL and report over 90% disease free survival in first CR.83 They justify early transplantation on the hypothesis that the IPI in ALCL does not correlate with prognosis. Because subsequent studies of ALCL have had prognosis correlate with the IPI and systemic ALCL has the best prognosis of PTCL with chemotherapy alone, a policy of transplantation in first CR for ALCL cannot be supported.
Until randomized trials prove differently, transplantation for NK/T cell neoplasms should be utilized similar to DLBCL as a part of salvage therapy in relapsed patients who are chemosensitive. The M. D. Anderson group reported a 3 year overall survival of 36% and PFS of 28% in a series of 36 relapsed patients with PTCL who underwent transplantation (29 autologous, 7 allogeneic).84 In a Vanderbilt series of 26 relapsed patients (ALCL–15, PTCL–9, NK–1, T-cell -1) who underwent transplant (18 autologous, 8 allogeneic) the 3 year EFS was 48% (+ 11%), which was similar to 38% (+ 6%) EFS in 84 patients with DLBCL.85 Of note, all 7 patients with ALK+ ALCL remained free of disease while 5 patients with primary cutaneous ALCL relapsed but remained alive.
Small numbers of patients with extranodal NK/T lymphoma, including hepatosplenic, SCPTCL, and nasal-type NK cell lymphomas, have undergone transplantation, usually as salvage therapy.86,87 Because the latter diseases have a worse prognosis than ALCL, they are more likely candidates for early transplantation, particularly in patients with a high IPI. The role of transplantation, primarily allogeneic, in ATLL has been performed in a few patients with mixed results due in part to the additional problem of eradicating HTLV-1.68,88
In summary, the T/NK neoplasms, other than ALK+ ALCL, have been more chemoresistant than the DLBCL. Mechanisms of drug resistance in T/NK lymphomas have included increased expression of the multidrug resistance proteins and p53.89,90 Available chemotherapy agents, including paclitaxel and topotecan, and new agents, such as flavopiridol and UCN-1, modulators of cyclin-dependent kinases, have activity in relapsed hematologic malignancies;91,92 however, there is little data regarding their efficacy in T/NK neoplasms. Additionally, monoclonal antibodies are likely to be effective when directed toward specific markers on a T/NK tumor, such as CD30 or the IL-2 receptor. Because of the rarity of these diseases, only cooperative groups or a few large centers will be able to adequately evaluate the role of new drugs, monoclonal antibodies, and transplantation.
Post-thymic T and NK cell neoplasms in the WHO and REAL classifications.
| * originally described as aggressive NK cell leukemia/lymphoma |
| ** Adult T-cell leukemia/lymphoma (ATLL) has varied clinical presentations that can be predominantly leukemic or lymphomatous Abbreviastions: HTLV, human T-cell lymphotrophic virus; AILD, angioimmunoblastic lymphadenopathy |
| Predominantly Leukemic Malignancies |
| T-cell prolymphocytic leukemia |
| T-cell granular lymphocytic leukemia |
| Aggressive NK-cell leukemia* |
| Adult T-cell lymphoma/leukemia (HTLV-1+)** |
| Predominantly Lymphoma |
| Peripheral T-cell lymphoma, unspecified |
| Angioimmunoblastic T-cell lymphoma (AILD-like) |
| Adult T-cell leukemia/lymphoma (HTLV-1+)** |
| Anaplastic large cell lymphoma (T- and null cell) |
| Predominantly Extranodal Malignancies |
| Indolent: |
| Mycosis fungoides/Sézary syndrome |
| Primary cutaneous anaplastic large cell lymphoma |
| Aggressive: |
| Extranodal NK/T cell lymphoma, nasal and nasal type |
| Blastic NK cell lymphoma |
| Enteropathy-type T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| * originally described as aggressive NK cell leukemia/lymphoma |
| ** Adult T-cell leukemia/lymphoma (ATLL) has varied clinical presentations that can be predominantly leukemic or lymphomatous Abbreviastions: HTLV, human T-cell lymphotrophic virus; AILD, angioimmunoblastic lymphadenopathy |
| Predominantly Leukemic Malignancies |
| T-cell prolymphocytic leukemia |
| T-cell granular lymphocytic leukemia |
| Aggressive NK-cell leukemia* |
| Adult T-cell lymphoma/leukemia (HTLV-1+)** |
| Predominantly Lymphoma |
| Peripheral T-cell lymphoma, unspecified |
| Angioimmunoblastic T-cell lymphoma (AILD-like) |
| Adult T-cell leukemia/lymphoma (HTLV-1+)** |
| Anaplastic large cell lymphoma (T- and null cell) |
| Predominantly Extranodal Malignancies |
| Indolent: |
| Mycosis fungoides/Sézary syndrome |
| Primary cutaneous anaplastic large cell lymphoma |
| Aggressive: |
| Extranodal NK/T cell lymphoma, nasal and nasal type |
| Blastic NK cell lymphoma |
| Enteropathy-type T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
Classification of cytotoxic cells according to immunophenotype, molecular, and functional parameters.
| Parameter . | T-cell . | NK-like T cell . | NK-cell . |
|---|---|---|---|
| * CD16, CD56, CD57 ** Some populations lack NK-cell antigens ‡ TCR gene rearrangement and protein expression | |||
| Abbreviations: TCR, T-cell receptor; MHC, major histocompatibility complex | |||
| Modified from Kinney MC, 1999 6 | |||
| NK-cell antigens* | - | + | +** |
| Cytoplasmic CD3 (epsilon chain) | + | + | +/- |
| Surface CD3 | + | + | - |
| CD4, CD8 | CD8+>>>CD4+ | CD8+>CD4-CD8->>CD4+ | CD4-CD8+/- |
| TCRαβ,γδ‡ | + | + | - |
| Cytotoxic granules | + | + | + |
| Killing mechanism | MHC restricted | Non-MHC restricted | Non-MHC restricted |
| Parameter . | T-cell . | NK-like T cell . | NK-cell . |
|---|---|---|---|
| * CD16, CD56, CD57 ** Some populations lack NK-cell antigens ‡ TCR gene rearrangement and protein expression | |||
| Abbreviations: TCR, T-cell receptor; MHC, major histocompatibility complex | |||
| Modified from Kinney MC, 1999 6 | |||
| NK-cell antigens* | - | + | +** |
| Cytoplasmic CD3 (epsilon chain) | + | + | +/- |
| Surface CD3 | + | + | - |
| CD4, CD8 | CD8+>>>CD4+ | CD8+>CD4-CD8->>CD4+ | CD4-CD8+/- |
| TCRαβ,γδ‡ | + | + | - |
| Cytotoxic granules | + | + | + |
| Killing mechanism | MHC restricted | Non-MHC restricted | Non-MHC restricted |
Characteristic immunophenotype of extranodal T/NK-cell neoplasms.
| Lymphoma Type . | T-cell Subset . | CD56 . | Cytotoxic Proteins . | CD30 . | EBV . | Cell Type . |
|---|---|---|---|---|---|---|
| Modified from Kinney MC, 19996; + = 50%-100%; +/- = 25%-49%; -/+ = 5%-24%; - = <5% | ||||||
| * most cases have a non-activated phenotype (TIA-1+, granzyme B-, perforin-). ** based on nodal cases | ||||||
| Abbreviations: SCPTCL, subcutaneous panniculitis-like T-cell lymphoma; ALCL, anaplastic large cell lymphoma; NT, not tested | ||||||
| Hepatosplenic | CD4-CD8->CD8+ | + | +* | -/+ | - | γδ>>αβ |
| SCPTCL | CD8+>CD4-CD8->CD4+ | -/+ | + | -/+ | - | αβ>γδ |
| ALCL, primary cutaneous | CD4+>CD4-CD8->CD8+ | -/+ | +/- | + | -** | αβ>γδ |
| Nasal/nasal type NK/T-cell | CD8+>CD4-CD8- | + | + | +/- | + | NK>γδ>αβ |
| Aggressive NK-cell leukemia | CD8+,CD4-CD8- | + | + | NT | -/+ | NK,T cell |
| Intestinal | CD4-CD8->CD8+>CD4+ | -/+ | + | +/- | αβ>γδ>>NK | |
| Blastic NK-cell lymphoma/leukemia | CD4 | + | + | NT | - | NK |
| Lymphoma Type . | T-cell Subset . | CD56 . | Cytotoxic Proteins . | CD30 . | EBV . | Cell Type . |
|---|---|---|---|---|---|---|
| Modified from Kinney MC, 19996; + = 50%-100%; +/- = 25%-49%; -/+ = 5%-24%; - = <5% | ||||||
| * most cases have a non-activated phenotype (TIA-1+, granzyme B-, perforin-). ** based on nodal cases | ||||||
| Abbreviations: SCPTCL, subcutaneous panniculitis-like T-cell lymphoma; ALCL, anaplastic large cell lymphoma; NT, not tested | ||||||
| Hepatosplenic | CD4-CD8->CD8+ | + | +* | -/+ | - | γδ>>αβ |
| SCPTCL | CD8+>CD4-CD8->CD4+ | -/+ | + | -/+ | - | αβ>γδ |
| ALCL, primary cutaneous | CD4+>CD4-CD8->CD8+ | -/+ | +/- | + | -** | αβ>γδ |
| Nasal/nasal type NK/T-cell | CD8+>CD4-CD8- | + | + | +/- | + | NK>γδ>αβ |
| Aggressive NK-cell leukemia | CD8+,CD4-CD8- | + | + | NT | -/+ | NK,T cell |
| Intestinal | CD4-CD8->CD8+>CD4+ | -/+ | + | +/- | αβ>γδ>>NK | |
| Blastic NK-cell lymphoma/leukemia | CD4 | + | + | NT | - | NK |
ALKgene dysregulation and the classification of anaplastic large cell lymphoma (ALCL).
| Abbreviations: SCV, small cell variant; EMA, epithelial membrane antigen; LyP, lymphomatoid papulosis; MF, mycosis fungoides; HD, Hodgkin's disease; HIV, human immunodeficiency virus |
| ALK-positive Group |
| Distinct clinicopathologic entity: |
| •young patients |
| •systemic disease |
| •variable histology (ALCL → SCV) |
| •T or null cell, EMA+ |
| ALK-negative Group |
| Heterogeneous group of lymphomas: |
| •primary cutaneous |
| •Hodgkin's related |
| •Secondary ALCL in patients with LyP, MF, HD |
| •CD30+ large B-cell lymphomas |
| •ALCL in HIV+ patients |
| Abbreviations: SCV, small cell variant; EMA, epithelial membrane antigen; LyP, lymphomatoid papulosis; MF, mycosis fungoides; HD, Hodgkin's disease; HIV, human immunodeficiency virus |
| ALK-positive Group |
| Distinct clinicopathologic entity: |
| •young patients |
| •systemic disease |
| •variable histology (ALCL → SCV) |
| •T or null cell, EMA+ |
| ALK-negative Group |
| Heterogeneous group of lymphomas: |
| •primary cutaneous |
| •Hodgkin's related |
| •Secondary ALCL in patients with LyP, MF, HD |
| •CD30+ large B-cell lymphomas |
| •ALCL in HIV+ patients |
Clinical features useful in distinguishing primary cutaneous anaplastic large cell lymphoma (ALCL) and lymphomatoid papulosis (LyP).
| Clinical Features . | LyP . | ALCL . |
|---|---|---|
| * > 3 cm more predictive of lymphoma; borderline lesions are usually intermediate in size, 1-2 cm. | ||
| Modified from Kinney and Kadin, 1999 42 | ||
| Type of lesion | Papules, nodules | Nodules, tumors |
| Number | Multiple | Single or grouped |
| Size | Usually <1 cm* | > 2 cm* |
| Sites of disease | Extremities, trunk | Extremities, head and neck |
| Regression | Yes, usually with a scar | ∼25% of cases |
| Clinical Features . | LyP . | ALCL . |
|---|---|---|
| * > 3 cm more predictive of lymphoma; borderline lesions are usually intermediate in size, 1-2 cm. | ||
| Modified from Kinney and Kadin, 1999 42 | ||
| Type of lesion | Papules, nodules | Nodules, tumors |
| Number | Multiple | Single or grouped |
| Size | Usually <1 cm* | > 2 cm* |
| Sites of disease | Extremities, trunk | Extremities, head and neck |
| Regression | Yes, usually with a scar | ∼25% of cases |
Pathologic features useful in distinguishing primary cutaneous anaplastic large cell lymphoma (ALCL) and lymphomatoid papulosis (LyP).
| Histology/ Immunophenotype . | LyP . | ALCL . |
|---|---|---|
| Abbreviations: EMA, epithelial membrane antigen; ALK, anaplastic lymphoma kinase | ||
| Modified from Kinney and Kadin, 199942 | ||
| Pattern of infiltration | Wedge-shaped perivascular/ periadnexal | More diffuse |
| Subcutaneous involvement | Absent or minimal | Present |
| Mixed inflammatory cells | Many | Few to many |
| CD30+ cells | Scattered single or small clusters | Large groups or sheets |
| EMA | Present in 10-30% of cases | Present in 10-30% of cases |
| ALK | Usually negative | Usually negative |
| Histology/ Immunophenotype . | LyP . | ALCL . |
|---|---|---|
| Abbreviations: EMA, epithelial membrane antigen; ALK, anaplastic lymphoma kinase | ||
| Modified from Kinney and Kadin, 199942 | ||
| Pattern of infiltration | Wedge-shaped perivascular/ periadnexal | More diffuse |
| Subcutaneous involvement | Absent or minimal | Present |
| Mixed inflammatory cells | Many | Few to many |
| CD30+ cells | Scattered single or small clusters | Large groups or sheets |
| EMA | Present in 10-30% of cases | Present in 10-30% of cases |
| ALK | Usually negative | Usually negative |
Classification of NK-cell neoplasms.
| Immature . | Mature . |
|---|---|
| Abbreviation: LGL, large granular lymphocyte; NK, natural killer | |
| *Previously called NK-cell leukemia/lymphoma, recently characterized as aggressive NK-cell leukemia to emphasize the predominance of the leukemic manifestations | |
| **The category myeloid/NK cell precursor acute leukemia is defined as a CD7+, CD56+, surface CD3-, myeloperoxidase-neoplasm with lymphoblastic morphology.70 | |
| Myeloid/NKcell precursor acute leukemia** | Leukemia: |
| Indolent | |
| LGL leukemia 21,71– 72 - | |
| Aggressive | |
| NK-cell leukemia* | |
| Blastic NK-cell lymphoma | Nasal/nasal type NK/Tcell lymphoma |
| Immature . | Mature . |
|---|---|
| Abbreviation: LGL, large granular lymphocyte; NK, natural killer | |
| *Previously called NK-cell leukemia/lymphoma, recently characterized as aggressive NK-cell leukemia to emphasize the predominance of the leukemic manifestations | |
| **The category myeloid/NK cell precursor acute leukemia is defined as a CD7+, CD56+, surface CD3-, myeloperoxidase-neoplasm with lymphoblastic morphology.70 | |
| Myeloid/NKcell precursor acute leukemia** | Leukemia: |
| Indolent | |
| LGL leukemia 21,71– 72 - | |
| Aggressive | |
| NK-cell leukemia* | |
| Blastic NK-cell lymphoma | Nasal/nasal type NK/Tcell lymphoma |
General comparison of immature and mature NK-cell neoplasms.
| Immature . | Mature . |
|---|---|
| *True NK LGL leukemia is not associated with EBV Abbreviations: TIA, EBV, Epstein-Barr virus; LGL, large granular lymphocyte | |
| Blastic morphology | Small, medium or large lymphocytes |
| Azurophilic granules inconspicuous or absent | Azurophilic granules present |
| TIA-1 usually negative | TIA-1+ |
| Lacks killer activity in vitro | Has killer activity in vitro |
| EBV- | EBV+* |
| Immature . | Mature . |
|---|---|
| *True NK LGL leukemia is not associated with EBV Abbreviations: TIA, EBV, Epstein-Barr virus; LGL, large granular lymphocyte | |
| Blastic morphology | Small, medium or large lymphocytes |
| Azurophilic granules inconspicuous or absent | Azurophilic granules present |
| TIA-1 usually negative | TIA-1+ |
| Lacks killer activity in vitro | Has killer activity in vitro |
| EBV- | EBV+* |
Characteristic features of CD56+ neoplasms with blast morphology.
| Feature . | Blastic NK-cell Lymphoma . | Aggressive NK-cell Leukemia* . | Myeloid/NK-cell Precursor Leukemia . |
|---|---|---|---|
| ++ = >50%; + = 25%-50%; +/- = 5%-25%; - = <5% | |||
| * Tumor cells vary from mature-appearing large granular lymphocytes (LGLs) to cells with a more blastic appearance | |||
| ** note: 13%-41% of AML overall are CD56+ | |||
| † the number of blasts may be small and involvement patchy compared to other leukemias | |||
| Marrow involvement | ++ | ++† | ++** |
| Skin disease | ++ | - | < 10% |
| Lymph node involvement | + | ++ | ++ |
| Hepatosplenomegaly | ∼15% | + | < 10% |
| Epstein-Barr virus | - | +/- | - |
| Cytoplasmic granules | +/- | + | - |
| Feature . | Blastic NK-cell Lymphoma . | Aggressive NK-cell Leukemia* . | Myeloid/NK-cell Precursor Leukemia . |
|---|---|---|---|
| ++ = >50%; + = 25%-50%; +/- = 5%-25%; - = <5% | |||
| * Tumor cells vary from mature-appearing large granular lymphocytes (LGLs) to cells with a more blastic appearance | |||
| ** note: 13%-41% of AML overall are CD56+ | |||
| † the number of blasts may be small and involvement patchy compared to other leukemias | |||
| Marrow involvement | ++ | ++† | ++** |
| Skin disease | ++ | - | < 10% |
| Lymph node involvement | + | ++ | ++ |
| Hepatosplenomegaly | ∼15% | + | < 10% |
| Epstein-Barr virus | - | +/- | - |
| Cytoplasmic granules | +/- | + | - |
Differential diagnosis of large granular lymphocyte (LGL) leukemia.
| . | LGL Leukemia . | Hairy Cell Leukemia . | CLL/SLL . | Follicular NHL . | Reactive Lymphocytosis . |
|---|---|---|---|---|---|
| Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; NHL, non-Hodgkins lymphoma | |||||
| Cytology (peripheral blood) | LGL | Hairy cells | Small lymphocytes | Cleaved lymphocytes | Atypical lymphocytes and LGL |
| Liver | Sinus and portal infiltrates | Sinus and portal infiltrates | Sinus and portal infiltrates | Portal infiltrates infiltrates Hepatocyte injury | Sinus and portal |
| Spleen | Red pulp infiltrate Plasmacytosis Follicular hyper- plasia of white pulp | Red pulp infiltrate Red cell “lakes” Reduction of white pulp | Primary expan- sion of white pulp Frequent infiltration of red pulp | White pulp infiltrate | Red pulp infiltrate (immunoblasts, neutrophils) |
| Bone marrow | Diffuse or nodular pattern Usually nonparatra- becular Often subtle involvement Maturation arrest | Diffuse pattern Frequent “dry tap” becular | Diffuse or nodular pattern Usually nonparatra- | Usually nodular pattern | Usually diffuse pattern Plasmacytosis ParatrabecularSometimes granulomas |
| . | LGL Leukemia . | Hairy Cell Leukemia . | CLL/SLL . | Follicular NHL . | Reactive Lymphocytosis . |
|---|---|---|---|---|---|
| Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; NHL, non-Hodgkins lymphoma | |||||
| Cytology (peripheral blood) | LGL | Hairy cells | Small lymphocytes | Cleaved lymphocytes | Atypical lymphocytes and LGL |
| Liver | Sinus and portal infiltrates | Sinus and portal infiltrates | Sinus and portal infiltrates | Portal infiltrates infiltrates Hepatocyte injury | Sinus and portal |
| Spleen | Red pulp infiltrate Plasmacytosis Follicular hyper- plasia of white pulp | Red pulp infiltrate Red cell “lakes” Reduction of white pulp | Primary expan- sion of white pulp Frequent infiltration of red pulp | White pulp infiltrate | Red pulp infiltrate (immunoblasts, neutrophils) |
| Bone marrow | Diffuse or nodular pattern Usually nonparatra- becular Often subtle involvement Maturation arrest | Diffuse pattern Frequent “dry tap” becular | Diffuse or nodular pattern Usually nonparatra- | Usually nodular pattern | Usually diffuse pattern Plasmacytosis ParatrabecularSometimes granulomas |
Series of peripheral T/NK cell lymphoma.
| Site . | No. Pts . | PTCL NOS (%) . | ALCL (%) . | AILD (%) . | Angiocentric (%) . | Rare subtypes (%) . | Comments . |
|---|---|---|---|---|---|---|---|
| Abbreviations: PTCL, peripheral T cell lymphoma; NOS, not otherwise specified; ALCL, anaplastic large cell lymphoma; AILD, angioimmunoblastic type PTCL; OS, overall survival; DLBCL, diffuse large B cell lymphoma; DFS, disease free survival; IPI, International Prognostic Index; PFS, progression free survival; ATLL, adult T cell leukemia/lymphoma | |||||||
| Spain32 | 174 | 95 (55) | 30 (17) | 22 (13) | 4 (8) | 13 (7) | ALCL had the best survival (65 mo median); the others were in 20-25 mo range with the in testinal as the predominant rare subtype at only 4 mos. |
| France8 | 288 | 160 (55) | 60 (21) | 68 (24) | - | - | The 5 yr OS for T-ALCL was 64% compared to 53% for DLBCL and 35% for other PTCL. |
| Italy33 | 167 | 78 (47) | 62 (37) | 19 (11) | 2 (1) | 6 (4) | ALCL had a better DFS (66% vs 38%, p=0.0001) than the remaining PTCL. |
| United States34,35 | 78 | 53 (68) | 8 (10) | 7 (9) | 9 (12) | 1 (1) | IPI correlated with 5 yr OS: 76% low, 32% low- intermediate, 28% high-intermediate, 9% high risk. |
| 92 | 28 (30) | 40 (43) | 13 (14) | - | 11 (12) | OS was 49% and PFS was 22% at 5 yr. ALCL had best prognosis (58% OS, 40% PFS) compared to other subtypes. | |
| Korea36 | 125 | 53 (42) | 5 (4) | 5 (4) | 59 (47) | 3 (2) | Angiocentric were primarily nasal/nasopharyngeal origin. PTCL, NOS, had a worse survival compared to DLBCL. |
| Japan 37 | |||||||
| Kyushu | 265 | 83 (27) | 22 (6) | 38 (10) | 28 (8) | 3 (1) | ATLL accounted for 181 (52%) in Kyushu and 47 (15%) in other areas, for a combined 238 cases in Japan. |
| Other | 315 | 130 (41) | 27 (9) | 39 (12) | 55 (17) | 17 (5) | |
| Site . | No. Pts . | PTCL NOS (%) . | ALCL (%) . | AILD (%) . | Angiocentric (%) . | Rare subtypes (%) . | Comments . |
|---|---|---|---|---|---|---|---|
| Abbreviations: PTCL, peripheral T cell lymphoma; NOS, not otherwise specified; ALCL, anaplastic large cell lymphoma; AILD, angioimmunoblastic type PTCL; OS, overall survival; DLBCL, diffuse large B cell lymphoma; DFS, disease free survival; IPI, International Prognostic Index; PFS, progression free survival; ATLL, adult T cell leukemia/lymphoma | |||||||
| Spain32 | 174 | 95 (55) | 30 (17) | 22 (13) | 4 (8) | 13 (7) | ALCL had the best survival (65 mo median); the others were in 20-25 mo range with the in testinal as the predominant rare subtype at only 4 mos. |
| France8 | 288 | 160 (55) | 60 (21) | 68 (24) | - | - | The 5 yr OS for T-ALCL was 64% compared to 53% for DLBCL and 35% for other PTCL. |
| Italy33 | 167 | 78 (47) | 62 (37) | 19 (11) | 2 (1) | 6 (4) | ALCL had a better DFS (66% vs 38%, p=0.0001) than the remaining PTCL. |
| United States34,35 | 78 | 53 (68) | 8 (10) | 7 (9) | 9 (12) | 1 (1) | IPI correlated with 5 yr OS: 76% low, 32% low- intermediate, 28% high-intermediate, 9% high risk. |
| 92 | 28 (30) | 40 (43) | 13 (14) | - | 11 (12) | OS was 49% and PFS was 22% at 5 yr. ALCL had best prognosis (58% OS, 40% PFS) compared to other subtypes. | |
| Korea36 | 125 | 53 (42) | 5 (4) | 5 (4) | 59 (47) | 3 (2) | Angiocentric were primarily nasal/nasopharyngeal origin. PTCL, NOS, had a worse survival compared to DLBCL. |
| Japan 37 | |||||||
| Kyushu | 265 | 83 (27) | 22 (6) | 38 (10) | 28 (8) | 3 (1) | ATLL accounted for 181 (52%) in Kyushu and 47 (15%) in other areas, for a combined 238 cases in Japan. |
| Other | 315 | 130 (41) | 27 (9) | 39 (12) | 55 (17) | 17 (5) | |
Nucleoside analogs in cutaneous T cell lymphoma.
| Drug/Author . | No. of Patients . | Regimen . | Response Rate (%) . | CR (%) . | Comments . |
|---|---|---|---|---|---|
| Abbreviations: SWOG, Southwest Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; SS, Sezary syndrome; MF, mycosis fungoides; PTCL, peripheral T cell lymphoma; CR, complete response; PR, partial response | |||||
| Fludarabine | |||||
| Von Hoff61 | 31 | 25 mg/m2/d x 5 | 19 | 3 | SWOG: 30% response in good risk |
| 2′-deoxycoformycin | |||||
| Ho62 | 44 | 4 mg/m2 q week x 3, then q 2 wk x 3, q mo x4 | 36 | 2 | EORTC: median response 4.5 mo for SS and 8.3 mo for MF |
| Kurzrock63 | 24 | 3.75-5.0 mg/m2/d q d X 3d q 3 wks | 71 | 25 | Median response was 2 mo in tumor stage MF and 3.5 mo in SS. Three pts with PTCL responded. |
| 2-CdA | |||||
| Saven64 | 15 | 0.05-0.15 mg/kg/d x 7 d | 47 | 20 | Median response of 5 mo |
| Kuzel65 | 21 | 0.1 mg/kg/d x 7 d or x 5 d | 28 | 14 | Median response 4.5 mo for CR, 2 mo for PR |
| Gemcitabine | |||||
| Zinzani66 | 44 | 1.2 gm/m2 d 1,8,15 q mo x 3 cycles | 70 | 11.5 | No difference in response between PTCL (N=14) and MF; median response 15 mo for CR, 10 mo for PR |
| Drug/Author . | No. of Patients . | Regimen . | Response Rate (%) . | CR (%) . | Comments . |
|---|---|---|---|---|---|
| Abbreviations: SWOG, Southwest Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; SS, Sezary syndrome; MF, mycosis fungoides; PTCL, peripheral T cell lymphoma; CR, complete response; PR, partial response | |||||
| Fludarabine | |||||
| Von Hoff61 | 31 | 25 mg/m2/d x 5 | 19 | 3 | SWOG: 30% response in good risk |
| 2′-deoxycoformycin | |||||
| Ho62 | 44 | 4 mg/m2 q week x 3, then q 2 wk x 3, q mo x4 | 36 | 2 | EORTC: median response 4.5 mo for SS and 8.3 mo for MF |
| Kurzrock63 | 24 | 3.75-5.0 mg/m2/d q d X 3d q 3 wks | 71 | 25 | Median response was 2 mo in tumor stage MF and 3.5 mo in SS. Three pts with PTCL responded. |
| 2-CdA | |||||
| Saven64 | 15 | 0.05-0.15 mg/kg/d x 7 d | 47 | 20 | Median response of 5 mo |
| Kuzel65 | 21 | 0.1 mg/kg/d x 7 d or x 5 d | 28 | 14 | Median response 4.5 mo for CR, 2 mo for PR |
| Gemcitabine | |||||
| Zinzani66 | 44 | 1.2 gm/m2 d 1,8,15 q mo x 3 cycles | 70 | 11.5 | No difference in response between PTCL (N=14) and MF; median response 15 mo for CR, 10 mo for PR |
Overall survival 228 PTCL (non-ALCL) and 60 T-ALCL patients compared with 1,595 DLBCL patients.8
Overall survival 228 PTCL (non-ALCL) and 60 T-ALCL patients compared with 1,595 DLBCL patients.8
The different age distribution (years) of ALK+ anaplastic large cell lymphoma (ALCL) and ALK- ALCL.13
The different age distribution (years) of ALK+ anaplastic large cell lymphoma (ALCL) and ALK- ALCL.13
The difference in overall survival between ALK+ ALCL and ALK- ALCL.23