Abstract
Transfusion therapy for inherited anemias and acquired refractory anemias both improves the quality of life and prolongs survival. A consequence of chronic transfusion therapy is secondary iron overload, which adversely affects the function of the heart, the liver and other organs. This session will review the use of iron chelating agents in the management of transfusion-induced secondary iron overload.
In Section I Dr. John Porter describes techniques for the administration of deferoxamine that exploit the pharmacokinetic properties of the drug and minimize potential toxic side effects. The experience with chelation therapy in patients with thalassemia and sickle cell disease will be reviewed and guidelines will be suggested for chelation therapy of chronically transfused adults with refractory anemias.
In Section II Dr. Nancy Olivieri examines the clinical consequences of transfusion-induced secondary iron overload and suggests criteria useful in determining the optimal timing of the initiation of chelation therapy. Finally, Dr. Olivieri discusses the clinical trials evaluating orally administered iron chelators.
I. Pharmacokinetics, Metabolism and Toxicity of Deferoxamine and Other Iron Chelators
John P. Porter, MD*
Department of Hematology, University College London, 98 Chenies Mews, London WC1 6HX
Dr. Porter is a consultant for Novartis' safety monitoring committee.
Because iron is essential for many physiological functions, there will inevitably be a relatively narrow therapeutic margin between iron toxicity resulting from excess iron and toxicity from excessive chelation. The metabolism and pharmacokinetics of chelators are critical to their therapeutic success because both their efficacy and toxicity are affected by these variables.
Goals of Chelation Therapy
A. Achievement of safe tissue iron levels
The principal goal of chelation therapy is to decrease tissue iron to concentrations where iron-mediated toxicity cannot occur. Only a small fraction of body iron is available for chelation at any given time, as the majority of storage iron is not effectively chelated at clinically achievable chelator concentrations. The achievement of safe tissue iron levels therefore takes many months or years with current chelation regimens. A safe level of tissue iron is unlikely to be the same for all tissues, as myocardial iron in patients dying from the cardiac complications is typically only a fraction of the liver iron concentration. Furthermore, although cardiac toxicity is the most common cause of death in transfusional iron overload, little is known about the relationship between myocardial iron levels and prognosis. The majority of prognostic information is derived from associations of liver iron concentrations with prognosis, levels above 15 mg/g dry weight being associated with a high risk of cardiac disease.1
In order to achieve iron balance (0.4-0.5 mg/kg daily iron excretion in a transfusion dependent individual) two key iron pools need to be accessed by chelators. The first is the intracellular labile iron pool, which in iron overload is chiefly derived from lysosomal catabolism of ferritin. Labile intracellular iron is also derived from iron uptake of transferrin or non-transferrin bound iron (NTBI) into cells. Hepatocytes are the major cell of iron storage in advanced iron overload, and liver iron closely reflects total body iron (total body iron stores in milligrams per kilogram of body weight = 10.6 x hepatic iron concentration in milligrams per gram of liver, dry weight).2 The liver is therefore a key target for chelation therapy. Fecal iron excretion with deferoxamine (DFO) is derived from chelation of hepatocellular iron from this pool of labile intracellular iron.3–,5 The second major source of chelatable iron is that derived from red cell catabolism in macrophages.3,4 In a healthy individual, 20 mg of iron is released by macrophages in liver, spleen, and marrow every day; this is increased during ineffective erythropoiesis but decreased by hypertransfusion in thalassemia syndromes.5 In transfusional iron overload, transferrin is generally saturated so that NTBI plasma iron species are typically present at concentrations of 1-10μM.6 Transit iron released from macrophages is the major source of urinary iron achieved with DFO therapy.4,5 It is not certain whether DFO binds this iron pool within macrophages or immediately after release from macrophages, but the latter appears more likely.
B. Iron detoxification
Because it may take many months or several years to accomplish the prime goal of decreasing tissue iron to safe values using currently available chelation regimes, a second key goal of iron chelation therapy, until the first objective is achieved, is iron detoxification. Iron (III) (ferric iron) has six coordination sites, which need to be chelated completely if the generation of free radicals through redox cycling is to be prevented. Chelators, which coordinate all six sites using a single molecule (hexadentate chelators) such as DFO, tend to form more stable iron-chelate complexes than ligands, which require more than one molecule. Chelators that possess only two co-ordination sites (bidentate chelators) tend to dissociate from iron at low concentrations, resulting in partial co-ordination and the potential to generate free radicals under these conditions. Iron that is not coordinated by physiological ligands such as transferrin or stored within the ferritin shell is potentially available to participate in the generation of harmful free radicals. Within cells this is present as labile iron and within plasma as NTBI. The exact forms of these iron species are incompletely understood, but the latter probably consists of citrate and albumin bound species. The detoxification of these iron species by chelators requires a biodistribution that allows access to these pools and that the iron-chelate complexes be both stable and rapidly excreted.
It is important to realise that tissue injury can be halted even in the face of very high tissue iron levels using continuous infusion of DFO.42 Thus whilst it may take months or years of chelation treatment to decrease bound iron to ‘safe’ levels, demonstrable benefit to cardiac performance occurs in many cases within days or weeks of continuous DFO therapy.42 In patients at high risk, either from consideration of liver iron concentrations, or because of falling ventricular ejection fractions or cardiac dysrhythmias, continuous intravenous (IV) or subcutaneous (SC) DFO treatment should be considered.
C. Wide therapeutic safety margin
The toxicity of iron chelators may result either from the excessive chelation of iron or other metals needed for essential metabolic functions or from the toxicity of unstable iron-chelate complexes. A key therapeutic challenge is to chelate excess iron pools without removing iron or other metals such as zinc from necessary metabolic pools. Chelators with small dimensions and high lipid solubility tend to interact with these metabolic pools more rapidly than larger and more hydrophilic molecules.7 Such inhibitory reactions are concentration- as well as time-dependent; the pharmacokinetics, distribution and metabolism of chelators therefore play an important role in determining whether these enzymes are inhibited. Iron chelators are cytotoxic to dividing cells at concentrations of 1-10 μM depending on the cell type involved and the duration of exposure to chelators.8,9 In order to achieve the widest therapeutic safety margin, chelator regimens need to be designed to minimize the inhibition of key enzymes and cytotoxic effects. The chelator should also have a high specificity of iron (III) relative to other metals such as zinc and should possess high stability of the iron-chelate complex, as demonstrated by a high pM value: (where pM is defined as –log of the uncoordinated iron concentration measured at pH 7.4, in a solution of 10 μM ligand and 1 μM iron [III]).10
Deferoxamine
A. Pharmacology and pharmacokinetics
Hexadentate chelators tend to have molecular weights that are too large to allow efficient absorption from the gut, and DFO (MW 657) therefore cannot be administered orally. The advantage of the hexadentate structure is the relatively high stability for iron (III) reflected by the high pM value of 27.7.10 When this drug was first evaluated for the treatment of iron overload, it was given as daily intramuscular injections, which produced significant urinary iron excretion, but this was generally insufficient to maintain negative iron balance.11 It was not until the introduction of IV infusion and subsequently SC infusions that effective negative iron balance was obtained.12 Detailed pharmacokinetic knowledge lagged behind observations of the efficacy of prolonged infusions, but it became clear that the short plasma half-life together with the finite transiently chelatable iron pools favored a chelation regimen where the drug was delivered over a prolonged period.
Pharmacokinetics of DFO has been studied mostly following IV injection or infusion. Early studies examined both IV bolus injections at 10 mg/kg and 24 hour continuous IV infusions at 100 mg/kg.13 Peak plasma concentrations of 80-130 μM were obtained following the IV bolus with an initial half life of 5-10 minutes. The proportion and maximal plasma concentration of iron bound DFO (ferrioxamine) was higher in iron overloaded patients than in healthy volunteers. Steady state ferrioxamine (FO) concentrations with IV infusion at 100 mg/kg were also higher in iron-loaded subjects, 12.9 μM, than in controls, 2.7 μM. The clearance of FO, injected as 59ferrioxamine, was noted to be slow, although this was not quantified. Later studies using IV DFO and high performance liquid chromatography (HPLC) analysis showed that elimination from plasma has two components, with an initial half-life of 0.3 hour and a terminal half-life of 3 hours.14 This study did not differentiate between DFO and FO, and it is likely that the rapid phase represented elimination of the former and the slower terminal phase the elimination of latter. During the IV infusion of 50 mg/kg/day in thalassemia major patients, steady state concentrations (DFO+FO) were generally obtained between 6-12 hours with a mean concentration of 7.4 ± 2.73 μM.14
Subcutaneous infusion at a daily dose 40 mg/kg over 8-12 hours has become the standard schedule of delivery for over 20 years.15,16 However the pharmacokinetics of subcutaneous DFO were unknown until its inclusion as a control arm of a recent multicenter study designed to evaluate a depot preparation of DFO. During an 8 hour infusion of standard DFO at 40 mg/kg the mean plateau concentration of DFO+FO of 10.1 μM was obtained within 4 and 8 hours after starting the infusion. After the infusion finished, plasma levels fell rapidly, with an initial t1/2 of 0.56 hour and a terminal t1/2 of 9.8 hours.17 The latter is likely to represent FO elimination.
B. Biodistribution and metabolism
DFO is hydrophilic, and this property together with its high molecular weight means that uptake into cells and subcellular compartments is generally slow relative to hydroxypyridinones (such as deferiprone), taking several hours for equilibration.18,19 Uptake into hepatocytes is rapid, however, probably by a facilitated process, making access to intrahepatic iron relatively efficient.20 Due to the positive charge of both DFO and FO, egress from cells by diffusion will be slow.21 DFO is relatively stable for several hours in plasma, and the majority of metabolism takes place within hepatocytes by oxidative deamination of the N-terminus, yielding metabolite B as the predominant product.21 The iron free form of metabolite B can diffuse back into plasma rapidly, due to its negative charge,21 where it is rapidly cleared from plasma (t1/2 = 1.3 hours) and eliminated in the urine.14 Levels of metabolite B are generally less (AUC 191 ± 106 μM) than those of DFO (354±131 μM) at standard clinical doses,14 but its relative proportions may be greater when excessive DFO doses are given (see below).
C. Clinical toxicity.
Toxicity from DFO in thalassemia major is unlikely provided that doses do not exceed 40 mg/kg/day, that DFO is not introduced at too young an age (see below), and that the dose is reduced as iron loading falls. Unfortunately this information has been gathered by the empirical clinical experience of giving greater doses and observing unforeseen toxicity. In other conditions where iron overload and distribution may be difficult to determine, such as sickle cell disorders, particular caution with dosing and monitoring is advisable.
Retinal toxicity:
The first significant toxicity to be noted, namely retinal and optic nerve disturbances, sometimes associated with pigmentary retinal changes, were described in patients receiving very high doses of DFO (125 mg/kg/day).22 Symptoms include blurred vision, loss of central vision, night blindness and optic neuropathy.23 The risk may be higher in patients with diabetes or other factors affecting the blood-retinal barrier,24 so these groups should be monitored more carefully. Symptoms and electroretinographic disturbances generally resolve over 1-2 months of stopping treatment provided these are identified sufficiently quickly. Failure to do so can lead to permanent retinal damage. Treatment can usually be resumed later at a reduced dose with close monitoring by electroretinography. It is advisable to perform regular assessment of retinal function, including yearly electroretinographic assessment for patients with diabetes on intensive continuous chelation or in patients where a rapid fall in iron loading is achieved.
Auditory toxicity:
High frequency sensorineural hearing loss was initially described in approximately 25% of well-chelated patients on high dose DFO regimens23 and may be reversible if diagnosed early.25 The risk is greatest in patients with low degrees of iron overload receiving high doses of DFO. By keeping the therapeutic index [the mean daily dose of DFO (mg/kg) divided by the serum ferritin (μg/L)]25 below 0.025, reducing the dose as the ferritin falls, together with yearly monitoring of audiometry, the risks can be minimized.
Effects on growth and bone:
The need for a correct balance between under and over-treatment of iron overload is perhaps best demonstrated by consideration of growth. Iron overload in the pituitary leading to hypogonadotrophic hypogonadism is the major cause of growth retardation in patients receiving high transfusion regimens.26 Paradoxically, over-treatment with chelation retards growth: the risk factors include young age, years at commencement of treatment (< 3 years), higher doses of DFO > 40mg/kg27–,29 and lower levels of iron overload. It is advisable to monitor height velocity as well as sitting and standing height twice yearly and adjust dosing as necessary. A quick resumption in growth follows reduction in DFO dosing, without the need to stop treatment.27
Rickets-like bony abnormalities have been described in association with decreased growth,28 and radiographic abnormalities of the distal ulnar, radial and tibial metaphases appear to be associated features. Vertebral growth retardation or milder changes involving vertebral demineralization and flatness of vertebral bodies have also been noted in well-chelated patients.30 It may be advisable to undertake regular surveillance for the toxic effects of DFO on bone31 with annual radiologic assessment of the thoracolumbar-sacral spine as well as the forearm and knees, and to reduce the dose of the chelator if significant changes are noted.
Localized reactions:
Local reactions with skin reddening and soreness occurring immediately or after the infusion has finished may be seen at the subcutaneous infusion site. These are often caused by DFO being reconstituted above the recommended concentration of 10%. On occasions when local reactions remain a problem, the instillation of a small dose of hydrocortisone (5–10 mg/infusion) may be helpful. Desensitization regimens used for serious reactions have also proved effective in severe cases of localized reactions (see below)
Infections:
There is an increased risk of Yersinia infection in iron overload, and this risk increases further with DFO treatment as Yersinia does not make a natural siderophore and uses iron from FO to facilitate its growth.32 Patients who present with diarrhea, abdominal pain or fever should stop DFO until Yersinia infection can be reasonably excluded by appropriate stool samples, blood cultures and serological testing. The growth of other organisms (e.g. Klebsiella) may also be facilitated by FO and it is wise to withhold DFO in a febrile patient until the source of the fever has been identified.
Rare effects:
Systemic reactions, with fever, muscle aches and arthralgia are uncommon and anaphylaxis may occasionally occur. Successfully desensitization regimens are described.33,34 Renal impairment, has been reported in occasional patients given high doses,35 and reversible changes in the glomerular filtration rate may be seen at lower doses.36 Very high doses (10-20 mg/kg/h) have been associated with a fatal respiratory syndrome37 and pulmonary fibrosis.38 Lens opacities occurring after high doses improved when the chelator was withdrawn.22 In non iron-overloaded patients, DFO potentiated the action of the phenothiazine derivative prochlorperazine, leading to reversible coma in two patients.39 Bolus intravenous doses may lead to nausea, vomiting, hypotension with acute collapse or even transient aphasia. Thrombocytopenia has been reported in two patients on renal dialysis.40
D. Relevance of pharmacokinetics and metabolism to toxicity of iron and deferoxamine
Because DFO is cleared rapidly from the plasma within minutes after SC or IV infusion ceases,14,17 continuous infusion has theoretical advantages. Continuous infusion will not only theoretically maximize iron excretion but also will minimize iron toxicity, as NTBI is found in plasma within minutes of stopping intravenous infusion.41 Continuous IV infusion has been shown to produce excellent long-term survival results in high-risk cases with cardiac complications.42 Thus although the 8-10 hour infusion has become ‘standard’ therapy and can maintain iron balance, infusions of longer duration have theoretical advantages for iron detoxification and should be encouraged where patient compliance is achievable. Because of the short plasma half-life, the use of intravenous DFO at the time of blood transfusion has minimal impact on iron balance or iron detoxification. Other reasons for avoiding this approach are that inadvertent intravenous DFO boluses can occur, and the interpretation of pyrexias during transfusions can be obscured. Metabolism is essentially intracellular, so that as the availability of chelatable intracellular iron decreases, the proportion of metabolite B increases. Furthermore, because DFO but not FO enters the hepatocyte freely, the increased availability of extrahepatic chelatable iron will also decrease metabolite B formation.43 Thus, in principle the presence of metabolite B can be used as a surrogate marker for excessive DFO dosing and potential toxicity. A trial conducted to test this hypothesis showed that metabolite B is directly correlated with the therapeutic index [the mean daily dose of DFO (mg/kg) divided by the serum ferritin (μg/L)], consistent with the notion that decreased availability of chelatable iron results in increased intracellular DFO metabolism.44 There was no evidence for an inherent qualitative difference DFO metabolism in patients at high risk of developing toxic complications from DFO. In principle, the presence of high proportions of metabolite B could be used as a trigger for dose adjustment in high risk patients receiving high doses of continuous IV DFO.
Deferiprone
A. Pharmacology and pharmacokinetics.
Deferiprone (1,2 dimethy-3-hydroxypyridin-4-one) is a member of a family of hydroxypyridin-4-one (HPO) chelators45 that require three molecules fully to bind iron (III), each molecule providing two co-ordination sites (bidentate chelation). The pM of deferiprone for iron (III) (pM = 20) is less than that of DFO, which reflects the lower stability of the iron-chelate complex. Other metals such as Ga3, Al3+ and In3+ are bound tightly with relative affinities for other metals being Cu2+ > Zn 2+ > Ca 2+ > Mg 2+.46 The molecular weight of deferiprone is approximately one-third that of DFO and this together with its neutral charge and relative lipophilicity account for its rapid absorption from the gut. These same properties also allow more rapid access by deferiprone and related HPOs to intracellular iron,47 to labile intracellular iron48 as well as more rapid access to intra-lysosomal iron pools18 and to iron containing enzymes.49,50
Deferiprone appears in plasma within 5 to 10 minutes of ingestion, the peak concentrations (Cmax) occurring within 1 hour, reaching levels in excess of 300 μM after oral ingestion of a 50 mg/kg dose.51,52 However, these levels are short-lived with an elimination t1/2 of 1.52 hours. Unlike DFO, where approximately half of iron excretion is fecal, there is little fecal iron excretion with deferiprone.53– 55
B. Metabolism
Deferiprone is metabolized to the inactive glucuronide that is the predominant form recovered in the urine.51,56 The peak concentration of the glucuronide typically occurs about 30 minutes after the peak of the native compound. It is likely, though unsubstantiated, that the variability in response to deferiprone is at least partly accounted for by variability in the glucuronidation rate between individuals. There is considerable genetic heterogeneity for glucuronidation rates of a number of drugs, and a study of the relationship between rates of glucoronidation and efficacy might identify patients most likely to respond to deferiprone.
C. Toxicities
Neutropenia and Agranulocytosis: Agranulocytosis is the most serious unwanted effect of this drug so far identified. This was initially reported in 3-4% of patients treated with deferiprone; mild neutropenia was found in an additional 4%.57,58 A prospective multicenter safety trial in 187 patients at a dose of 75 mg/kg in three divided doses showed monitoring blood counts weekly and stopping the drug at the first signs of neutropenia reduced the risk of agranulocytosis (absolute neutrophil count < 500/μL) to as low as 0.6 per hundred patient years.59 It may be difficult to interpret whether neutropenia in a patient receiving deferiprone is part of an independent fluctuation in the neutrophil count (which is more likely in unsplenectomized patients) or is the first sign of agranulocytosis induced by deferiprone. Agranulocytosis may last from 4 to 124 days.60,59 Growth factors have been used in some cases of severe or protracted neutropenia, but their value is uncertain. Reintroduction of deferiprone leads to a rapid fall in the neutrophil count in some patients and, therefore, is contraindicated. Avoidance of deferiprone in patients in whom stem cell or progenitor function is compromised would also seem advisable, such as those with Diamond-Blackfan anemia.61
Arthropathy:
Painful swelling of the joints, particularly the knees, occurs in 6-39% of patients.62,59,57 This complication usually but not always resolves after stopping therapy. Arthritis was the most common side effect in the Indian study in which many of the patients were heavily iron overloaded62 and was also more common in patients with high degrees of iron loading in the multicenter study.59
Other Unwanted Effects:
Other unwanted effects include nausea (8%), zinc deficiency (14%) and fluctuation in liver function tests (44%).58 Treatment had been discontinued in 13-30% of patients in various studies.57,58,63 Isolated cases of disturbances of immune function such as fatal systemic lupus, increased antinuclear antibodies and rheumatoid factors,64 and a fatal varicella infection65 have been reported but not found in longitudinal studies.66,59 Progression of audiometric disturbances was reported in 5 of 9 patients switched from DFO to deferiprone, but in 7 patients without audiometric disturbances prior to switching no new abnormalities occurred.67
Liver fibrosis.
In a retrospective analysis, fibrosis was reported to progress in 5 of 12 patients on deferiprone and in none of 12 age-matched control subjects receiving regular DFO.68 The estimated median time to progression of fibrosis was 3.2 years. Four of the 5 patients with increasing fibrosis were seropositive for hepatitis C, but the frequency of antibody to hepatitis C was the same (6/14) in the deferiprone-treated group and the DFO-treated group (5/12). While there are as yet no prospective data that address this issue, a number of investigators have reported no excess fibrosis that they could not attribute to active hepatitis C. For example liver biopsies taken at 2-4 years followup in patients who were hepatitis C RNA negative showed no fibrosis in 11 of 12 and mild fibrosis in 1 of 12 patients, whereas in 5/5 patients who were hepatitis C RNA positive cirrhosis was present.63 A DFO control group was not reported, however. Another study found hepatic fibrosis in 7 of 11 patients on long-term deferiprone, which was greater in the hepatitis C positive than the hepatitis C negative group.69 A number of abstracts have reported a lack of progression of fibrosis, the most recent being a 3.5-year follow-up in 56 patients.70
D. Relationship of pharmacokinetics and metabolism to efficacy and toxicity
Rapid glucuronidation significantly limits the efficacy and toxicity of a number of HPOs but there are significant interspecies differences. For example 1,2 diethyl-3-hydroxypyridin-4-one (CP94) is significantly more active than deferiprone in the rat but has no activity or toxicity in the guinea pig. This difference is due to rapid glucuronidation of the dimethyl derivative in the guinea pig.71 In humans, this compound is unfortunately glucuronidated much more rapidly than deferiprone,72 but a number of derivatives have been identified which are not glucuronidated.46 The rate of inactivation by glucuronidation of a number of HPOs in animal models appears to be decreased in the presence of iron overload.73 The short plasma half life and rapid glucuronidation of deferiprone means that even with three daily doses, there are intervals where blood levels are subtherapeutic. By analogy with DFO, more continuous exposure to chelation is predicted to induce greater iron excretion: indeed preliminary findings suggest that such a strategy can lead to increased iron excretion with deferiprone.74
The relevance of metabolism and plasma concentrations of deferiprone to agranulocytosis is under investigation but is presently unclear. It is not known whether increasing the dose from 75 mg/kg/day to 100 mg/kg/day, as has been advocated for patients whose liver iron concentrations fail to respond,75 will increase the risk of agranulocytosis, and a large study would be needed to clarify this question. There is debate about whether agranulocytosis in humans is dose dependent as it is in laboratory animals;9 it is presumed to be idiosyncratic by some investigators.60,76 In laboratory animals agranulocytosis is associated with bone marrow hypoplasia, possibly through a cytotoxic mechanism.9 In humans, bone marrow findings have not been frequently reported, but hypoplasia has been noted in some cases.60,76 In some patients an immune mechanism may be involved, as suggested by a report of agranulocytosis associated with a vasculitic syndrome and disturbances of immune function.77
The relevance of the plasma concentration of iron-chelate complexes to toxicity is also unclear. These have the potential to participate in the generation of hydroxyl radicals, thereby increasing the risk of lipid peroxidation and inflammation.78 The higher frequency of arthropathy in deferiprone treated patients who are relatively iron overloaded suggests that the iron-chelate complexes may be responsible.62,59 It has also been suggested that these may cause DNA damage in the presence of high concentrations of intracellular iron,79 but this has not been demonstrated in a clinical setting.
Other Chelators.
New developments in chelation therapy include hydroxypyridinones with higher pM values, less inhibition of metalloenzymes and slower metabolism than deferiprone.7,80 Clinical evaluation of a new class of oral chelators has begun with detailed pharmacokinetic studies.81 Combined use of deferiprone with DFO has been advocated82 and may increase iron excretion through an iron ‘shuttling’ mechanism.83 It will be important to establish that this does not increase cytotoxic effects or the shuttling of other metals at clinically relevant concentrations of these chelators.
II. Iron Chelating Therapy
Nancy F. Olivieri, MD*
Hospital for Sick Children, Hematology/Oncology Department, 555 University Avenue, Room 9413, Toronto ONT M5G 1X8, Canada
Iron Overload
In untransfused patients with severe β-thalassemia, abnormally regulated iron absorption results in increases in body iron burden that may, depending on the severity of erythroid expansion, vary between 2 and 5 grams per year.3,4 Regular transfusions may double this rate of iron accumulation. Although most clinical manifestations of iron loading do not appear until the second decade of life in inadequately chelated individuals, evidence from serial liver biopsies in young patients indicates that the deleterious effects of iron are mediated much earlier. After approximately one year of transfusions, iron is deposited in parenchymal tissues, where it may cause significant toxicity as compared to that within reticuloendothelial cells.5 As iron loading progresses, the capacity of serum transferrin, the main transport protein of iron, to bind and detoxify iron may be exceeded. Thereafter, the non-transferrin-bound fraction of iron within plasma may promote generation of free hydroxyl radicals, propagators of oxygen-related damage.5 The effectiveness of an iron-chelating agent depends in part on its ability to bind non-transferrin bound plasma iron over sustained periods of time, thereby ameliorating iron-catalyzed toxicity of free radicals.
Clinical Impact of Iron Overload
In the absence of chelating therapy, iron accumulation results in progressive dysfunction of the heart, liver and endocrine glands.1 In response to iron loading, human myocytes in vitro upregulate the transport of non-transferrin-bound iron,6 thereby possibly aggravating cardiac iron loading. Extensive iron deposits are associated with cardiac hypertrophy and dilatation, myocardial fiber degeneration and, rarely, fibrosis.7 In many patients abnormal function is observed in the absence of symptoms.8 In transfused, unchelated patients, symptomatic cardiac disease is observed after about ten years following the start of transfusions9 and may be aggravated by myocarditis10 and pulmonary hypertension.11,12 Survival is determined by the magnitude of iron loading within the heart.13,14
Iron-induced liver disease is a common cause of death in transfused patients.15 Within two years following the start of transfusions, collagen formation16 and portal fibrosis17 are observed; in the absence of chelating therapy, cirrhosis may develop in the first decade of life.18,19 As in cultured heart cells, upregulation of transport of non-transferrin-bound iron is observed in cultured hepatocytes,20 possibly aggravating iron loading in vivo.
In transfused patients, iron loading within the anterior pituitary results in disturbed sexual maturation in approximately 50% of males and females.21 Diabetes mellitus is observed in about 5% of adults.21,22 Chronic iron deposition also damages the thyroid, parathyroid, adrenal glands, and exocrine pancreas23–,25 and may provoke pulmonary hypertension, right ventricular dilation, and restrictive lung disease.26,27
Iron Chelation Therapy
Iron overload may be treated or prevented with a chelating agent capable of complexing with iron and promoting its excretion. The only iron-chelating agent presently available for clinical use in the US is DFO B, a trihydroxamic acid produced by Streptomyces pilosus with relative specificity for ferric iron. Poorly absorbed from the gastrointestinal tract and rapidly metabolized in the plasma, DFO is usually given by prolonged parenteral infusion. Several studies have permitted the design of regimens of nightly SC DFO infusions using portable ambulatory pumps.1,28 Iron bound by DFO is rendered virtually inactive: hence, the drug can prevent or reverse the effects of free radical formation and lipid peroxidation.28,29
Evidence of the clinical effectiveness of DFO:
Net negative iron balance is feasible during treatment with deferoxamine over a long period; adequate therapy has led to survival curves that approximate to those of the normal population.1,2,13,14,30–,35 The magnitude of body storage iron is the primary determinant of survival.13,14,30 A recent survey of transfused thalassemia patients reported mortality of approximately 50% prior to the age of 35 years;31 this analysis included patients born 20 years before the implementation of DFO as well as many who had not received DFO prior to adulthood. The survival of this study's most recent birth cohort31 is consistent with reports from other centers.
Adequate DFO prevents early death from cardiac disease;13,14 maintenance of body iron burden corresponding to hepatic iron concentrations less than 15 mg/g dry weight is associated with a greatly decreased risk of this complication.13 The prevalence of clinical cardiac disease in ideally chelated patients over the age of 15 years was 9%;14 in a slightly younger cohort of patients this figure was 2%.32 Hepatic iron can be maintained at near normal concentrations with modern regimens of DFO. Moreover, DFO arrests the progression of hepatic fibrosis to cirrhosis (a critical event associated with an increased risk of death in iron overload33) even when it is administered in regimens that stabilize, rather than reduce, body iron burden.34 A favorable effect of sustained reduction in body iron is also suggested by a relatively low prevalance of thyroid, parathyroid, and adrenal abnormalities in the modern era.2 In parallel, early and intensive DFO may improve the incidence of normal sexual maturation21 but has not been observed to reverse established abnormalities. Similarly, while DFO is effective in the prevention of diabetes mellitus,13 there is no evidence that this complication can be reversed with therapy. Finally, the effect of transfusion and chelating therapy on pulmonary function in iron overload requires further study.
The most common difficulty associated with long-term DFO is erratic compliance with therapy.1,35,36 Protocols for alternate means of administration have been developed in patients unable or unwilling to administer full doses of DFO. Implementation of short-term intravenous therapy frequently induces rapid reduction in body iron burden, which may itself improve a patient's outlook and his or her subsequent compliance.41 Other approaches include administration of twice daily intramuscular DFO, a regimen that can arrest the progression of hepatic fibrosis at doses that stabilize, but do not reduce, body iron burden.34 Alternatively, DFO administered by twice-daily SC injections which may induce urinary iron excretion equal to the same dose administered by prolonged SC infusion71,72 (and appear to induce greater urinary iron excretion than does the oral chelating agent deferiprone), may be tolerated by the patient. Finally, the balance studies described below which have compared the orally active chelating drug deferiprone and DFO indicate that SC DFO administered two to three times per week achieves iron excretion greater than that induced by daily deferiprone, without the uncertainty of the latter drug's toxicity. In summary, because of the rarity of genuine allergic reactions to the drug, there are very few patients who cannot tolerate some modified regimen of DFO.36 The observation that even low doses of DFO arrest progressive hepatic fibrosis justifies attempts to provide modified regimens to patients. Difficulties with compliance do not appear to be limited to parenteral therapy: in carefully selected cohort of patients who had declared their unwillingness or inability to administer DFO” and who were treated with deferiprone over one year, compliance with this oral agent was reported to vary between 40% and 100%.
Optimal body iron concentrations in patients with thalassemia major:
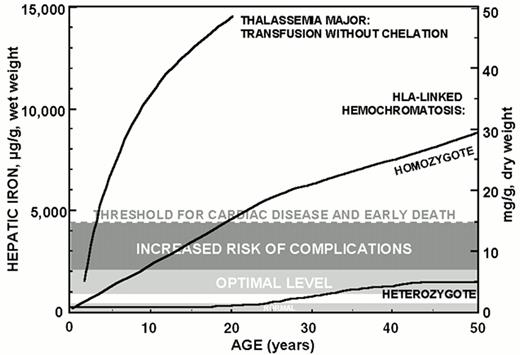
Data accumulated over the past ten years permit a quantitative approach to the management of iron overload and guidelines for the control of body iron burden in chelated patients. Maintenance of hepatic iron levels exceeding 15 mg/g liver, dry weight, in thalassemia patients is associated with a heightened risk of cardiac disease and early death.13 Slightly lower iron burdens corresponding to hepatic iron levels between 7-15 mg/g liver, dry weight, are associated with an increased risk of other complications of iron overload, including hepatic fibrosis, in homozygotes for hereditary hemochromatosis33,37 and in patients with thalassemia.1,38 By contrast, in about one-quarter of those heterozygous for hereditary hemochromatosis, body iron stores do not increase beyond about 2 to 4 times the upper limit of normal levels (corresponding to hepatic iron levels between 3.2-7 mg/g liver, dry weight) associated with a normal life expectancy.39 Clearly, the toxic manifestations of iron overload depend not only on the amount of excess iron but also on several other factors including the rate of iron accumulation, the duration of exposure to increased iron, the partition of iron between reticuloendothedlial stores and more hazardous deposits in parenchymal cells, ascorbate status and other factors influencing the distribution of iron between macrophage and parenchymal sites, and the influence of other hepatotoxins including alcohol and viral hepatitis.40 Nonetheless, the data suggest that a conservative goal for iron chelation therapy in transfusion-dependent patients is maintenance of body iron corresponding to hepatic storage iron levels of about 3.2-7 mg/g liver, dry weight, the range identified in asymptomatic heterozygotes for hereditary hemochromatosis. Patients with body iron burdens of 7-15 mg/g liver, dry weight, require more intensive subcutaneous iron chelation therapy.33,37 Patients with body iron burdens higher than 15 mg/g liver dry weight are candidates for protocols of intravenous DFO.1,41
Serum ferritin concentrations are a more commonly used, but less reliable, means to estimate body iron. The serum ferritin concentrations corresponding to the optimal range for hepatic iron shown in Figure 1 are not clearly defined. Serum ferritin concentration of approximately 2,500 μg/L, maintained over a period of approximately 15 years, has been used as a threshold level to identify, retrospectively, patients at increased risk of clinical cardiac disease.14 Further evidence is emerging that truly safe levels of body iron with respect to the development of complications of iron overload are lower than this estimate.38,42
Thalassemia “intermedia”
Iron loading secondary to increased gastrointestinal iron absorption in patients with thalassemia “intermedia” is less accelerated than that of transfusional iron overload in thalassemia major3 and may be similar to that found in homozygotes for hereditary hemochromatosis. Striking elevations of hepatic iron concentration, in parallel with modestly elevated levels of serum ferritin, have been observed in adults with thalassemia intermedia, and direct determination of body iron burden is indicated in any patient with an elevated serum ferritin concentration. Chelating therapy should be initiated if the hepatic iron concentration exceeds 7 mg/g dry weight liver tissue. Usually, in untransfused patients, the reduction of hepatic iron is rapid, and if compliance is reasonable, deferoxamine therapy is rarely required for more than 18 months.
Initiation of chelating therapy:
Uncertainties exist regarding the optimal age to start chelation therapy (see Deferoxamine Toxicity). Tissue iron concentrations have been determined in very few children early in the course of regular transfusions, prior to initiation of chelating therapy and appear to be highly variable: some children appear to acquire tissue iron loads that exceed thresholds of risk (7 mg/g) after 10-12 transfusions, while in others, concentrations may be much lower than this. The mean hepatic iron concentration in one cohort of patients with thalassemia major after approximately 1.3 years of transfusions, was 8.5 mg/g.43 Hepatic iron concentrations should, ideally, be measured, using tissue obtained at biopsy, after approximately one year of regular transfusions to identify children who do, or do not, require treatment with DFO at this important time point in their clinical course. The level of hepatic iron that should prompt therapy is approximately 5-6 mg/g liver, dry weight, which is in the range that should be maintained during chronic iron-chelating therapy. Clearly, delays in therapy may result in unnecessary accumulation of tissue iron, while treatment at levels less than this may be associated with a increased incidence of DFO toxicity. If a liver biopsy is not possible one year after the start of regular transfusion, treatment with SC DFO, not exceeding 25-35 mg/kg body weight/night, should be initiated at that time. In older patients it is usually possible to maintain iron balance with no more than 50 mg/kg body weight/night, administered five nights each week.
“Rescue” of patients with intravenous DFO:
Regimens of intravenous ambulatory DFO administered through implantable venous access ports or Hickman lines are associated with rapid reduction of body iron burden.44 Long-term intravenous DFO is associated with improvement in clinical cardiac disease, good compliance in most patients, a low incidence of DFO toxicity, and an acceptable incidence of line infections and of thrombosis.41
Assessment of body iron burden:
Both direct and indirect means for the assessment of body iron are available; no single indicator or combination of indicators is ideal for the evaluation of iron status in all clinical circumstances. The measurement of plasma or serum ferritin is the most commonly used indirect estimate of body iron stores.1,45 Interpretation of ferritin levels may be complicated by a variety of conditions that alter concentrations independently of changes in body iron burden, including ascorbate deficiency, fever, acute infection, chronic inflammation, acute and chronic hepatic damage, hemolysis and ineffective erythropoiesis45. Reliance on ferritin alone usually leads to inaccurate assessment of body iron burden in individual patients. Similarly, the serum iron, transferrin, transferrin saturation and transferrin receptor concentration, or urinary iron excretion following an infusion of DFO do not quantitatively reflect body iron stores. Studies to determine the usefulness of measurements of the iron content of serum ferritin in patients with iron overload show conflicting results;46,47 the usefulness of this modality requires further study. The availability of a simple assay for monitoring non-transferrin-bound plasma iron could provide a useful measurement of iron status but has not been applied widely.48
A variety of studies have been directed at imaging tissue iron by computed tomography, nuclear resonance scattering, and magnetic resonance imaging (summarized in reference 45). Studies of the correlation obtained between hepatic iron levels determined at biopsy and by magnetic resonance imaging have reported wide confidence intervals. Hence, present MRI techniques do not distinguish patients with dangerous levels of body iron (those with hepatic iron concentrations exceeding 15 mg iron/g dry weight tissue) from those who require standard approaches to chelating therapy (those whose hepatic iron values exceeding 7 but less than 15 mg/g); the overlap between MRI-determined hepatic iron values between the ranges is too great to guide approaches to therapy.49 MRI measurements have also been shown to be altered by the presence of hepatic fibrosis and therefore, in many patients with elevated hepatic irons in whom fibrosis is a common finding, cannot provide measurements of tissue iron quantitatively equivalent to those determined at tissue biopsy.50
Measurement of hepatic iron concentration is the most quantitative, specific, and sensitive method for determining body iron burden.45 Iron in biopsy samples is assessed most frequently using atomic absorption spectroscopy. In patients with severe thalassemia, liver biopsy of adequate weight permits evaluation of the non-heme storage iron concentration, the pattern of iron accumulation, and the extent of inflammation, fibrosis and cirrhosis. The perceived dangers and discomforts of liver biopsy and the impression that liver iron concentrations do not reflect accurately body iron burden may explain the reluctance of many centers to undertake regular liver biopsies to monitor body iron burden. The frequency of complications appears to be lower in children, in whom a 0.5% rate of major complications has been reported,51,52 than in adults.53 Quantitation of liver iron from a single liver biopsy has limited level in long-term monitoring of body iron burden,38 emphasizing the importance of regular monitoring of hepatic iron in the management of patients with iron overload. Although uneven distribution of hepatic iron has been reported in some studies54 a recent study demonstrated unequivocally that the iron concentration of adequate biopsies of non-cirrhotic livers is a reliable indicator of total body iron stores.55
Transjugular liver biopsy has been undertaken in many centers, most often in patients with bone marrow failure syndromes in whom reduced platelet counts present a practical issue with respect to the safety of a percutaneous procedure. In this author's experience, such samples are frequently not adequate for the quantitation of iron, but increased experience may result in improvements in sampling through this approach.
Magnetic susceptometry using a superconducting quantum interference device (SQUID) magnetometer provides a direct measure of hepatic storage iron that is based on a fundamental physical property of ferritin and hemosiderin.45,56 Magnetic susceptometry is available presently in two sites, one in the United States45 and one in Germany.57 The correlations between hepatic iron concentration determined by SQUID and those measured from biopsy specimens in these two centers do not appear to be equivalent.45,56–,58 The development of high-transition-temperature superconductors that can operate at liquid nitrogen temperatures promise to make SQUID susceptometers widely available at low cost.45
The balance between the effectiveness and toxicity of DFO is described in another section in this review.
Iron Chelation in Sickle Cell Disease
As the indications for transfusions in patients with sickle cell disease have expanded59,60 iron overload has now complicated the course of many patients with this disorder.61 Although tissue iron loading,62,63 hepatic fibrosis and cirrhosis64,65 were reported in early pathologic studies of this disorder, iron-induced tissue damage was hypothesized to be unusual in sickle cell disease,66 ostensibly because inflammation associated with this disorder purportedly fixes a large proportion of iron in reticuloendothelial cells. Similarly, a recent study found elevated hepatic iron levels but only modest histologic changes in biopsies obtained from transfused, chelated patients with sickle cell disease. It should be noted that some patients had been transfused for very short periods of time, most under ten years,52 and that, even in patients poorly compliant with DFO, cardiac symptoms do not present during the first decade of transfusions in patients with thalassemia.9 Hence, it is not surprising that in patients who are likely to become transfusion dependent later in life, such as those with sickle cell disease, the onset of iron-associated complications will be later than in those with thalassemia. Other clinical and pathologic studies have observed diabetes7 and serious liver and cardiac dysfunction7,67 in iron-loaded patients with sickle cell disease. Similar proportions of elevated body storage iron concentrations and hepatocellular damage have been observed one to three years following the initiation of transfusions damage in patients with both thalassemia and sickle cell disease.68 In sickle cell disease, as in other disorders, the serum ferritin cannot be relied upon to provide an estimate of body storage iron.58,69 Larger prospective studies should examine the hypothesis that patients with sickle cell disease are able to accommodate iron burdens exceeding those of other chronic anemias. In the meantime the management of these patients should be similar to that of other transfused patients.70
Experimental Therapies
Bolus injections of subcutaneous deferoxamine:
DFO administered by twice-daily SC injections has been reported to induce urinary iron excretion equal to the same dose administered by prolonged subcutaneous infusion.71,72 If confirmed, these observations could lead to the implementation of regimens that offer an alternative to prolonged infusions.
Orally active iron chelators:
The expense and inconvenience of DFO has led to continued search for orally active iron chelators. The only agent to reach extended clinical trials is the orally active agent 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone; L1),73 evaluated in several short- and long-term studies over the last decade. Most trials have estimated changes in body iron using serum ferritin concentration. A meta-analysis of these studies76 has concluded that they demonstrate the effectiveness of deferiprone. In the largest study,74,75,93 increases in serum ferritin were reported in approximately half of patients remaining on study,75 and nearly half of the dropouts (representing more than 30% of the total cohort) discontinued deferiprone because of increasing serum ferritin concentrations.75 Only a few studies of deferiprone have evaluated tissue iron stores.44,77–,83 Support for two studies that had evaluated prospectively serial liver biopsies was terminated prematurely by the corporate sponsor in 1996,84,85 but independent follow-up has provided information about the long-term effectiveness of deferiprone in thalassemia major. A recent review86 has concluded that all peer-reviewed papers reporting long-term changes in storage iron have shown inadequate effectiveness of deferiprone in a substantial proportion of patients.78–,83 The poor correlation between serum ferritin and hepatic iron concentrations in these studies emphasizes the importance of determination of storage iron in the evaluation of this chelating agent. Overall, in these studies, after extended periods of deferiprone hepatic iron has exceeded the threshold for cardiac disease and early death13 in 18-65% of patients and, on average, that for increased risk of other complications33 in approximately 70% of patients. However, several reports76 published in non-peer-reviewed conference proceedings remain highly supportive of the effectiveness and safety of deferiprone.
In a randomized trial that compared changes in hepatic iron in deferiprone- and DFO-treated patients, 90% of patients underwent follow-up assessment of hepatic iron.87 The proportion of deferiprone-treated patients in whom final hepatic iron exceeded the threshold for premature death increased nearly 5-fold over two years, while in most patients treated with DFO, hepatic iron remained within optimal range. These findings confirm those of iron-balance studies comparing the short-term effectiveness of deferiprone relative to that of DFO.88
Recent short-term studies have attempted to circumvent the reduced effectiveness of deferiprone by combining the drug with intermittent DFO with the goal of achieving iron balance.89–,91 DFO has been administered in full therapeutic doses in one study;89 with less regular DFO supplementation, deferiprone was not sufficiently effective in most patients.91 Until combination therapy is evaluated in controlled clinical trials in which body iron burden is evaluated quantitatively, it is not clear that combinations of deferiprone and DFO offer benefits over those of DFO as a single agent.
Toxicity of deferiprone:
The major previously recognized adverse effects of deferiprone in humans include embryotoxicity, teratogenicity, neutropenia and agranulocytosis,92 although. the incidence of neutropenia and agranulocytosis is not clear.74,75,93 Recently, the potential for deferiprone to produce cellular toxicity has been confirmed experimentally in studies of hepatocytes94 and myocyte cultures.95 In gerbils, chronic co-administration of iron dextran and 1,2-diethyl-3-hydroxypyridin-4-one, a closely related hydroxypyridinone, resulted in increased iron accumulation in the liver and heart, worsening of hepatic fibrosis, and development of cardiac fibrosis;96 studies with deferiprone have confirmed these findings in gerbils97 and guinea pigs.98 In the only human trial to obtain serial systematic liver biopsies over six years, deferiprone treatment was associated with progression of hepatic fibrosis over a median period of 3.2 years.78 At another center, nearly 30% of deferiprone-treated patients demonstrated, over two to three years, significant progression of hepatic fibrosis, at three times the rate observed in deferoxamine-treated patients.83 An increase in serum alanine aminotransferase levels was observed in the most recent reports of a large trial.75 Liver histology, however, determined in less than 20% of patients, was reported as showing no progression of fibrosis.99 This may be because the median period to progression of hepatocellular dysfunction and fibrosis may exceed three years.78 Taken together, the pattern of findings based upon in vitro studies, animal testing, and human trials provides biological plausibility to a relationship between deferiprone and accelerated hepatic fibrosis for which no other established cause has been identified. The prolonged period over which progression of fibrosis has developed indicates that extended observation of adequate numbers of patients will be required to permit meaningful assessment of this risk.86 The findings that several patients have died as a result of cardiac disease during therapy with deferiprone79 indicate that the drug's impact on this complication must also be studied further.100
In my view, before deferiprone can be considered for clinical use, careful evaluation of its long-term toxicity in controlled clinical trials with prospective evaluation of hepatic iron and histology is mandatory, particularly in view of the grave prognostic implications of the progression of liver and cardiac disease in thalassemia patients.86
In summary, iron-chelating therapy with deferoxamine has altered dramatically the prognosis of patients with transfusional iron overload. The development of alternative strategies of therapy, including safe and effective orally active iron chelators, remains a high priority. Careful controlled studies of the risks and benefits of any new therapy are required before widespread implementation of new therapies.