Abstract
In this update, selected clinical features of sickle cell disease and their management are reviewed. In addition, the current status of interventions that have curative potential for sickle cell disease is discussed, with particular attention focused on indications, methodology, recent results, and challenges to wider clinical application.
In Section I, Dr. Nienhuis describes recent improvements in vector technology, safety, and replacement gene expression that are creating the potential for clinical application of this technology.
In Section II, Dr. Vichinsky reviews our current understanding of the pathophysiology and treatment of pulmonary injury in sickle cell disease. The acute and chronic pulmonary complications of sickle cell disease, modulators and predictors of severity, and conventional and novel treatment of these complications are discussed.
In Section III, Dr. Walters reviews the current status of hematopoietic cell transplantation for sickle cell disease. Newer efforts to expand its availability by identifying alternate sources of stem cells and by reducing the toxicity of transplantation are discussed.
I. Gene Therapy for Sickle Cell Disease
Arthur W. Nienhuis, MD*
St. Jude Children’s Research Hospital, 332 N. Lauderdale, Rm. C7045, Memphis, TN 38105
The ability to efficiently insert a gene into repopulating hematopoietic cells and to achieve regulated expression in specific hematopoietic lineages would create many therapeutic opportunities.1 Indeed, sickle cell anemia has long been recognized as a potential candidate for the development of gene therapy approaches. Despite considerable effort over nearly 20 years, progress toward the goal of implementing gene therapy for sickle cell anemia has been slow. The relatively low efficiencies of gene transfer with conventional, murine oncoretroviral vectors coupled with the requirement for very high level globin gene expression in differentiating erythroblasts have been major barriers to rapid progress. Several convergent avenues of research to be reviewed in this section have culminated in the recent demonstration that the sickling phenotype can be corrected by retroviral vector mediated gene transfer into repopulating stem cells in murine models of sickle cell anemia.2 Development of these models required considerable effort in that both the mouse α- and β-globin genes had to be deleted and the human α and βS genes had to be introduced into the germline and coexpressed at relatively equal levels.3 Another major breakthrough that facilitated progress was the adoption of lentiviral vectors based on human immunodeficiency virus (HIV) for globin gene transfer.4 This proof-of-principle demonstration that the sickling phenotype can be corrected by gene transfer has set the stage for critically evaluating the remaining problems to be solved before gene therapy for sickle cell anemia can be introduced into the clinic.
Stem Cell Targeted Gene Transfer with Viral Vectors
Although HIV-based lentiviral vectors currently appear the most promising for the development of gene therapy for sickle cell disease, much has been learned regarding strategies for optimizing gene transfer into stem cells using murine oncoretroviral vectors. Other systems are also being developed or have been tested for this purpose and merit a brief description.
Murine oncoretroviral vectors
The principles of retroviral vector mediated gene transfer emerged during the study of murine oncoretrovirus vectors beginning in the early 1980s. Steps involved in vector entry and genome integration are shown in Figure 1 . Molecular cloning and DNA sequencing provided insights into the organization of these relatively simple viral genomes. Packaging lines expressing the structural proteins were engineered, and vector genomes were developed in which the viral structural proteins were replaced with genes of interest while retaining the sequences necessary for packaging, integration, and expression of the vector genome.5 Such vectors were then shown to be capable of gene transfer into a physiologically relevant target population, namely primary, multipotential, hematopoietic stem cells.6 Modern versions of oncoretroviral vector producing cells have the coding sequences for viral structural proteins segregated onto at least 2 expression elements to reduce the probability that genetic recombination could result in the emergence of potentially tumorigenic, replication-competent retroviruses (RCR).7
Refinements in the use of murine oncoretroviral vectors have allowed for successful correction of genetic defects in several murine models of human hematopoietic disorders.2,4,8,9 Attempts to extend these results to studies in large animal models and human clinical trials have been much less successful, however.1 The difference in gene transfer efficiency between mouse and primate stem cells undoubtedly reflects several biological differences with respect to ex vivo replicative potential and preservation of subsequent engraftment capability.
Two major barriers to the use of oncoretroviral vectors to insert genes into human stem cells have been the focus of much research. The first pertains to the quiescent status of these cells: oncoretroviral vectors require nuclear mitosis within hours of introduction of the retroviral genome into a target cell to allow the relatively unstable genome to gain access to nuclear chromatin and undergo genome integration.10,11 Various cytokine combinations have been used both in vivo and ex vivo to trigger stem cells into entering the cell cycle. The second barrier to stem cell targeted gene transfer is the relatively low level of expression of the receptor protein used to initiate cell entry.12 The basic amino acid transporter utilized by ecotropic vector particles is expressed at relatively high levels on primitive murine hematopoietic cells, but the human homolog of this receptor protein does not support entry of vector particles bearing the ecotropic envelope protein. The receptor for the amphotropic envelope protein used in large animal models and human studies, a phosphate transporter, is expressed at much lower levels than the ecotropic receptor on primitive human hematopoietic cell populations.12 Alternative envelope proteins including that of the gibbon ape leukemia virus (GALV)13 and that of the feline endogenous virus (RD114)14 have been used to generate particles that have demonstrably higher transduction efficiency in various experimental systems.
Large animal models, including rhesus monkeys,15 baboons,13 and dogs,16 have been useful for evaluating gene transfer into repopulating stem cells during the past decade. Several general conclusions have emerged: (1) peripheral blood cells or bone marrow collected after administration of cytokines contain stem cells that are transduced more efficiently than those found in steady state bone marrow;17 (2) the coating of tissue culture plates with a fibronectin fragment, which colocalizes vector particles and target cells, enhances transduction efficiencies;18 and (3) the inclusion of relatively high concentrations of stem cell factor and Flt-3 ligand (Flt3L) along with other cytokines in the culture medium used for prestimulation of the target cells and during transduction increases gene transfer into repopulating stem cells.19 Studies in all 3 animal species have documented frequencies of genetically modified cells in peripheral blood of 5-20% with the highest frequencies during the earliest weeks following transplantation followed by a decline to lower levels during the first year. Furthermore, there is considerable variation in the frequency of genetically modified cells among transplant recipients ranging from less than 1% to more than 10% under identical transduction conditions.15
Using the principles established from studies in animal models, oncoretroviral vectors have now been used to successfully treat a form of human severe combined immune deficiency.20 In this disorder, the gene-corrected cells have a strong selective advantage as previously shown in murine models,9 allowing a positive therapeutic outcome despite the relatively low efficiency of gene transfer into human stem cells and the lack of myeloablation prior to the clinical gene therapy procedure.
Lentiviral vectors
Recent studies have suggested that lentiviral vectors, derived with components of the HIV genome, transduce human stem cells more efficiently than do oncoretroviral vectors.21,22 The lentiviral vector preintegration complex can move through the nuclear membrane without mitosis11 and is relatively stable,23 increasing the window of opportunity for genome integration. Early studies established that HIV-based vectors were superior to traditional oncoretroviral vectors at introducing genetic information into primitive human hematopoietic cells from cord blood that are able to establish hematopoiesis in immunodeficient mice.21 Higher transduction efficiencies have also been documented in studies of such cells in bone marrow24 or peripheral blood following cytokine administration.22
More effort will be required to optimize transduction with stem cells using lentiviral vectors. Although cell division is not required, activation of cells from G0 to G1 phase of the cell cycle is necessary to achieve conversion of the RNA genome into its double-stranded, preintegrative form.25 Thus, many of the principles that have emerged in studies in large animal models using murine oncoretroviral vectors will be applicable to the ultimate clinical application of lentiviral vectors. The internal components of such vectors can be pseudotyped with a broad spectrum of envelope proteins;22 additional studies will be needed to determine which of these is most useful in generating particles for human stem cell targeted gene transfer.
To date, most studies utilizing lentiviral vectors have relied on the generation of infectious particles by a transfection of tissue culture cells with several plasmids.26 The organization of the system that we have developed for globin gene transfer is shown in Figure 2 . Distribution of the coding sequences for the several proteins required for particle assembly onto several plasmids virtually eliminates the possibility that replication-competent retrovirus can be generated during packaging of the vector genome. Furthermore, vectors have been engineered to eliminate all unnecessary HIV sequences and to have a self-inactivating design in that transcriptional regulatory sequences have been removed from the 3′ long terminal repeat (LTR).22,26,27 During the steps that result in genome integration, the 5′ LTR of the integrated provirus is generated using the deleted 3′ LTR; thus, the integrated proviral genome is largely devoid of transcriptional regulatory sequences that can interfere with expression of the transgene. Such considerations are of particular importance in the design and utilization of vectors containing globin genes with associated regulatory elements necessary to achieve high-level expression in differentiating erythroid cells.
Recombination events among homologous sequences in transfected plasmids are well documented28 and reflect a potential safety hazard that can in principle be eliminated by integration of the plasmid sequences in well-characterized, stable vector producing cell lines. However, the cellular toxicity of several of the HIV proteins has made the development of such cell lines rather difficult, although inducible promoters have been used to create some potentially useful lines.29
Lentiviral vectors other than those based on HIV-1 might also be evaluated for therapeutic gene transfer in preclinical studies including those based on simian immunodeficiency virus30 and feline immunodeficiency virus.31 Some have argued that such viruses, which are not known to be pathogenic in humans, might be more appropriate for clinical use than HIV-based vector systems would be. However, given the complexities of primate lentiviruses and the certainty that pathogenic strains that arose by recombination and mutation have passed into humans,32 others have argued that HIV-based vectors may in fact be safer.33 Certainly, the genetic elements of the HIV genome and the biology of viral infection are well known, and active therapeutic agents for HIV are available.
Other vectors
Another retroviral vector being considered for gene therapy is based on human foamy virus (HFV), which is a member of the spumavirus family. Among the desirable features of such vectors for hematopoietic applications are the ability to integrate into a wide variety of cells types; resistance to serum inactivation; a relatively large packaging capacity; and enhanced ability, relative to oncoretroviral vectors, to transduce nondividing cells. Progress is being made in developing a stable packaging system for HFV-based vectors, and recent data establish the ability of such vectors to transduce cells capable of establishing lymphoid and myeloid hematopoiesis in immunodeficient mice.34
Vectors based on DNA viruses such as adeno-associated virus (AAV) and adenovirus are useful for transducing nonmitotic cells including differentiated cells in vivo, but their vector genomes integrate inefficiently or not at all, making them unsuitable for use for introduction of genetic information into proliferating hematopoietic cells.1 Of potential interest are composite vectors composed of elements from adeno and AAV vectors that do integrate genetic elements into hematopoietic cells.35 Other nonintegrating vectors such as those based on SV40 or herpes simplex may have specific applications in the hematopoietic system but again seem unsuitable for long-term gene therapy applications.1
Selection Systems for In Vivo Amplification of Genetically Modified Hematopoietic Cells
Despite the improved transduction efficiencies of repopulating stem cells that are likely to be achieved with lentiviral vectors, an effective strategy for selectively amplifying a genetically modified population in vivo, analogous to that which occurs naturally in immunodeficient patients,20 is likely to be desirable. Indeed, the use of a selection system may obviate the need for complete myeloablation, thereby increasing the safety of clinical gene therapy applications for hemoglobin disorders.
Several approaches have been explored for amplifying genetically modified stem cell populations by in vivo selection with drugs.36 Substantial amplification has been documented in mice by using a variant dihydrofolate reductase gene and a combination of trimetrexate and a nucleoside transporter inhibitor for selection,37 but this selection system has worked less well in the nonhuman primate model and was associated with severe drug-induced myelosuppression in a murine model of β-thalassemia intermedia (D. Persons and B. Sorrentino, unpublished data). Another system based on the multidrug resistance gene (MDR) has also been explored for positive in vivo selection, but the fact that enforced expression of MDR1 gene expands stem cells and leads to a myeloproliferative syndrome in mice38 has discouraged further development of this system.
Another approach to the in vivo expansion of genetically modified cells has been the use of fusion proteins, which contain a growth receptor signaling domain and a domain that permits activation by small molecules that reversibly dimerize these fusion proteins.39,40 Multipotential progenitor cells from mouse bone marrow have been expanded by using the thrombopoietin receptor signaling domain.39 However, the same system, when used to expand primary human hematopoietic cells, led to preferential expansion of erythroid cells.41 Concern exists that these fusion proteins could perturb hematopoiesis in a nonphysiological way.
Variant, O6-benzylguanine (BG) resistant, methylguanine methyltransferase (MGMT) genes have also been evaluated for in vivo selection of genetically modified cells. MGMT is an alkyl transferase, which repairs cellular DNA damage at the O6 position of guanine produced by methylating agents such as temozolomide (TMZ). MGMT proteins with specific amino acid changes, such as P140K and G156A, remain active in repairing DNA but are resistant to BG. Therefore, BG can be used to inactivate endogenous MGMT and further enhance alkylator-mediated cell death. In normal mice, a genetically modified hematopoietic cell population has been amplified from 5% to more than 90% by serial drug treatment.42 Amplification of a minority population of MGMT expressing normal stem cells has also been achieved in the presence of a majority of thalassemic stem cells without myeloablation (D. Persons, A. Nienhuis, and B. Sorrentino, unpublished data). A concern about the use of drugs such as temozolomide is the possibility of predisposition to the development of a malignancy, which must be balanced against the potential benefits of this approach.
Phenotypic Correction of Thalassemia and Sickle Cell Anemia in Murine Models
Lentiviral vectors containing a globin gene with introns linked to regulatory elements from the locus control region (LCR) have been used to successfully correct the thalassemia and sickle cell anemia phenotypes in murine models of these human disorders.2,4 The LCR extends from 5 kb to 25 kb upstream from the human ε globin gene.43 Most of the LCR activity in conferring high-level gene expression relatively independent of chromosomal integration position resides in a series of 300 bp to 500 bp regions identified by their nuclease hypersensitivity (HS). Inclusion of these elements in retroviral vectors became generally feasible when lentiviruses were used to construct globin gene vectors. The well-developed rev-dependent mechanisms of lentiviruses for nuclear to cytoplasmic transport of unspliced RNA and the elimination of cryptic polyadenylation sites have allowed passage of the complex vector genome necessary to achieve high-level globin gene expression. In mice with a sickle syndrome, a decrease in the number of irreversibly sickled cells, an improvement in the sickling propensity of red cells in response to lowered oxygen tension, and an increase in kidney concentration function have been documented in 2 mouse strains with sickle cell anemia in which an antisickling variant of human β-globin was expressed at 10-50% of the level of the endogenous human or mouse β-globin chains.2
These early studies, while encouraging, leave a number of problems to be resolved. Mice homozygous for human hemoglobin S (HbS) typically have a relative deficiency in β chain synthesis3 so that part of the improvement of anemia in 1 of the 2 mouse strains studied may have reflected correction of the thalassemia component of the phenotype.2 The red cells of the other mouse strain studied had a complex globin composition that included a “super sickling” human variant as well as the normal mouse globins, and thus are not directly analogous to human sickle blood cells. In addition, the conditions used for bone marrow cell transduction resulted in integration of 2 or 3 vector genomes per transduced stem cell, improving the chances that at least 1 genome was expressed at therapeutic levels in the relatively small number of animals studied to date. In our laboratory, study of a larger number of animals in the thalassemia model documented a very general correlation between copy number and correction of the thalassemia phenotype, although variable expression levels from individual copies of the vector genome clearly implied integration position effects that influenced the level of expression (D.A. Persons, P.W. Hargrove, E.R. Allay, H. Hanawa, A.W. Nienhuis, unpublished data). Some animals with an average of 2 or 3 vector copies per cell nonetheless had lower levels of expression and modest correction of the thalassemia phenotype.
Given the refractoriness of human stem cells to transduction even with lentiviral vectors, integration of multiple vector genomes in each transduced stem cell seems unlikely and thus the effects of integration position on achieving therapeutic levels of globin gene expression are likely to be even more problematic than in the mouse models. There are a number of potential strategies to ensure adequate globin gene expression from a majority of integrated vector genomes. The LCR elements included in the vectors studied to date are relatively small (Figure 2) and do not include all of the sequences that flank the hypersensitive sites that are needed for optimizing the potential of the LCR to enhance gene expression.44 A well-characterized insulator element from a chicken β-globin locus45 has been shown to protect from integration position effects in studies with oncoretroviral vectors46 and could, in principle, be incorporated into lentiviral vectors in an effort to achieve the same effect. As outlined above, our approach to developing a clinically applicable strategy for gene therapy for human globin disorders includes the use of the MGMT selection system to amplify a genetically modified population of cells. An important hypothesis yet to be tested is that selection of genetically modified stem cells based on MGMT expression from the vector genome may yield a population that generates erythroblasts having a higher probability of globin gene expression because of amplification of cells in which the vector genome is in a favorable chromosomal position for gene expression.
Prospects for Clinical Gene Therapy Protocols for Sickle Cell Anemia
Although the early clinical protocols to evaluate gene therapy in patients with sickle cell disease are likely to be Phase I studies designed to evaluate toxicity, the patients who will be offered the opportunity to participate are likely to be severely affected and thus have some potential to benefit from the intervention. Such patients are likely to be adults with severe disease including severe anemia and low endogenous levels of fetal hemoglobin. Aspirated bone marrow is likely to be the preferred source of stem cells for transduction as the neutrophilia which accompanies cytokine mobilization of stem cells into peripheral blood may trigger severe vaso-occlusive crises.47 Two parameters that are likely to be important are the levels of an antisickling hemoglobin required to achieve a therapeutic benefit and the proportion of circulating red cells that express that variant. Longitudinal, natural history studies suggest that even modest amounts of hemoglobin F (HbF) improve outcome in patients with sickle cell disease48 so that a target value of 20% of an antisickling variant would seem reasonable. The complexity of the mouse models makes it difficult to directly infer the required amount of HbF.49 Absolute protection from sickling crises would presumably require replacement of all endogenous red cells but, again, a reasonable goal might be 30-50% in an initial clinical trial since mixed chimerism after non-myeloablative stem cell transplantation is associated with clinical improvement. As noted above, achieving that level even with the use of lentiviral vectors may be unlikely even with significant myeloablation, so the incorporation of strategies to selectively amplify the genetically modified population of cells seems desirable.
The focus of ongoing preclinical work leading to gene therapy trials for sickle cell disease using lentiviral vectors based on HIV-1 will likely include the following:
Identification of the appropriate stem cell target population, presumably bone marrow derived, and optimization of transduction of that population.
Further optimization of vector design to improve the probability of globin gene expression from each integrated genome.
Characterization of selection systems in large animal models and potentially in clinical trials for malignancy.
Continued characterization of new antisickling variants.
The development of systems for production of lentiviral vector particles from stable producer cell clones and their characterization to allow scale-up for clinical trials with appropriate quality control.
Substantial consideration has already been given to biosafety issues related to lentiviral vector production,36 and an initial gene therapy protocol involving the use of HIV-based vectors is under review. 50 Reliable strategies for ensuring the absence of replication-competent retrovirus of any type from materials to be used for clinical trials have been judged to be mandatory. Another issue remaining to be evaluated is the risk of mobilization of the vector genome in the context of subsequent infection with HIV, for which there is always a finite risk in patients who participate in clinical trials.
Concurrent with efforts to bring HIV-based vectors to the clinic are continued efforts to develop other vector systems. Given the magnitude and potential synergies of these efforts, ultimate success in the development of gene therapy for sickle cell anemia seems likely.
II. Understanding the Pathophysiology and Treatment of Pulmonary Injury in Sickle Cell Disease
Elliott Vichinsky, MD*
Children’s Hospital & Research Center, Oakland; Director, Hematology/Oncology; Prof. of Pediatrics, University of California, San Francisco; 747 - 52nd St., Oakland, CA 94609
Acute chest syndrome (ACS) is the second most common cause of hospitalization of patients with sickle cell disease (SCD) and is responsible for up to 25% of deaths in this population.1 Repeat events have been associated with an increased risk of chronic lung disease and pulmonary hypertension.2 This report will review the clinical course, etiology, treatment, and pathophysiology of sickle lung disease.
Definition
The term ACS is used to describe acute pulmonary disease in SCD. A distinct entity is recognized because infectious and noninfectious pulmonary events are complicated by sickling, resulting in a more severe clinical course. The diagnosis of ACS is made when a new infiltrate on chest radiography is accompanied by acute respiratory symptoms. Uniform use of this definition has only recently occurred. Therefore, past reports have different clinical descriptions. For instance, the Cooperative Study of Sickle Cell Disease defined ACS as a new pulmonary infiltrate, with or without symptoms.3 In contrast, the recent multicenter ACS study requires a new pulmonary alveolar consolidation involving at least one complete lung segment associated with acute symptoms.1
Incidence and Risk Factors
Although the incidence of ACS is high, it is most likely underestimated. Recent studies have demonstrated that clinical judgment alone may miss or delay the diagnosis of pulmonary disease.4 This fact has led to the recommendation that any SCD patient presenting with fever or chest pain, even without pulmonary symptoms, be followed radiographically.
In the Cooperative Study of Sickle Cell Disease study, 3751 patients were followed prospectively for a total of 19,867 person-years.5 Of these, 1085 (29%) had at least one episode of ACS.3 Six percent had 5 or more episodes. ACS occurred more often in sickle cell anemia (12.8 per 100 person-years) and HbS-β0 thalassemia (9.4 per 100 person-years) than in HbSC disease (5.2 per 100 person-years) and HbS-β+ thalassemia (3.9 per 100 person-years). The incidence of ACS is age-dependent, with rates of 24.5 events per 100 patient-years in young children with HbSS, decreasing to approximately 8.8 events per 100 patient-years in older adults (Table 1 ).
Several risk factors influence the frequency of ACS. Hemoglobin type, fetal hemoglobin level, anemia, steady state white count, age, seasons, and bronchoreactive lung disease strongly predict frequency.1,3,5 ACS incidence is inversely proportionally to fetal hemoglobin (HbF) levels. An increase of HbF from 5% to 15% decreases the incidence of ACS by half in all age groups. HbF differences may also explain why the beta globin haplotypes (CAR, Benin, Senegal) also affect the incidence of ACS. The incidence of ACS is inversely proportional to the degree of anemia. Patients with high baseline hemoglobin have a 2-fold increase in incidence of ACS compared to those with low hemoglobin. The incidence of ACS is directly proportional to the steady state white blood cell counts. This is most likely secondary to the associated inflammatory state with alteredin, cytokine production, endothelial adhesion, and thrombosis. The rate of ACS is affected by the season of the year.5 Seasonal variation in young children is most striking, with a lower rate of ACS in summer and an increasing rate into the winter. Other age groups follow a similar pattern, but one that is less pronounced. ACS rates are significantly higher in children aged 0 to 4 than in adults during December.
Bronchoreactive lung disease is very common in sickle cell patients and is a risk factor for ACS. It has been noted in 43% of children with sickle cell anemia during the steady state, and it often worsens during the course of each event.6,7 Lung function studies in these patients indicate airway hyperresponsiveness, which progresses to obstructive lung injury. The high rate of bronchoreactive lung disease may be secondary to ongoing subclinical pulmonary injury from sickling.
Associated Events Analysis
Pain and febrile events are prodromes of ACS.5 Thirty percent of patients experience a painful event within 2 weeks of onset of ACS. These painful events increase with age (Figure 3 ). In contrast, a febrile illness often precedes the development of ACS in children. Surgery is also a risk factor; 25% of patients who undergo major surgery are shown to develop postoperative ACS.8
Presenting Symptoms
Half of the patients are initially admitted for a diagnosis other than ACS.1 Admission diagnosis in adults is usually a painful event, and in children, a febrile illness. Patients who were not admitted for ACS develop positive x-rays and clinical findings of ACS on average 2.5 days after admission.
Fever, cough, and chest pain are the most common presenting symptoms. The frequency of presenting symptoms is age-dependent, with fever and cough being more common in young children, while the incidence of chest pain, shortness of breath, and hemoptysis increases with age. There is no significant difference in the frequency of presenting symptoms between hemoglobinopathies. Presenting symptoms observed during a patient’s first ACS event were predictive of symptoms during subsequent events.
Physical Exam
The most frequent physical exam findings are rales and dullness to percussion. Wheezing is age-dependent and common in children. Notably, the second most common auscultory finding is a normal lung exam, which occurs in 35% of patients. Half of adults presenting with ACS have an associated pain event requiring analgesics. Seven percent of adults have neurologic dysfunction upon presentation.
Laboratory
Radiographic findings also vary with age. Children have a higher frequency of upper and middle lobe disease. However, most patients progress to multilobar involvement involving the lower lobes. Pleural effusion occurs in a majority of patients. Hemoglobin and white blood cell counts show significant changes compared to steady-state values. Hemoglobin drops an average of 1 g/dL, and white blood cell counts double. Platelet counts often decrease during hospitalization. A platelet count of less than 200,000 mm3 is a strong predictor of morbidity and mortality. At diagnosis, mean oxygen saturation is approximately 92%, but it usually falls further during hospitalization. Nearly one fifth of patients present with a PaO2 less than 60 mm Hg. The average forced expiratory volume at diagnosis is approximately 53% of the predicted value. At least 25% of patients show significant improvement in pulmonary function testing with bronchodilators.
Hospital Course
Initial laboratory and clinical findings usually worsen following the diagnosis of ACS. With aggressive therapy, most patients become afebrile and stabilize in approximately 3 days. During the first few days of hospitalization, many patients become unstable. Rapid deterioration can develop in a previously stable patient. Approximately 15% of all patients require intubation, and this percentage increases with age. Eleven percent of patients develop neurologic events and multiorgan failure. Despite these serious complications, with intense support most patients recover. The mortality rate is age-dependent. The average mortality rate for the group is 3%, rising to 9% for patients over 18 years of age.
Complications
Older patients are more likely to have complications, including respiratory failure and neurologic events, as well as death. In addition to age, early predictors of respiratory failure are multilobar pneumonia, a platelet count below 200,000 mm3, and history of cardiopulmonary disease.
Neurologic events increase with age and occur in 25% of all adult patients. The most common types of neurologic injuries are coma, seizures, anoxic brain injury, and stroke. The mortality rate for those patients with a neurologic event is 23%, with most patients eventually recovering neurologic function. Those patients with thrombocytopenia and hypoxia are at greatest risk for developing neurologic complications and require early intervention. Relative thrombocytopenia, previously associated with neurologic ischemic insults, reflects hypoxia, tissue injury, and platelet consumption.9 In addition, fat embolism and infections with Chlamydia pneumoniae and Mycoplasma pneumoniae may also play a direct role in brain injury.1 Invasion into vascular endothelium by Chlamydia pneumoniae has been demonstrated.
Etiology of Acute Chest Syndrome
The pathogenesis of ACS is complex, likely influenced by a number of factors. These may include the production of proinflammatory cytokines, oxidants, and procoagulant proteins; the alteration of cellular adhesive molecules; injury of vascular endothelium; increased neutrophil activity; and thrombosis.1,10 15 Several catalysts initiate this complex cycle, each influencing the overall final event. However, because of the influence of sickling and its secondary pathology, the clinical picture of each etiology is not distinct. Although the most common precipitating etiology includes infection, pulmonary fat embolism, and pulmonary infarction, often more than one factor is involved. In a recent multicenter ACS study, which included diagnostic bronchoscopy, 70% of episodes had a specific etiology, with infection followed by embolism and infarction (Table 2 ).
In the multicenter ACS study, at least 27 unique pathogens were identified, including bacterial, viral, and mixed infections (Table 3 ). The wide spectrum of bacterial infections requires broad antibiotic coverage. Furthermore, the occurrence of unusual organisms, such as Legionella pneumophila, pseudomonas species, and salmonella species, requires a rethinking of the etiology in nonresponding patients. C. pneumoniae is the most commonly occurring organism. Although simply a community-acquired illness in healthy individuals, this is a serious infection in people with SCD. In the multicenter ACS study, 20% of patients with C. pneumoniae developed respiratory failure, and 12% had a neurologic event. Two thirds of patients experienced a concomitant painful event. Seventeen percent of the patients with C. pneumoniae infection in our study had evidence of coinfection with other organisms. Seventy-one percent of these coinfections were with M. pneumoniae. Some patients have recurrent ACS caused by C. pneumoniae. Recurrent episodes of C. pneumoniae infection have been described previously in immunocompromised patients, but they are uncommon. There are several mechanisms by which infection with C. pneumoniae may contribute to ACS. C. pneumoniae infects and activates large numbers of macrophages. These macrophages directly cause tissue injury through the activation of complement, hydrogen peroxide, and acid hydrolases and induce the production of proinflammatory cytokines. Furthermore, preliminary evidence suggests C. pneumoniae may cause brain injury in ACS patients by direct invasion of vascular endothelium.1
M. pneumoniae is a known respiratory pathogen and is the second most common infectious cause of ACS. Previous studies of pneumonia in the general population reported rates of M. pneumoniae infection from 9% to 27%.1M. pneumoniae occurs in all age groups, but it is not typically suspected in young children. It is usually benign in the general population. In SCD, even infants can have a serious illness with M. pneumoniae. In the multicenter ACS trial, multilobar disease, hypoxia, and wheezing were common.1 The reason for the severe clinical course of these infections appears to be the ability of M. pneumoniae to induce sickling and vascular injury. Following infection, an inflammatory response with lymphocyte infiltration and proinflammatory cytokine production leads to damage of vascular endothelium, alteration of cellular adhesion properties, and progressive regional hypoxia. Depressed cellular immunity in patients with M. pneumoniae is a possible factor in the high rate of viral coinfection. The findings suggest that all patients should have a treatment regimen that includes macrolides or similar antibiotics to cover M. pneumoniae.
Viral infections are commonly associated with ACS as sole organism or a coinfection.1 Many relatively benign viruses are more virulent in sickle cell patients. Parvovirus appears to be especially dangerous.16 B19 parvovirus infection is associated with many complications in SCD, including nephritis, red cell aplasia, stroke, and joint pain.17 Only recently has it been identified as a cause of ACS and pulmonary fat embolism (PFE).18 These pulmonary events are often associated with hypoxia, severe pain, fever, and bone marrow necrosis. The patients may be young. Some patients may not have the severe reticulocytopenia typical of these infections. Autopsy studies on fatal cases have noted bone marrow necrosis with fat emboli in the lungs.16,19
After infections, PFE is the most common cause of ACS and probably accounts for up to a quarter of all episodes.20 Its clinical course is so variable that diagnosis without bronchoscopy is very difficult.19,21 Tissue infarction, when it occurs in the intramedullary cavity of the bone during a painful event, can generate the equivalent source of fat that accompanies a major fracture. Once in the lung, the degradation of fat causes a marked inflammatory response, including interleukin-1, tumor necrosis factor, and upregulation of endothelial VCAM-1.10,11,13,22 Postmortem studies of SCD patients have found that the incidence of PFE may vary from 13% to 75%.
The classic clinical presentation of severe bone pain followed by respiratory distress is commonly seen in SCD, suggesting that patients admitted for vaso-occlusive crisis (VOC) may be in the prodromal phase of PFE. Secretory phospholipase A2 (sPLA2) is an inflammatory mediator and liberates free fatty acids, which are responsible for the acute lung injury of PFE. sPLA2 levels are dramatically elevated in most cases of ACS and rise before pulmonary symptoms develop (Table 4 ).23 Preliminary data suggest that patients with VOC and elevated sPLA2 levels should be transfused to prevent the development of ACS.
Pulmonary infarction is likely the sole cause of ACS in 20% of cases, but it is a major factor in all events. Hypoxia causes adhesion and thrombosis in the pulmonary microcirculatory system, resulting in tissue infarction.24 Activated white cells, elevated cytokines, procoagulants, and adhesive molecules, which are normally high in steady-state SCD, have been found to rapidly increase in hypoxia, amplifying lung injury.11,15,25-,27 Regional perfusion abnormalities are associated with pulmonary vascular constriction and result in sickle cell-cell entrapment. These trapped sickle erythrocytes adhere to human vascular endothelial cells via the endothelial receptors, further decreasing flow. Because of the existence of a hypercoagulable state, thrombosis easily occurs in these areas of decreased flow. The hemolysis of trapped red cells in these areas releases heme, which results in the formation of reactive oxygen species that damage membranes. The process is exacerbated by a deficiency of cytoprotective mediators, such as nitric oxide (NO).14 Hypoxia and sickle cells inhibit NO production by decreasing NO synthase in the endothelium. Arginine, the substrate to NO, is often depleted in the steady state and becomes a rate-limiting step during ACS.28- 30 The overall effect of these interacting factors is an unpredictable and often severe pulmonary event, which may be precipitated by any trigger of hypoxia such as infection, fat embolism, hypoventilation, narcosis, sleep apnea, and bronchoreactive lung disease.
Treatment
To minimize lung injury, a comprehensive plan aimed at both the catalyst and the host response is needed. Patients should receive the influenza and pneumococcal vaccines, and be evaluated for respiratory syncytial vaccine prophylaxis. Those admitted for pain crises should be considered to be in the prodromal phase of ACS and require incentive spirometry, daily monitoring for pulmonary disease, and, if available, monitoring with sPLA2.31,32
The treatment of ACS is undergoing significant change because of the growing list of infectious agents identified in ACS and the recognition of iatrogenic complications, such as fluid overload and narcosis.33 Prevention of further respiratory compromise should be part of a treatment plan for all patients hospitalized with ACS. Vigorous hydration for VOC episodes was originally recommended to reduce the effects of dehydration, but it now appears that this hydration may actually result in an increased incidence of pulmonary edema and worsen respiratory distress, particularly in older patients. Close monitoring of the vital signs, including oxygen saturation, is mandatory. All patients should be instructed to use incentive spirometry at least every 2 hours while awake. Doing so can significantly reduce pulmonary infiltrates in patients initially admitted for VOC because of the counteracting effect of atelectasis.31 Proper pain control utilizing patient-controlled analgesia devices reduces the risk of narcotic-induced hypoventilation.
Once a patient has ACS, broad-spectrum antibiotics, including a macrolide, are required. Because bacterial and community-acquired infections occur in all age groups, including patients without high fevers, all patients require antibiotics. Unresponsive, deteriorating patients may suffer from unusual or resistant organisms and require more extensive antibiotic coverage, as well as bronchoscopy. The use of adrenergic bronchodilators is indicated in many patients with ACS.6,7 Patients have a substantial improvement in peak expiratory flow rates after bronchodilator challenge. Use of nebulized albuterol therapy should be attempted in all patients initially, because benefit can be documented even in patients who do not have audible wheezing. Although albuterol therapy is a generally safe adjunct to ACS management in adults, its cardiac and hypertensive effects require close monitoring.
Oxygen therapy is indicated in all hypoxic ACS patients, as well as those with clinical distress. Pulse oximetry provides a noninvasive method of monitoring oxygen saturation. However, owing to the effects of the oxygen dissociation curve, pH, and hemoglobin level, pulse oximetry may be unreliable.34 We therefore recommend blood gas confirmation in patients with suspected hypoxia or for those who appear toxic.
Transfusions are very efficacious in reversing hypoxia in ACS and are recommended for treatment of most cases of acute lung disease.1,35 In the multicenter ACS study, two thirds of patients were transfused and demonstrated a rapid improvement in their oxygenation. Following transfusion, hypoxic patients with ACS showed rapid improvement in their oxygenation. The average PaO2 of 63 mm Hg rose to 71 mm Hg after transfusion. Although exchange transfusion has been advocated to decrease the risk of increased viscosity, simple transfusions also appear safe and efficacious in less severe cases. The worsening anemia that accompanies acute pulmonary disease may lessen the effect of hyperviscosity and allow cautious simple transfusions to be helpful. Transfusion products should consist of leukocyte-poor packed cells matched for Rh, C, E, and Kell antigens. The use of these products has lowered red cell antibody formation to less than 1%. How transfusions produce such immediate benefit in ACS is unknown, but possible mechanisms include a decrease in cell-cell interactions due to dilution of sickle cells, correction of ventilation/perfusion mismatching, or restoration of a more normal inflammatory cytokine balance.
Mechanical ventilation is required for patients with life-threatening respiratory failure. In the multicenter ACS study, 81% of individuals receiving mechanical ventilation recovered. These data indicate that patients in respiratory failure should be aggressively supported. Treatment should include the use of extracorporeal membrane oxygenation (ECMO) and high-frequency ventilators.36
There are several promising new and experimental therapies for ACS. The use of glucocorticoids was recently reported to decrease length of hospitalization.37 Steroids most likely modify ACS by altering the inflammatory process, including decreased endothelial cell adhesion and inhibition of phospholipase A2. However, a higher frequency of readmission and the long-term risk of aseptic necrosis in this population suggest that routine use of steroids should await further study.
NO is a very promising therapy for ACS.10,12,13,38,39 NO causes relaxation and vasodilation of the blood vessel by affecting smooth muscle function. Reduced NO results in pulmonary vasoconstriction. Inhalation of NO causes vasodilation and improves oxygenation in neonates and adults with pulmonary hypertension. Preliminary use in ACS has been encouraging. Inhaled NO has a dramatic effect on general physiology.40 It decreases intrapulmonary shunting, inhibits red cell endothelial adhesion, has generalized anti-inflammatory effects, decreases vascular leak syndrome, and has a bronchodilator effect.41 There is a concern about NO production of peroxynitrite, a potent oxidant that can cause vascular injury, as well as a rebound effect. However, NO inhalation or supplementation of its substrate, arginine, will have a role in the future management of ACS.28- 30
Non-ionic surfactant (Flocor) appears to reduce blood viscosity and inhibit red cell endothelial adhesion, resulting in a beneficial effect in painful events.42,43 Recent studies in a transgenic sickle cell mouse model suggest it may prevent hypoxic lung injury.43 Pilot studies in the treatment of ACS have been initiated.44
Hypercoagulation appears to play a role in SCD pathophysiology, but at present use of anticoagulation therapy in the management of patients with recurrent ACS events remains poorly studied. Improvement in long-term survival in patients treated with anticoagulants for pulmonary hypertension suggests thrombosis is an important contributing factor.45,46 Chronic transfusion, hydroxyurea, and bone marrow transplantation appear promising for prevention of recurrence in selected patients.47 Hydroxyurea resulted in a 50% reduction in ACS among adults treated in the multicenter ACS trial, whereas prophylactic transfusion almost eliminated the risk of pulmonary complications.48 Currently, recurrent ACS is the most common indication for bone marrow transplantation, and the results in young patients have been excellent.49 Early identification of patients with progressive lung disease through pulmonary function testing is imperative in order to prevent end-stage lung disease.
Pulmonary Hypertension in SCD
Despite improved treatment of ACS, pulmonary hypertension is a common problem in adults with SCD. The true frequency is unknown. Recent reports found a frequency between 5% and 60% in adult patients.50 The difference in incidence occurs because some studies report only symptomatic patients and others report asymptomatic patients with laboratory findings of pulmonary hypertension.2 The definition of pulmonary hypertension includes right ventricular overload with tricuspid regurgitation velocity exceeding 30 mm Hg. The early stages of pulmonary hypertension are asymptomatic and therefore not usually detected. These cases can progress to irreversible damage, associated with chest pain, dyspnea, resting hypoxemia, cardiac ischemia, and sudden death. Early diagnosis is therefore essential. The etiology of pulmonary hypertension in SCD is multifactorial. Chronic scarring related to ACS is only one risk factor.50 Hypoxia-induced production of vasoactive substances, which alter pulmonary vascular tone and cause vascular muscle wall proliferation, is important.10,13 The inflammatory state common in SCD causes increased vascular adhesion and production of oxygen radicals.51,52 A hypercoagulable state causes pulmonary thrombosis of the constricted, injured vessels, resulting in an overall picture of progressive obliteration of the vascular bed.53 The NO deficiency inherent to SCD prevents the body’s protective reactions from occurring.54 Finally, a region on chromosome 2 encoding bone morphogenetic receptor type 2 has been identified as underlying many cases of sporadic primary pulmonary hypertension. It is possible that this mutation and other genetic modifiers may affect the frequency of pulmonary hypertension in SCD.55
Appropriate management of adult sickle cell patients requires screening with echocardiography. Once pulmonary hypertension is diagnosed, chronic transfusion and oxygen to prevent resting hypoxemia are usually indicated. Alternatively, in mild cases, treatment with hydroxyurea has been proposed. The availability of new pharmacological treatments offers hope for treating this fatal complication effectively.55 Inhaled NO selectively dilates the pulmonary vascular bed. Oral arginine supplementation upregulates natural NO production and may reverse pulmonary hypertension.28-,30 Sildenafil is a selective inhibitor of type V phosphodiesterase. It is a useful adjunct therapy to NO or prostacyclin because it inhibits rebound pulmonary hypertension. Recently, endothelin receptor blockades have been used.55 Also promising is orally available bosentan, which reduces pulmonary artery pressure and improves exercise capacity.55 Adjunctive anticoagulation may also increase long-term survival.45,46
In summary, pulmonary injury in SCD is a multifactorial problem that requires intervention for prevention, treatment, and follow-up. Because the survival rate in even the most severe cases is good, aggressive treatment is always indicated. The growing problem of pulmonary hypertension requires routine screening with echocardiography of all adults and early treatment of those with elevated pulse artery pressures.
III. Stem Cell Transplantation for Sickle Cell Disease: How and When to Intervene?
Mark C. Walters, MD*
Children’s Hospital & Research Center, Oakland, University of California, San Francisco, 747 - 52nd St., Oakland, CA 94609
Since the beginning of a national effort to establish and sustain comprehensive centers for sickle cell anemia 30 years ago, a generation of children and adults have benefited from advances in supportive and interventional therapies and from access to services that grew from this commitment to improve care.1,2 As a result, today the vast majority of infants diagnosed with sickle cell anemia by state newborn screening programs and enrolled in comprehensive care programs will survive to adulthood, at a rate that appears indistinguishable from that of African Americans who do not inherit this disorder.3 Thus, the key features of sickle cell anemia are shifting from events characterized by a series of life-threatening acute episodes in childhood, each having the potential for early mortality,4 to ongoing complex management issues of a chronic illness of adulthood, characterized by an inexorable accrual of significant health problems that adversely affect the quality of life.5 Against this backdrop, attitudes about the role of hematopoietic cell transplantation are also shifting.6 Initially performed in those who had co-existent hematological malignancies, these first cases served primarily to demonstrate that allogeneic transplantation had the potential for curing sickle cell anemia.7 The next phase of investigations targeted symptomatic patients for enrollment and focused on characterizing the consequences of transplantation and the quality of cure among those who survived free of sickle erythropoiesis.8-,10 As a consequence, in standard practice, transplantation today is reserved almost exclusively for those who have clinical features that portend a poor outcome or significant sickle-related morbidity, in part because of the toxicity of this intensive therapy. However, follow-up studies after transplantation confirm the sustained benefit of donor erythropoiesis, which significantly improves the quality of life of those who survive with stable engraftment of donor cells.10,11 Thus, current investigations of transplantation for sickle cell anemia are focused on methods that might reduce its toxicity and identify suitable alternate donors, which together might expand the availability of this beneficial therapy. This article reviews the results of conventional myeloablative transplantation for sickle cell anemia with discussion of the long-term follow-up evaluations, discusses recent data from pilot trials to establish stable donor-host mixed chimerism after nonmyeloablative preparation before allogeneic transplantation, and describes preliminary results of transplantation utilizing alternate stem cell sources.
Current Results and Indications
The worldwide experience of transplantation for sickle cell disease has been updated in several recent reviews.12-,14 In these reviews, the transition of transplantation from an experimental intervention reserved for the most ill patients to one in which increasingly younger children with early signs of sickle-related morbidity are targeted has been observed. Several series in Europe and North America have reported very similar results after HLA-identical sibling transplantation.8,11,15 The principal aim of these multicenter clinical studies was to define more completely the risks and benefits of this therapy and to characterize the natural history of those surviving free of sickle cell disease. The results of transplantation were best when performed in children with SCD who had HLA-identical sibling donors. Anecdotal reports of transplantation for adults with sickle cell anemia suggest a poor outcome.13 Even though many children who received allografts had significant sickle-related complications, such as stroke and recurrent episodes of ACS, the disease-free survival was very good, approximately 80 to 85% in several series. However, 5 to 10% of patients died of complications related to transplantation, with graft-versus-host disease (GVHD) and its treatment noted as the leading cause of death. Still, in response to these observations, today most clinicians consider transplantation as a reasonable treatment option for patients who have significant sickle-related complications (Table 5 ).
The problem of disease recurrence after transplantation, however, remains a key obstacle to success. The cumulative incidence of graft rejection accompanied by autologous recovery was approximately 10% in several published series.8,11,15 In our multicenter investigation of transplantation for SCD, there was an association between graft rejection and the pretransplant administration of iron chelation therapy, suggesting that pretransplant exposures to blood products promoted an immunological response that interfered with donor engraftment.11 This contrasts with graft rejection rates that are very low among recipients with hematological malignancies who undergo HLA-identical sibling transplantation.16 Thus, the high frequency of graft rejection observed in sickle cell patients implies that immunological barriers that might include suppression of host natural killer (NK) cells and T-lymphocytes are not reliably overcome by ablative pretransplantation therapy. The problem of rejection might be approached by several different strategies. If, in fact, sensitization to minor histocompatibility antigens mediated by host memory T-cells is a key determinant of rejection, targeting younger patients with few or even no transfusion exposures might optimize outcomes. When transfusions are necessary in transplant candidates, it is important to administer γ- irradiated, leukocyte-reduced blood products to reduce the risk of sensitization to minor histocompatibility antigens.17 Alternatively, it is possible that targeted immunosuppressive therapy that inhibits the host-versus-graft (HVG) reaction before and after transplantation, either with or in lieu of ablative pretransplantation conditioning, might be employed to promote engraftment. Finally, the identification and enrichment of donor cellular populations that facilitate engraftment without causing GVHD is another strategy that could be pursued. It is likely that novel approaches crafted to overcome the problem of graft rejection are more likely to succeed than empirical trials of unrelated, or related but HLA-mismatched, donor transplantation for sickle cell anemia. However, there is a compelling rationale to conduct these investigations in light of the paucity of HLA-identical sibling donors for sickle cell recipients.
To consider transplantation for those not yet exposed to red blood cell (RBC) transfusions, it is necessary to understand the natural history of sickle-related pathology after allogeneic transplantation and the reliability of indicators that predict the clinical severity and early mortality of SCD. Protection from sickle-related complications after transplantation was observed in several investigations with up to 8 years of follow-up.8,10,11,18,19 Specifically, patients did not have painful vaso-occlusive crises, ACS, splenic or hepatic sequestration, or bony infarction. Moreover, among younger patients, recovery of splenic function was documented.20 As a result, it has become a standard of current practice to consider allogeneic bone marrow transplantation (BMT) for children who have HLA-identical sibling donors and who have experienced significant complications, such as those listed in Table 5. Less certain is how to apply clinical severity predictors that appear to be associated with a morbid outcome and early mortality. An analysis by the Cooperative Study of Sickle Cell Disease identified predictors of adverse outcomes, defined as death, stroke, and recurrent episodes of pain and ACS (≥ 2 and ≥ 1 event(s)/year for 3 consecutive years, respectively) before 10 years of age.21 These predictors included the onset of early dactylitis (before 1 year of age), leukocytosis, and severe anemia (Hgb level < 7 g/dL during the second year of life) (Figure 4 ).22 While these features may be clinical manifestations of the expression of genetic and epigenetic factors that synergistically magnify the impact of sickling, the complexity of linking these as yet undefined but potentially measurable modifiers to a clinical severity index remains elusive. However, if these predictors have reproducible and meaningful utility, they might provide a means for identifying and treating presymptomatic children with high-risk features by transplantation and thereby reduce the risk of graft rejection.
Transplantation for Central Nervous System Disease
Another avenue for pursuing prospective transplantation candidates before extensive transfusion exposures is to consider the often debilitating effect of brain injury, with the view that transplantation is a therapeutic and pre-emptive intervention for this serious complication. The central nervous system (CNS) injury associated with SCD, appropriately, has been a focus of intense investigation. While stroke is an overt manifestation of CNS injury that affects approximately 5-10% of patients, a more pervasive and subtle injury caused by endothelial damage and ischemia is increasingly apparent among children, and a more detailed view of the problem among adults is very likely to emerge.23,24 Prospective investigations by magnetic resonance imaging (MRI) have documented that CNS lesions progress in number and that these injuries correlate with functional abnormalities measured by neuropsychological testing instruments.25,26 Silent neurovascular lesions have predictive value with regard to stroke but have perhaps optimal predictive value when combined with transcranial Doppler evaluations.27-,29 The utility of RBC transfusions in preventing stroke and silent cerebral infarction among these high-risk patients has been demonstrated, and the duration of transfusion is the subject of a current multicenter clinical investigation.28,29 Stabilization of neurovascular disease as measured by cerebral MRI has been observed after successful allogeneic transplantation, raising the possibility that it too might be employed to prevent stroke and halt progression of CNS injury.10,18 This potential use was illustrated dramatically by our multicenter investigation of transplantation for SCD. Among 31 patients who were enrolled because they had stroke or other significant neurovascular disease before transplantation, 1 patient died of intracranial hemorrhage and 1 patient with graft rejection had a second stroke. Overall, the Kaplan-Meier probabilities of survival and stroke-free survival in this group were 97% and 93%, respectively, with a median follow-up of 32.2 (range, 2-95) months after transplantation. Partial recovery from pre-existing vascular injury also has been reported and improved cerebral arterial cross-sectional diameter noted in one study involving a small number of subjects after transplantation. Together these observations strongly suggest that patients who have stable engraftment of donor cells after transplantation also benefit by protection from subsequent CNS vascular events.
These observations have not prompted the universal application of transplantation for children with sickle cell anemia who have an HLA-identical sibling donor, in part because of the acute toxicity of transplantation, which includes a risk of dying, and its long-term effects, which include risks of infertility and secondary malignancies.8,10 In addition, clinical severity predictors and stroke risk have not been used routinely to indicate prospective transplantation candidates because there are therapeutic alternatives that are perhaps safer and more widely available.30,31 However, the use of these predictors might become acceptable if the toxicity of transplantation could be reduced without significantly altering its efficacy. Thus, current transplantation investigations have focused on reducing its toxicity by employing nonmyeloablative pretransplantation therapy with the aim of facilitating the stable engraftment of a sufficient number of donor cells to elicit a clinical effect. If successful, this strategy might make the option of transplantation available to presymptomatic children at one extreme of the spectrum of clinical severity, and to adult patients who have significant comorbid conditions that preclude conventional transplantation at the other. The justification resides in the prediction that risk-benefit considerations might be favorable for both the low-risk pediatric and high-risk adult recipients.
Stable Mixed Chimerism
A likely outcome after nonmyeloablative stem cell transplantation is stable mixed chimerism, a condition that was observed after conventional myeloablative transplantation for hemoglobinopathies. This condition has the potential for considerable ameliorative effect, an observation that has been particularly well documented for β-thalassemia major and also other hereditary disorders.32-,37 Approximately 10% of children with SCD and thalassemia major developed stable mixed chimerism after conventional HLA-identical sibling hematopoietic cell transplantation (HCT).11,34 Among those with β-thalassemia major, stable mixed chimerism persisted for 211 years after HCT. These patients remained transfusion independent, with hemoglobin levels that varied from 8.314.7 g/dL. The level of donor chimerism ranged from 2590%. From our multicenter investigation of BMT for sickle cell anemia, 13 of 50 patients with successful allografts developed stable mixed chimerism. The level of donor chimerism, measured ≥ 6 months after transplantation in peripheral blood, varied between 90% and 99% in 8 patients. Five additional patients had a lower proportion of donor cells (range, 11-74%). Among these 5, hemoglobin levels varied between 11.2 and 14.2 g/dL (median, 11.3; mean, 12.0). In patients who had donors with a normal hemoglobin genotype, the sickle hemoglobin (HbS) fractions were 0%, 0%, and 7%, which corresponded to donor chimerism levels of 67%, 74%, and 11%, respectively (Figure 5 ). Among patients who had donors with sickle trait, the HbS fractions were 36% and 37%, which corresponded to donor chimerism levels of 25% and 60%, respectively. Thus, allograft recipients with stable mixed chimerism had HbS levels similar to donor levels, and only one patient required an RBC transfusion beyond 90 days after transplantation. None of the patients experienced painful events or other clinical complications related to SCD after transplantation. These observations are consistent with the idea that chimerism even with a minority of donor cells might have a curative effect, and that full engraftment of donor cells is not a requirement for successful transplantation.
Nonmyeloablative Transplantation
The results after nonmyeloablative transplantation remain limited and incomplete. There have been two approaches, one utilizing a minimally toxic regimen first developed in a large animal model and translated successfully into human trials for older adult patients with hematological malignancies and a second, reduced-intensity regimen that retains a moderate degree of the myelosuppressive effect of transplantation.38,39 Representative examples of the two regimens are described in Table 6 . The former causes minimal myelosuppression and thus can be administered in the outpatient setting. This minimally toxic regimen relies on postgrafting immunosuppression to prevent GVH and HVG reactions and thereby promotes engraftment of donor cells. In contrast, the latter approach relies on reduced-intensity preparation to suppress the HVG reaction and promote engraftment. This reduced-intensity regimen is associated with hospitalization and accompanied by a risk of regimen-related toxicity, albeit at a reduced level.
A survey of the experience to date utilizing these two alternative nonmyeloablative regimens is presented in Table 6 (Iannone et al, August 2002, unpublished data).40-,43 All patients received HLA-identical sibling allografts, utilizing either granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC) or bone marrow. All 10 recipients of a minimally toxic regimen developed mixed chimerism initially, but tolerance was not sustained after post-grafting immunosuppression was discontinued in 8 of 10 patients. However, most patients were treated in the outpatient setting, none developed life-threatening transplant-related complications, and GVHD occurred infrequently and was mild in this predominantly pediatric cohort. Patients reverted to a sickle cell anemia phenotype after nonfatal graft rejection. Alternatively, those who received reduced-intensity conditioning regimens benefited from augmented pregrafting immunosuppression that facilitated engraftment of donor cells, and only 1 of the 7 recipients experienced graft rejection. Acute and chronic GVHD occurred more frequently in this older cohort and, in particular, among those who received PBSC allografts and developed full donor chimerism. Thus, the problem of transplant-related mortality was not eliminated by the reduced-intensity conditioning regimen, especially among older recipients. A possible strategy to overcome the adverse outcomes of graft rejection in the first group and transplant-related mortality in the second was suggested by the experience of 3 patients who developed stable mixed chimerism after receiving a minimally toxic regimen supplemented by rabbit anti-thymocyte globulin (ATG) (Thymoglobulin, Sangstat Medical Corp., Fremont, California).43 Preliminary data demonstrate that all 3 patients had stable engraftment of donor cells, and that the fraction of donor cells was sufficient to support a phlebotomy program after transplantation in a patient with thalassemia major and transfusional iron overload. A multicenter collaborative clinical trial to assess the safety and efficacy of this regimen is ongoing.
The observations of mixed chimerism after nonmyeloablative preparation also confirmed the clinical benefit of this condition, even when it was transitory. This benefit is illustrated by 2 patients who had initial engraftment of donor cells that resulted in mixed hematopoietic chimerism, although the level of donor chimerism declined after postgrafting immunosuppression was withdrawn.44 Despite the decline, the HbS fraction remained < 30% during the period when there was a low level of donor chimerism. The unexpectedly high HbA levels may reflect an enrichment of normal donor RBC because of their longer life span. However, it is also possible that a selective advantage for donor erythroid cells began at an earlier stage of erythroid development, even before significant hemoglobinization of the maturing erythrocyte had occurred. To investigate this, serial blast forming unit-erythroid (BFU-E) and colony forming unit-granulocyte macrophage (CFU-GM) colonies were obtained from marrow samples, and donor contribution was determined. In both patients, there was overrepresentation of donor erythroid progenitors compared with myeloid counterparts (Figure 6 ). These findings suggested that the clinical benefits of mixed chimerism after nonmyeloablative transplantation for sickle cell anemia resulted from an extended life span of mature donor erythrocytes and a selective advantage for donor erythroid progenitors in the marrow. Thus, it appears possible to achieve a clinical benefit even when the cellular compartment expressing normal hemoglobin represents a fraction of all marrow cells.
Development of Alternate Donors
The utilization of alternate stem cell sources is another obvious but unproven method to expand transplantation for SCD. Almost all transplantation cases to date have utilized bone marrow from HLA-identical siblings, and thus the experience of unrelated and HLA-mismatched related donor transplantation for sickle cell anemia is very limited. But comparison with results following alternate donor transplantation for β-thalassemia major suggests that it might be premature to consider these alternates except in special circumstances. A recent survey of HLA-mismatched related donor transplantation showed actuarial survival and graft failure rates of 65% and 55%, respectively, among 29 patients with β-thalassemia major.45 The results of HLA-matched unrelated donor HCT were better in a contemporary group of 32 patients with β-thalassemia major, who had a probability of disease-free survival of 66% after pretransplant preparation with busulfan (BU) and cyclophosphamide (CY), alone (n = 4) or in combination with thiotepa (TT) (n = 28).46 These 2 series illustrate the challenges of graft rejection and transplant-related mortality when there is HLA disparity between the donor and recipient. However, refinement in identifying allelic differences between unrelated donors and their recipients has directed the selection of donors to minimize the risks of GVHD and rejection.47,48 This refinement in identifying differences was also illustrated by results of transplantation for β-thalassemia major after extended HLA haplotype matching between unrelated donor-host pairs was performed. Among 22 recipients who were HLA identical for at least 1 extended haplotype, 19 patients survived, 17 free of thalassemia major.46 The identification of minor histocompatibility antigen differences that contribute to GVHD and transplantation outcome might also affect the selection of optimal unrelated donors.49 Of course, by increasing the resolution for optimal HLA-matching between donor-recipient pairs, a greater stringency of donor selection is very likely to restrict even further the donor availability for ethnic groups such as African Americans. Thus, recent results of haploidentical related donor transplantation are particularly promising, with their new insights about lymphocyte populations that facilitate engraftment.50,51 These new insights include the important discovery that donor alloreactive NK cell populations inhibit GVHD and promote engraftment.52 A similar facilitative effect by a lymphocyte population in the bone marrow that is distinct from lymphocytes in the blood has been reported recently.53 Finally, efforts to utilize parental hematopoietic stem cell donors by T-cell depletion and CD34+ cell megadose transplantation and to induce anergy by cosimulatory blockade have both yielded promising preliminary results,54- 56 although a minority of patients survive, in part because of a high rate of infection-related deaths. With refinement, it is likely that these methodologies will be applied to nonmalignant disorders such as sickle cell anemia.
Umbilical cord blood (UCB) is another source of hematopoietic stem cells undergoing investigation to support transplantation for SCD. UCB has several unique properties that make it potentially useful in this setting. Studies of CD34+ cells isolated from UCB demonstrate enhanced generation of committed hematopoietic progenitor cells compared to similar cells isolated from bone marrow.57 In addition, UCB is immunologically naive compared to adult peripheral blood, a factor that facilitates its consideration for transplantation when there is donor-host HLA disparity. The lower incidence of severe GVHD associated with HLA-mismatched UCB transplantation may be related to lower levels of hematopoietic cytokines and lymphokines produced by UCB-derived T cells compared to adult peripheral blood,58 as well as to decreased T-cell alloreactivity.59 Of course, abrogation of the allogeneic effect combined with a limiting number of hematopoietic stem cells together act to increase the risk of engraftment failure, a key consideration when developing UCB transplantation for SCD.
Results after UCB Transplantation
There are, however, other considerations that might mitigate the problem of graft rejection after UCB transplantation. Cell dose and HLA compatibility are important factors that contribute to engraftment and successful outcome after UCB transplantation.60,61 Recent experience of unrelated cord blood transplantation in adults suggests that cell dose considerations can be overcome in part by careful selection of candidate units, thus making UCB an important alternate stem cell source for pediatric and adult recipients.62 While unrelated UCB transplantation has been performed for a variety of genetic conditions, unrelated UCB transplantation has been attempted in few children with sickle cell anemia. Early reports of successful UCB transplantation for hemoglobinopathies, using related UCB donors, have been extended and confirmed by recent experience from the Eurocord registry.63 Forty-four patients (median age, 5 years; range, 1-20 years) with thalassemia (n = 33) or SCD (n = 11) received UCB transplantation from a related donor. All but 2 donors who were mismatched at a single HLA-A antigen were HLA-identical sibling donors. Twenty-six patients received BU and CY, either alone (n = 8) or in combination with ATG or antilymphocyte globulin (n = 18). In 17 patients, a conditioning regimen that contained either BU and CY or BU and fludarabine (FLU) was augmented by TT. One patient received a combination of BU, CY, and FLU. None of the patients died, and 36 of 44 children survived disease-free, with a median follow-up of 24 months (range, 3-76 months) after transplantation. Four patients experienced grade II acute GVHD, and 2 of 39 patients at risk developed limited chronic GVHD. The 2-year probabilities of event-free survival were 79% and 90% among patients with thalassemia and SCD, respectively.
One patient with SCD and 7 of 33 patients with thalassemia experienced disease recurrence after UCB transplantation. Three of 5 patients had sustained donor engraftment after a second conventional BMT from the same sibling donor. The impact of the conditioning regimen and the use of methotrexate (MTX) to prevent GVHD were considered in a univariate analysis of their associations with disease-free survival. This analysis showed that patients given MTX as part of GVHD prophylaxis had a significantly lower probability of surviving event-free compared to those who did not receive it (55% vs. 90%, respectively, P = 0.005). It also showed that receiving a non-MTX-containing regimen favorably affected the probability of myeloid recovery among all patients (P = 0.04) and in thalassemia patients (P = 0.06). In addition, pretransplant conditioning with BU and CY alone was associated with a lower probability of engraftment (P = 0.0003). Among thalassemia patients, the combinations of BU, TT, and CY or BU, TT, and FLU in the conditioning regimen were associated with a significantly higher probability of surviving event-free (94% for BU, TT, CY or BU, TT, FLU vs. 62% for BU/CY, P = 0.03). These results suggest that outcomes after UCB transplantation from sibling donors for hemoglobinopathies are similar to observations after BMT, with the potential for a lower rate of GVHD. The results also suggest that the problem of graft rejection is affected by the composition of pre- and postgrafting immunosuppressive regimens. To test these hypotheses, a prospective multicenter clinical trial of UCB transplantation from HLA-identical donors has been initiated.
Based in part on these observations, there is growing interest in facilitating UCB collection and storage from families who currently have a child with sickle cell anemia or hematological disorder and who are expecting another child. A national program to support cord blood collection for families who might benefit from UCB transplantation has been developed at the Children’s Hospital Oakland Research Institute.64 To date, this program has collected over 600 UCB donations from remote sites (primarily at community hospitals) in 42 states. High-quality UCB units that have a low bacterial contamination rate (less than 3%) and a cellular content sufficient to support transplantation (> 1.5 × 107 total nucleated cells/kg recipient weight collected in 90% of the cases) have been collected and processed by this program. On a very practical level, UCB stored in a public banking program is immediately available for use and does not require an extended waiting period. These considerations demonstrate the value of a national UCB banking resource that is available to families with children who might benefit from UCB transplantation and to researchers conducting prospective investigations of UCB transplantation.
Summary
Since the first report of successful BMT for sickle cell anemia 20 years ago, this curative therapy has emerged as an important option in treating individuals who inherit this disorder. While its curative potential distinguishes it from therapeutic alternatives, such as RBC transfusions and hydroxyurea, the acute and late toxicities of transplantation raise concerns about its wider use. These concerns are being addressed by ongoing efforts to develop nonmyeloablative transplantation regimens for sickle cell anemia. However, to date, an optimal regimen has not yet been identified. Ultimately, the future of transplantation for SCD will revolve around efforts to better understand the barriers to engraftment and how to overcome them. If these obstacles are eliminated, the possibility of transplantation from alternate stem cell sources such as UCB may become routine, thereby making transplantation available to more patients.
Within-person changes in acute chest syndrome (ACS) incidence according to patient age (for SS patients with at least 6 years of follow-up).
| . | . | Mean Incidence (Events/Pt-Yrs) . | Change in Incidence* . | ||
|---|---|---|---|---|---|
| Age at Study Entry (y) . | Sample Size . | First 3 yr . | Second 3 yr . | Mean . | P** . |
| * Incidence rate changes were obtained by subtracting a patient’s rate during the first 3 years of follow-up from his/her rate during the remainder of the follow-up period. | |||||
| ** Paired t- test (probability that the mean change is 0) | |||||
| Table reprinted with permission from Castro O et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84:643-9. | |||||
| 0-4 | 327 | 0.25 | 0.20 | -0.05 | .04 |
| 5-9 | 226 | 0.20 | 0.12 | -0.08 | .0004 |
| 10-14 | 200 | 0.10 | 0.10 | -0.01 | .81 |
| 15-19 | 168 | 0.07 | 0.10 | 0.03 | .08 |
| 20-24 | 140 | 0.14 | 0.14 | 0.0 | .99 |
| 25-29 | 108 | 0.09 | 0.10 | 0.02 | .53 |
| . | . | Mean Incidence (Events/Pt-Yrs) . | Change in Incidence* . | ||
|---|---|---|---|---|---|
| Age at Study Entry (y) . | Sample Size . | First 3 yr . | Second 3 yr . | Mean . | P** . |
| * Incidence rate changes were obtained by subtracting a patient’s rate during the first 3 years of follow-up from his/her rate during the remainder of the follow-up period. | |||||
| ** Paired t- test (probability that the mean change is 0) | |||||
| Table reprinted with permission from Castro O et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84:643-9. | |||||
| 0-4 | 327 | 0.25 | 0.20 | -0.05 | .04 |
| 5-9 | 226 | 0.20 | 0.12 | -0.08 | .0004 |
| 10-14 | 200 | 0.10 | 0.10 | -0.01 | .81 |
| 15-19 | 168 | 0.07 | 0.10 | 0.03 | .08 |
| 20-24 | 140 | 0.14 | 0.14 | 0.0 | .99 |
| 25-29 | 108 | 0.09 | 0.10 | 0.02 | .53 |
Characteristics of acute chest syndrome patients.*
| . | Age at First Episode of Acute Chest Syndrome . | ||||
|---|---|---|---|---|---|
| Characteristic . | All Pts.(n=537) . | 0-9 yr(n=264) . | 10-19 yr(n=145) . | >20 yr(n=128) . | P Value** . |
| * Because of rounding, percentages may not total 100. Data on one patient were excluded because the patient’s birth date was not known. | |||||
| ** P values were calculated by chi-square analyses. | |||||
| + Other reasons included a vaso-occlusive event, anemia, fever, infection, gastrointestinal or abdominal pain, asthma, urologic and orthopedic conditions, heart failure, and surgery. | |||||
| Reprinted with permission from Vichinsky E et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855-65. | |||||
| Sex (%) | |||||
| Male | 58 | 63 | 57 | 48 | 0.02 |
| Female | 42 | 38 | 43 | 53 | |
| Hemoglobinopathy (%) | |||||
| SS | 82 | 83 | 81 | 83 | 0.94 |
| Other | 18 | 17 | 19 | 17 | |
| Mean age at first episode of acute chest syndrome (yr) | 13.8 | 5.4 | 14.6 | 30.2 | <0.001 |
| Medical history (%) | |||||
| Vaso-occlusive event | 80 | 72 | 85 | 89 | <0.001 |
| Transfusion | 74 | 63 | 84 | 86 | <0.001 |
| Acute chest syndrome or pneumonia | 67 | 63 | 66 | 76 | 0.03 |
| Prophylactic antibiotic therapy | 57 | 81 | 51 | 13 | <0.001 |
| Major surgery | 40 | 26 | 48 | 61 | <0.001 |
| Neurologic disease | 16 | 10 | 17 | 29 | <0.001 |
| Sleep apnea | 14 | 14 | 17 | 11 | 0.41 |
| Red-cell antibodies | 12 | 7 | 13 | 21 | <0.001 |
| Aseptic necrosis or fracture | 12 | 5 | 11 | 26 | <0.001 |
| Asthma | 11 | 14 | 9 | 5 | <0.001 |
| Smoking | 10 | 0 | 4 | 36 | <0.001 |
| Renal disease or urinary tract infection | 7 | 3 | 7 | 14 | <0.001 |
| Chronic transfusion therapy | 5 | 5 | 5 | 7 | 0.64 |
| Cardiac disease | 4 | 1 | 3 | 12 | <0.001 |
| Chronic lung disease | 4 | 3 | 4 | 5 | 0.46 |
| Pulmonary edema | 4 | 1 | 3 | 9 | <0.001 |
| Clubbing | 2 | 1 | 0 | 5 | 0.002 |
| Deep-vein thrombosis | 1 | <1 | 0 | 3 | 0.01 |
| Tuberculosis | <1 | 0 | 0 | 1 | 0.20 |
| Reason for current admission (%) | |||||
| Acute chest syndrome | 52 | 61 | 46 | 42 | <0.001 |
| Other+ | 48 | 39 | 54 | 58 | |
| Symptoms at diagnosis of acute chest syndrome (%) | |||||
| Fever | 80 | 86 | 78 | 70 | <0.001 |
| Cough | 62 | 69 | 58 | 54 | 0.006 |
| Chest pain | 44 | 27 | 67 | 55 | <0.001 |
| Tachypnea | 45 | 47 | 67 | 55 | <0.001 |
| Shortness of breath | 41 | 31 | 44 | 58 | <0.001 |
| Pain in arms and legs | 37 | 22 | 44 | 59 | <0.001 |
| Abdominal pain | 35 | 38 | 36 | 29 | 0.24 |
| Rib or sternal pain | 21 | 14 | 26 | 30 | <0.001 |
| Reactive airway disease | 13 | 17 | 12 | 6 | 0.01 |
| Neurologic dysfunction | 4 | 3 | 3 | 7 | 0.11 |
| Cyanosis | 2 | 1 | 3 | 3 | 0.24 |
| Heart failure | 1 | 2 | 1 | 1 | 0.49 |
| . | Age at First Episode of Acute Chest Syndrome . | ||||
|---|---|---|---|---|---|
| Characteristic . | All Pts.(n=537) . | 0-9 yr(n=264) . | 10-19 yr(n=145) . | >20 yr(n=128) . | P Value** . |
| * Because of rounding, percentages may not total 100. Data on one patient were excluded because the patient’s birth date was not known. | |||||
| ** P values were calculated by chi-square analyses. | |||||
| + Other reasons included a vaso-occlusive event, anemia, fever, infection, gastrointestinal or abdominal pain, asthma, urologic and orthopedic conditions, heart failure, and surgery. | |||||
| Reprinted with permission from Vichinsky E et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855-65. | |||||
| Sex (%) | |||||
| Male | 58 | 63 | 57 | 48 | 0.02 |
| Female | 42 | 38 | 43 | 53 | |
| Hemoglobinopathy (%) | |||||
| SS | 82 | 83 | 81 | 83 | 0.94 |
| Other | 18 | 17 | 19 | 17 | |
| Mean age at first episode of acute chest syndrome (yr) | 13.8 | 5.4 | 14.6 | 30.2 | <0.001 |
| Medical history (%) | |||||
| Vaso-occlusive event | 80 | 72 | 85 | 89 | <0.001 |
| Transfusion | 74 | 63 | 84 | 86 | <0.001 |
| Acute chest syndrome or pneumonia | 67 | 63 | 66 | 76 | 0.03 |
| Prophylactic antibiotic therapy | 57 | 81 | 51 | 13 | <0.001 |
| Major surgery | 40 | 26 | 48 | 61 | <0.001 |
| Neurologic disease | 16 | 10 | 17 | 29 | <0.001 |
| Sleep apnea | 14 | 14 | 17 | 11 | 0.41 |
| Red-cell antibodies | 12 | 7 | 13 | 21 | <0.001 |
| Aseptic necrosis or fracture | 12 | 5 | 11 | 26 | <0.001 |
| Asthma | 11 | 14 | 9 | 5 | <0.001 |
| Smoking | 10 | 0 | 4 | 36 | <0.001 |
| Renal disease or urinary tract infection | 7 | 3 | 7 | 14 | <0.001 |
| Chronic transfusion therapy | 5 | 5 | 5 | 7 | 0.64 |
| Cardiac disease | 4 | 1 | 3 | 12 | <0.001 |
| Chronic lung disease | 4 | 3 | 4 | 5 | 0.46 |
| Pulmonary edema | 4 | 1 | 3 | 9 | <0.001 |
| Clubbing | 2 | 1 | 0 | 5 | 0.002 |
| Deep-vein thrombosis | 1 | <1 | 0 | 3 | 0.01 |
| Tuberculosis | <1 | 0 | 0 | 1 | 0.20 |
| Reason for current admission (%) | |||||
| Acute chest syndrome | 52 | 61 | 46 | 42 | <0.001 |
| Other+ | 48 | 39 | 54 | 58 | |
| Symptoms at diagnosis of acute chest syndrome (%) | |||||
| Fever | 80 | 86 | 78 | 70 | <0.001 |
| Cough | 62 | 69 | 58 | 54 | 0.006 |
| Chest pain | 44 | 27 | 67 | 55 | <0.001 |
| Tachypnea | 45 | 47 | 67 | 55 | <0.001 |
| Shortness of breath | 41 | 31 | 44 | 58 | <0.001 |
| Pain in arms and legs | 37 | 22 | 44 | 59 | <0.001 |
| Abdominal pain | 35 | 38 | 36 | 29 | 0.24 |
| Rib or sternal pain | 21 | 14 | 26 | 30 | <0.001 |
| Reactive airway disease | 13 | 17 | 12 | 6 | 0.01 |
| Neurologic dysfunction | 4 | 3 | 3 | 7 | 0.11 |
| Cyanosis | 2 | 1 | 3 | 3 | 0.24 |
| Heart failure | 1 | 2 | 1 | 1 | 0.49 |
Infectious pathogens isolated in 671 episodes of the acute chest syndrome.*
| Pathogen . | No. of Episodes . |
|---|---|
| * All infectious agents isolated during episodes of the acute chest syndrome are included. | |
| Reprinted with permission from Vichinsky E et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855-65. | |
| Chlamydia pneumoniae | 71 |
| Mycoplasma pneumoniae | 51 |
| Respiratory syncytial virus | 26 |
| Coagulase-positive Staphylococcus aureus | 12 |
| Streptococcus pneumoniae | 11 |
| Mycoplasma hominis | 10 |
| Parvovirus | 10 |
| Rhinovirus | 8 |
| Parainfluenzvirus | 6 |
| Haemophilus influenzae | 5 |
| Cytomegalovirus | 4 |
| Influenza A virus | 4 |
| Legionella pneumophila | 4 |
| Escherichia coli | 3 |
| Epstein-Barr virus | 3 |
| Herpes simplex virus | 3 |
| Pseudomonas species | 3 |
| Adenovirus | 2 |
| Branhamella species | 2 |
| Echovirus | 2 |
| Beta-hemolytic streptococcus | 2 |
| Mycobacterium tuberculosis | 2 |
| Enterobacter species | 1 |
| Klebsiella pneumoniae | 1 |
| Mycobacterium avium complex | 1 |
| Salmonella species | 1 |
| Serratia marcescens | 1 |
| Total | 249 |
| Pathogen . | No. of Episodes . |
|---|---|
| * All infectious agents isolated during episodes of the acute chest syndrome are included. | |
| Reprinted with permission from Vichinsky E et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855-65. | |
| Chlamydia pneumoniae | 71 |
| Mycoplasma pneumoniae | 51 |
| Respiratory syncytial virus | 26 |
| Coagulase-positive Staphylococcus aureus | 12 |
| Streptococcus pneumoniae | 11 |
| Mycoplasma hominis | 10 |
| Parvovirus | 10 |
| Rhinovirus | 8 |
| Parainfluenzvirus | 6 |
| Haemophilus influenzae | 5 |
| Cytomegalovirus | 4 |
| Influenza A virus | 4 |
| Legionella pneumophila | 4 |
| Escherichia coli | 3 |
| Epstein-Barr virus | 3 |
| Herpes simplex virus | 3 |
| Pseudomonas species | 3 |
| Adenovirus | 2 |
| Branhamella species | 2 |
| Echovirus | 2 |
| Beta-hemolytic streptococcus | 2 |
| Mycobacterium tuberculosis | 2 |
| Enterobacter species | 1 |
| Klebsiella pneumoniae | 1 |
| Mycobacterium avium complex | 1 |
| Salmonella species | 1 |
| Serratia marcescens | 1 |
| Total | 249 |
Predictive value of secretory phospholipase A2(sPLA2) in acute chest syndrome.
| . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy* (%) . |
|---|---|---|---|---|---|
| Abbreviations: PPV, positive predictive value; NPV, negative predictive value | |||||
| * The number of true positives and true negatives divided by the total number of patients. | |||||
| Reprinted with permission from Styles L et al. Secretory phospholipase A2 predicts impending acute chest syndrome in sickle cell disease. Blood. 2000;96:3276-78. | |||||
| sPLA2 alone | 100 | 67 | 55 | 100 | 76 |
| sPLA2 + fever | 100 | 87 | 75 | 100 | 90 |
| sPLA2 + chest pain | 50 | 80 | 50 | 80 | 71 |
| sPLA2 + respiratory symptoms | 67 | 100 | 100 | 88 | 90 |
| sPLA2 + ausculatory findings | 67 | 100 | 100 | 88 | 90 |
| . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy* (%) . |
|---|---|---|---|---|---|
| Abbreviations: PPV, positive predictive value; NPV, negative predictive value | |||||
| * The number of true positives and true negatives divided by the total number of patients. | |||||
| Reprinted with permission from Styles L et al. Secretory phospholipase A2 predicts impending acute chest syndrome in sickle cell disease. Blood. 2000;96:3276-78. | |||||
| sPLA2 alone | 100 | 67 | 55 | 100 | 76 |
| sPLA2 + fever | 100 | 87 | 75 | 100 | 90 |
| sPLA2 + chest pain | 50 | 80 | 50 | 80 | 71 |
| sPLA2 + respiratory symptoms | 67 | 100 | 100 | 88 | 90 |
| sPLA2 + ausculatory findings | 67 | 100 | 100 | 88 | 90 |
Indications for transplantation for sickle cell disease.
| Current Inclusion Criteria . |
|---|
| Abbreviations: CNS, central nervous system; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; RBC, red blood cell. |
| Patients with sickle cell disease (SS or Sβ°-thalassemia) less than 16 years of age |
| One or more of the following complications: |
| Stroke or CNS event lasting longer than 24 hours |
| Recurrent acute chest syndrome |
| Recurrent vaso-occlusive painful episodes or recurrent priapism |
| Impaired neuropsychologic function with abnormal cerebral MRI and angiography |
| Other Considerations for Inclusion Criteria |
| Stage I or II sickle lung disease |
| Sickle nephropathy (GFR 30-50% of predicted normal) |
| Osteonecrosis of multiple joints |
| RBC alloimmunization (≥2 antibodies) on chronic transfusion therapy |
| Increased cerebral arterial velocity by transcranial doppler |
| Current Inclusion Criteria . |
|---|
| Abbreviations: CNS, central nervous system; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; RBC, red blood cell. |
| Patients with sickle cell disease (SS or Sβ°-thalassemia) less than 16 years of age |
| One or more of the following complications: |
| Stroke or CNS event lasting longer than 24 hours |
| Recurrent acute chest syndrome |
| Recurrent vaso-occlusive painful episodes or recurrent priapism |
| Impaired neuropsychologic function with abnormal cerebral MRI and angiography |
| Other Considerations for Inclusion Criteria |
| Stage I or II sickle lung disease |
| Sickle nephropathy (GFR 30-50% of predicted normal) |
| Osteonecrosis of multiple joints |
| RBC alloimmunization (≥2 antibodies) on chronic transfusion therapy |
| Increased cerebral arterial velocity by transcranial doppler |
| Regimen . | Minimal-Toxicity Conditioning Regimen . | Reduced-Intensity Conditioning . |
|---|---|---|
| †Includes 2 patients with thalassemia major. | ||
| ‡Includes 1 patient with thalassemia major. | ||
| Abbreviations: ATG, antithymocyte globulin; CY, cyclophosphamide; Flu, fludarabine; Gr, grade; GVHD, graft-versus-host disease; Mel, melphalan; No., number; PBSC, peripheral blood stem cells; TBI, total body irradiation; TLI, total lymphoid irradiation; UCB, umbilical cord blood. | ||
| No. of patients | 10† | 7‡ |
| Patient age (median, yrs) | 10 (range, 3-28) | 22 (range, 7-56) |
| Conditioning regimen (dose) [no. of patients] | Flu (90-150)/TBI (200) [5]; Flu (125-150)/ATG/TBI (200)[5] | Flu(175)/BU(8)/ATG/TLI (500)[4]; Flu (120)/Mel(140)/ATG [2]; Flu(120)/ CY(120) [1] |
| Source of stem cells (no. of patients) | Marrow (8); PBSC (2) | Marrow (2); PBSC (3); UCB (1) |
| Induction of mixed chimerism | Yes (transient in 8) | Yes |
| No. with graft rejection/disease recurrence | 8 | 1 |
| No. with GVHD | Acute 1 (Gr I), chronic, none | Acute 3 (Gr II-IV), chronic, 3 (2 fatal) |
| No. of deaths | None | 2 |
| No. with event-free survival | 2 | 4 |
| Regimen . | Minimal-Toxicity Conditioning Regimen . | Reduced-Intensity Conditioning . |
|---|---|---|
| †Includes 2 patients with thalassemia major. | ||
| ‡Includes 1 patient with thalassemia major. | ||
| Abbreviations: ATG, antithymocyte globulin; CY, cyclophosphamide; Flu, fludarabine; Gr, grade; GVHD, graft-versus-host disease; Mel, melphalan; No., number; PBSC, peripheral blood stem cells; TBI, total body irradiation; TLI, total lymphoid irradiation; UCB, umbilical cord blood. | ||
| No. of patients | 10† | 7‡ |
| Patient age (median, yrs) | 10 (range, 3-28) | 22 (range, 7-56) |
| Conditioning regimen (dose) [no. of patients] | Flu (90-150)/TBI (200) [5]; Flu (125-150)/ATG/TBI (200)[5] | Flu(175)/BU(8)/ATG/TLI (500)[4]; Flu (120)/Mel(140)/ATG [2]; Flu(120)/ CY(120) [1] |
| Source of stem cells (no. of patients) | Marrow (8); PBSC (2) | Marrow (2); PBSC (3); UCB (1) |
| Induction of mixed chimerism | Yes (transient in 8) | Yes |
| No. with graft rejection/disease recurrence | 8 | 1 |
| No. with GVHD | Acute 1 (Gr I), chronic, none | Acute 3 (Gr II-IV), chronic, 3 (2 fatal) |
| No. of deaths | None | 2 |
| No. with event-free survival | 2 | 4 |
The retroviral life cycle.
Binding of the virus to the cell is mediated by specific interactions with the viral envelope and cellular receptors. The diploid RNA genome is released into the cytoplasm, where a double-stranded complementary DNA is formed through the action of viral reverse transcriptase. A circular double-stranded DNA intermediate is incorporated as part of the preintegration complex, which gains access to the nuclear contents either during mitosis or by direct nuclear transmigration in the case of lentiviruses. The proviral genome is subsequently integrated into the chromosomal DNA, a reaction mediated by the viral integrase protein. After integration, the provirus is stably passed to daughter cells following cell division.
The retroviral life cycle.
Binding of the virus to the cell is mediated by specific interactions with the viral envelope and cellular receptors. The diploid RNA genome is released into the cytoplasm, where a double-stranded complementary DNA is formed through the action of viral reverse transcriptase. A circular double-stranded DNA intermediate is incorporated as part of the preintegration complex, which gains access to the nuclear contents either during mitosis or by direct nuclear transmigration in the case of lentiviruses. The proviral genome is subsequently integrated into the chromosomal DNA, a reaction mediated by the viral integrase protein. After integration, the provirus is stably passed to daughter cells following cell division.
A lentiviral vector system for globin gene transfer.
Several modifications were introduced to improve vector production and/or enhance safety as the vector genome was assembled to derive pCL10.1.22 The 5’ LTR is chimeric in that the U3 region of the HIV-1 LTR is replaced with the CMV enhancer. A deletion in the U3 region of the 3’ LTR renders it self-inactivating; in addition, a portion of the R region and the U5 region have been removed and replaced with the rabbit β-globin gene polyadenylation site to enhance safety and improve the efficiency of vector production. The cPPT and CTS were added to improve vector integration. All coding sequences for accessory proteins have been eliminated from the vector. The γ-globin gene with a 720 bp deletion in the second intron was linked to the β-globin gene promoter (-130 to +54) and placed downstream from HS 2 (374 bp), 3 (898 bp), and 4 (445 bp) from the β-globin locus control region. The cassette is placed in the reverse transcriptional orientation of the vector transcript to prevent loss of the globin gene introns during passage of the vector genome (D. A. Persons, P. W. Hargrove, E. R. Allay, H. Hanawa, A. W. Nienhuis, unpublished data, 2002). The expression plasmid pCAGGS was used to construct the cassettes expressing HIV structural and regulatory proteins and the VSV-G envelope protein.22 This expression plasmid contains a powerful chimeric CMV enhancer/β-actin promoter, the β-globin large intron, which ensures splicing of most transcripts, and the rabbit β-globin polyadenylation sequence, which includes an RNA stability element in the 3’ untranslated region and the SV40 origin of replication to increase plasmid copy number in 293T cells. The accessory proteins, tat and rev, were expressed together on a plasmid separate from that expressing the GAG-POL-precursor polyprotein. The RRE element was included to enhance rev-dependent, nuclear-to-cytoplasmic transfer of RNA molecules encoding gag/pol and to modulate expression of rev and tat by fostering transport of unspliced transcripts.
Abbreviations: LTR, long terminal repeat; CMV, cytomegalovirus; cPPT, central polypurine tract; CTS, central termination sequence; HS, hypersensitive site; HIV, human immunodeficiency virus.
A lentiviral vector system for globin gene transfer.
Several modifications were introduced to improve vector production and/or enhance safety as the vector genome was assembled to derive pCL10.1.22 The 5’ LTR is chimeric in that the U3 region of the HIV-1 LTR is replaced with the CMV enhancer. A deletion in the U3 region of the 3’ LTR renders it self-inactivating; in addition, a portion of the R region and the U5 region have been removed and replaced with the rabbit β-globin gene polyadenylation site to enhance safety and improve the efficiency of vector production. The cPPT and CTS were added to improve vector integration. All coding sequences for accessory proteins have been eliminated from the vector. The γ-globin gene with a 720 bp deletion in the second intron was linked to the β-globin gene promoter (-130 to +54) and placed downstream from HS 2 (374 bp), 3 (898 bp), and 4 (445 bp) from the β-globin locus control region. The cassette is placed in the reverse transcriptional orientation of the vector transcript to prevent loss of the globin gene introns during passage of the vector genome (D. A. Persons, P. W. Hargrove, E. R. Allay, H. Hanawa, A. W. Nienhuis, unpublished data, 2002). The expression plasmid pCAGGS was used to construct the cassettes expressing HIV structural and regulatory proteins and the VSV-G envelope protein.22 This expression plasmid contains a powerful chimeric CMV enhancer/β-actin promoter, the β-globin large intron, which ensures splicing of most transcripts, and the rabbit β-globin polyadenylation sequence, which includes an RNA stability element in the 3’ untranslated region and the SV40 origin of replication to increase plasmid copy number in 293T cells. The accessory proteins, tat and rev, were expressed together on a plasmid separate from that expressing the GAG-POL-precursor polyprotein. The RRE element was included to enhance rev-dependent, nuclear-to-cytoplasmic transfer of RNA molecules encoding gag/pol and to modulate expression of rev and tat by fostering transport of unspliced transcripts.
Abbreviations: LTR, long terminal repeat; CMV, cytomegalovirus; cPPT, central polypurine tract; CTS, central termination sequence; HS, hypersensitive site; HIV, human immunodeficiency virus.
Age-specific associated events within the 2 weeks preceding the first episode of acute chest syndrome.
Reprinted with permission from Vichinsky E et al. Acute chest syndrome in sickle cell disease: clinical presentations and course. Blood. 1997;89:1787-92.
Age-specific associated events within the 2 weeks preceding the first episode of acute chest syndrome.
Reprinted with permission from Vichinsky E et al. Acute chest syndrome in sickle cell disease: clinical presentations and course. Blood. 1997;89:1787-92.
Estimated likelihood of severe sickle cell disease before 18 years of age as predicted by severe anemia (Hgb <7 g/dL in the second year of life), leukocyte count, and the occurrence of dactylitis before 1 year of age.22
Reprinted with permission from Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in the newborn cohort of the Cooperative Study of Sickle Cell Disease (CSSCD): extended follow-up to 18 years. Blood. 2000;96:12a.
Estimated likelihood of severe sickle cell disease before 18 years of age as predicted by severe anemia (Hgb <7 g/dL in the second year of life), leukocyte count, and the occurrence of dactylitis before 1 year of age.22
Reprinted with permission from Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in the newborn cohort of the Cooperative Study of Sickle Cell Disease (CSSCD): extended follow-up to 18 years. Blood. 2000;96:12a.
Serial determinations of donor chimerism and HbS fractions after transplantation.
(A) The fraction of donor cells in the blood (closed circles) and HbS fraction (open triangles) are depicted for 3 patients who had HbAA donors.(B) The fraction of donor cells and HbS in the blood are depicted for 2 patients who had HbAS donors.11
Abbreviations: BMT, bone marrow transplantation.
Reprinted with permission from the American Society for Blood and Marrow Transplantation. Walters MC, Patience M, Leisening W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–673.
Serial determinations of donor chimerism and HbS fractions after transplantation.
(A) The fraction of donor cells in the blood (closed circles) and HbS fraction (open triangles) are depicted for 3 patients who had HbAA donors.(B) The fraction of donor cells and HbS in the blood are depicted for 2 patients who had HbAS donors.11
Abbreviations: BMT, bone marrow transplantation.
Reprinted with permission from the American Society for Blood and Marrow Transplantation. Walters MC, Patience M, Leisening W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–673.
Bone marrow chimerism after nonmyeloablative transplantation for sickle cell disease.
The percentage of donor progenitor colonies in the bone marrow (BFU-E [open bars] and CFU-GM [closed bars]) after transplantation is depicted in 2 patients.44
Abbreviations: BMT, bone marrow transplantation.
Reprinted with permission from Walters M, Woolfey A, Torok-Storb B, et al. Blood. 2001;98:490a.
Bone marrow chimerism after nonmyeloablative transplantation for sickle cell disease.
The percentage of donor progenitor colonies in the bone marrow (BFU-E [open bars] and CFU-GM [closed bars]) after transplantation is depicted in 2 patients.44
Abbreviations: BMT, bone marrow transplantation.
Reprinted with permission from Walters M, Woolfey A, Torok-Storb B, et al. Blood. 2001;98:490a.

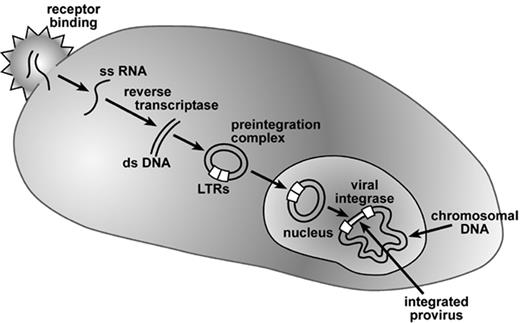
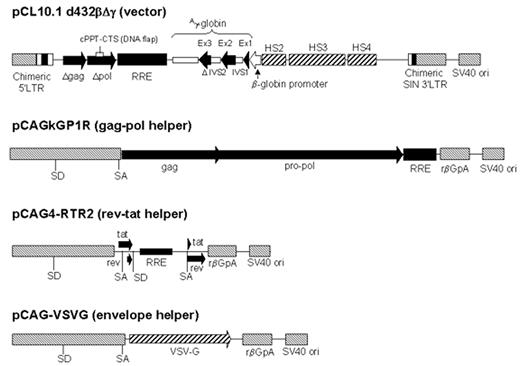
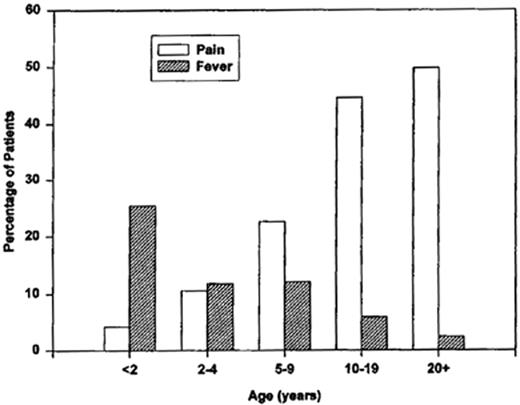
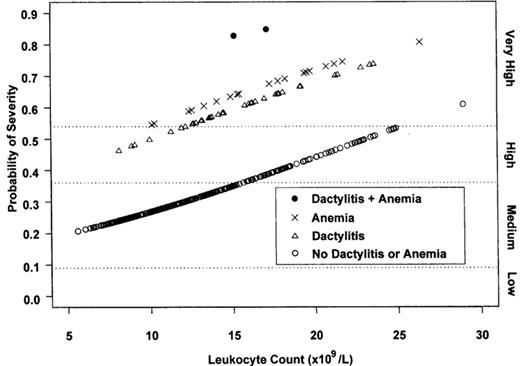

![Figure 6. Bone marrow chimerism after nonmyeloablative transplantation for sickle cell disease. / The percentage of donor progenitor colonies in the bone marrow (BFU-E [open bars] and CFU-GM [closed bars]) after transplantation is depicted in 2 patients.44. / Abbreviations: BMT, bone marrow transplantation. / Reprinted with permission from Walters M, Woolfey A, Torok-Storb B, et al. Blood. 2001;98:490a.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2002/1/10.1182_asheducation-2002.1.10/3/m_walters_fig6.jpeg?Expires=1767733962&Signature=zLyINIwwzvBPF4PR574c6qS7KTG1oblTHA2BF4MxBtmlmTdFgEgdBOBw0F7d7xCaOMh8GIb~py9uMnTJ1egOrEyAt7nymkF2VHXhMPtkKguoVmUAH1vAKHkhae-AUZbibxIBOCqHVNlVOC0OanYn2Cq1qyvxZ691VOexOIsnOHPj-vuJ~YbeQR8u3133AEG-IUsQgBZgz46P3QvFmSWIKKCdznpH7epXgivJJBCeyNd9YXkAnu90QCndGp6xDmWg0rldOG2Meu5S-batijRW1df9P5n9xJ5MECvyJNpqxtxV8drAJk0HPUR6kbK1YZPyQv2DNscHZXFXtAtp6CSplQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)