Abstract
This chapter presents updated information on the trends and patterns of non-Hodgkin’s lymphoma (NHL) diagnoses as well as new information on chemotherapeutic and immunotherapeutic options for NHL treatment.
In Section I, Dr. Brian Chiu summarizes the current knowledge regarding the etiologic factors and patterns of NHL as well as suggests future epidemiologic studies based on these preliminary results.
In Section II, Dr. Bruce Cheson and colleagues outline new chemotherapeutic and small molecule antineoplastic agents with unique mechanisms of action such as protease inhibitors, farnesyl transferase or histone deacetylase inhibitors, and antisense oligonucleotides.
In Section III, Dr. Julie Vose reviews the anti-lymphoma effects of monoclonal antibodies, radioimmunoconjugates, idiotype vaccines, and immunologic enhancing adjuvants with respect to mechanisms of action, clinical trials, and their potential for patient therapy.
I. Epidemiology of Non-Hodgkin’s Lymphoma
Brian C.-H. Chiu, PhD*
University of Nebraska Medical Center, 984350 Nebraska Medical Center, Omaha, NE 68198-4350
Non-Hodgkin’s lymphoma (NHL) is composed of many histologically and perhaps biologically distinct lymphoid malignancies, each with poorly understood but putatively distinct etiologies. This review presents the demographics of NHL, describes the recent changes in incidence, and summarizes the current knowledge about the possible etiology of NHL, with a focus on NHL subtypes where data are available.
Incidence Rates
Present incidence of NHL
It is estimated that approximately 53,900 new cases of NHL (28,200 males and 25,700 females) will be diagnosed in the United States in 2002,1 representing 4% of all cancers. The annual incidence rate of NHL in 1995-1999, estimated from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, was 19.1 cases per 100,000 persons.1 The overall incidence rate is about 50% higher for males (23.6 per 100,000) than for females (15.4 per 100,000),1 and this increased risk in men is seen in other countries as well.2 The difference between male and female incidence rate is more marked in younger than older individuals.1 During the period of 1995-1999, the NHL incidence rates were 35% higher among whites (19.9 per 100,000) than blacks (14.7 per 100,000). Rates of NHL historically have been about 40% higher in urban counties than in rural counties, but the excess has become smaller (about 15-25%) in recent years and is attributed to a more rapid increase in the incidence of NHL in rural areas.3
The majority of NHL cases arise in lymph nodes, but primary extranodal disease now accounts for 20-30% of all cases.4,5 The most frequent primary extranodal sites are the stomach, small intestine, skin, and brain. Histologically, diffuse lymphomas account for about 40% of NHL1 and occur more frequently among males than females at middle age and among whites more than blacks at older ages.5 Follicular lymphomas account for about 20% of NHL,1 are twice as common in whites as in blacks, but occur almost equally in men and women.5 The majority of new cases (50%) are intermediate-grade lymphomas, followed by low-grade (30%) and high-grade (10%) lymphomas.5
The incidence rates of NHL in the United States tend to exceed those of most other countries.2 The difference is particularly striking for follicular lymphoma, which constitutes 20% of cases in Western countries but is relatively rare in developing countries, China, and Japan.6,7 Geographical differences in etiologic or host factors may play a role in these international differences.7,8
Recent changes in the incidence rate
Data from the U.S. SEER program indicate that the incidence of NHL has increased substantially since the early 1970s, from 10.2 per 100,000 in 1973 to 18.5 in 1990, an increase of 81% overall and an increase of about 3.6% per year.1 Recent SEER data suggest that, after steady increases of 3-4% per year in the 1970s and 1980s, overall NHL incidence rates leveled off in the 1990s, except among black females.1,9 From 1990 through 1999, the incidence rates decreased 0.8% per year for both white males and black males. However, during the same period, NHL incidence rates increased 0.9% and 3.6% per year for white females and black females, respectively.1
The rapid increase in NHL incidence in the 1970s and 1980s has been exceeded only by the increase in lung cancer in women and malignant melanomas in both sexes. The increase has been seen for males and females, for whites and blacks, and in all age groups, except for children.2,5 The increase has been largest among those aged 65 years and older. The increase in incidence has been more rapid in rural than urban regions of the United States,3 and may be associated with exposures to pesticides (see below).
Since 1973, the incidence rate of all subtypes of NHL, according to the Working Formulation, have increased, with the largest rate of increase occurring among high-grade lymphomas, which includes most AIDS-related lymphoma.2 High-grade lymphoma is twice as common in males as in females. The incidence of high-grade lymphoma tripled among males and doubled among females from 1978 through 1999.1,5 Extranodal disease has increased more rapidly than nodal disease has.3 Incidence rates increased 3.0-6.9% per year for extranodal cases, compared to 1.7-2.5% per year for nodal cases, with the largest increase occurring in the brain and other areas of the nervous system (224%). The increase in extranodal lymphomas is, in part, a consequence of the application of modern immunophenotypic and genotypic methods, which have led to the reclassification of so-called pseudolymphomas as monoclonal B-cell lymphomas.10 The dramatic increase in NHL of the central nervous system warrants investigation, although it contributed only slightly to the long-term NHL trends.11
Histologically, diffuse NHL has seen the largest increase in the past 2 decades, particularly the diffuse large-cell and immunoblastic subtypes (rose by 50-100%).4,5 Diffuse small cleaved cell NHL was the only subtype that declined,5 and this decrease probably reflects a change in diagnosis.11
The reasons for these increases are poorly understood. After accounting for the impact of changes in diagnosis and well-established risk factors (see below), there remains an unexplained increase in incidence of more than 40% over the past 40 years.12 The following section will review some of the established and postulated risk factors for the development of NHL.
Etiologic Factors
Immunodeficiency
Immunodeficiency, including both congenital and acquired conditions, is the strongest risk factor known to increase NHL risk.8 NHL is the most frequent malignancy in young persons with ataxia telangiectasia or the Wiskott-Aldrich syndrome, as well as in children with X-linked lymphoproliferative syndrome or combined immunodeficiency.13 Epstein-Barr virus (EBV) appears to be an important co-factor. In addition, host defects in immunoregulation resulting in an imbalance of cytokine production and genetic defects resulting in imprecise and/or ineffective rearrangement of immunoglobulin and T-cell receptor genes during lymphopoiesis likely contribute to the development of NHL.13 However, these congenital immunodeficiency disorders are rare and thus have not contributed substantially to the increasing incidence of NHL.
Patients who are treated with immunosuppressive drugs following solid organ or bone marrow transplantation are at substantially increased risk (30 to 50 times) for NHL.14 Chronic antigenic stimulation induced by the graft and the accompanying immunosuppression are probable mechanisms.2 Polyclonal B-cell proliferation is often seen first in transplant patients, and is occasionally reversible when the immunosuppressive therapy is stopped. It may persist and evolve into a monoclonal disease. Loss of control of persistent EBV infection caused by the immunosuppressive therapy appears to be part of this process.2 However, in spite of the significant increase of organ transplants in the United States in the past decade, these transplant-related NHL are relatively uncommon and explain very little of the NHL increase in the general population.5
The risk of NHL in persons infected with HIV is more than 100-fold that of the general population.15 The incidence of NHL increased 20-fold between 1973-1979 and 1988-1990 in the city of San Francisco, in which approximately one-fourth of all single young men were infected with HIV-1 as of 1984.8 These lymphomas are typically of B-cell origin with high-grade histology, are primarily diffuse large cell or Burkitt’s lymphoma, and frequently occur in extranodal sites, such as the brain.2,8 However, except possibly in San Francisco in the most recent years, AIDS accounts for a relatively small fraction of NHL.12 Even with San Francisco excluded from the US data, though, there still is an increased incidence of 2.6% per year in males and 2.0% per year in females.16
An excess risk of developing NHL has also been reported among patients with a variety of diseases of the immune system, including rheumatoid arthritis, Sjögren’s syndrome, systemic lupus erythematosus, and celiac disease.2 The estimated risks were lower in population-based studies.17 These conditions are also unlikely to contribute dramatically to rising secular trends in NHL because these diseases are rare and their incidence has either not increased or risen only moderately over time.5
Infectious organisms other than HIV
Infectious organisms, including human T-cell lymphotrophic virus type I (HTLV-I), EBV, Helicobacter pylori, and possibly hepatitis C virus (HCV), have been postulated to play a role in the development of NHL because infection can result in immune system stimulation or dysregulation.
Human T-cell lymphotrophic virus (HTLV), types I and II.
HTLV-I is a retrovirus that is endemic in southern Japan and the Caribbean basin.8 Infection with HTLV-I, especially in early childhood, is strongly related to adult T-cell leukemia/lymphoma.18 The cumulative lifetime risk for development of adult T-cell leukemia/lymphoma in infected individuals is estimated to be approximately 5%.19 Because HTLV-I has a unique geographic distribution, is a relatively rare infection, and causes a specific and rare subtype of NHL, HTLV-I infection is unlikely to account for much of the current increase in NHL.16,18
Epstein-Barr virus.
Unlike HTLV-I, EBV infection is a highly prevalent infection in the adult population, with approximately 90% of individuals in developed countries having evidence of previous infection by age 40.8 The association between EBV and lymphoma is well described for both Burkitt’s lymphoma and immunodeficiency-associated lymphoma. Nearly all cases of the endemic Burkitt’s lymphoma (in Africa and New Guinea) can be shown to contain EBV viral genomic DNA, but the frequency is less than 20% in sporadic cases (in the United States and other developed countries).8,16 Even though EBV can be identified in many lymphomas, its impact on NHL trends is expected to be small, because the infection is nearly ubiquitous and the prevalence of infection has not changed for generations.
Helicobacter pylori.
Chronic gastric infection with H. pylori has been linked to the development of low-grade, mucosa-associated lymphoid tissue (MALT) lymphoma in the stomach.20 Seropositivity to H. pylori was associated with a 6-fold increased risk of gastric lymphoma in a prospective study.21 These findings suggest that chronic antigenic stimulation and/or inflammation may be important in the development of MALT lymphoma, and perhaps other forms of NHL as well.8 However, the declining prevalence of H. pylori infection in the United States suggests that this infectious organism is unlikely to play an important role in the upward NHL trends.
Hepatitis C virus.
The association of HCV with some B-cell NHL has been demonstrated by multiple case reports.22,23 A recent therapeutic study that showed regression of splenic lymphoma with villous lymphocytes after treatment of HCV infection further supports that HCV infection may play a role in lymphomagenesis.24 However, the findings from epidemiologic studies of a relationship between HCV and NHL are mixed. A positive association between HCV and B-cell NHL has been found in some case-control studies25,26 but not others.27 A study in Southern Italy showed a higher incidence of HCV infection in aggressive NHL than in low-grade NHL,25 whereas other studies report a higher risk for low-grade NHL.26
Familial aggregation
A history of NHL or other hematolymphoid cancer in close relatives has been repeatedly shown to increase the risk of NHL by 2- to 3-fold,28,29 a stronger association than that estimated for most of the other suspected risk factors. The aggregation is associated with an inherited defect of immune function in some instances, although in many families no such abnormality can be discerned.8 Lymphomas may also cluster within families, not because of an inherited genetic susceptibility, but because of shared environmental determinants. Studies28–,30 have shown that pesticide-related risks and occupational exposure to benzene were greater among individuals with a positive family history of cancer. Ward and colleagues31 found that the risks of NHL in people with a low intake of vitamin C and carotene were greater among individuals with a family history of cancer, particularly a history of hematolymphoid cancer. Chiu and coworkers32 also found that alcohol use is associated with an increased risk of NHL among only men with a family history of hematolymphoid cancer. These findings are intriguing and suggest that the risk of developing NHL may be determined by interactions between environmental and genetic factors.
Blood transfusion
Studies that have examined the association between a history of blood transfusion and the risk of NHL have produced contradictory findings. Cohort studies have supported the hypothesis that previous allogeneic blood transfusion may increase the risk of developing NHL, but several case-control studies published subsequently failed to replicate the findings.33 Blood transfusion has also been specifically associated with low-grade NHL34,35 or with extranodal high-grade NHL.34
This increased risk has been attributed to the immunosuppressive effects of allogeneic blood transfusion, as well as the increased susceptibility of those persons who receive transfusions to infections caused by blood-borne organisms.36 Increases in the use of blood transfusion since the 1950s have coincided with the increase in the incidence of NHL.33
Agricultural and pesticide exposures
An association between NHL and pesticide exposures has been observed repeatedly, but not consistently. Results from a number of epidemiologic studies suggest that the excess risk of NHL is related to the use of phenoxyacetic acid herbicides, organophosphate insecticides, triazine herbicides, and fertilizers.37 A nest case-control study found a strong positive association between serum concentrations of polychlorinated biphenyls and risk of NHL.38 However, risk estimates vary widely among studies, and in some studies the risk was not increased at all.
When NHL subtype was evaluated, herbicides increased the risk of follicular NHL in a study conducted in Nebraska,39 whereas modest increases for small lymphocytic NHL (associated with certain crops and pesticide categories) and diffuse NHL (linked with specific herbicides and pesticides) occurred among farmers in Iowa and Minnesota.40 A recent study evaluated the pesticide-NHL association according to t(14;18) status and found that t(14;18)-positive NHL was associated with farming and exposures to dieldrin, lindane, atrazine, and fungicides, in marked contrast to null or inverse associations for the same exposures and t(14;18)-negative NHL.41
Although the fraction of agricultural workers in the population is small and has been decreasing over time, the pesticide exposure of the general population has been increasing. The use of lawn-care pesticides is increasing at a rate of 5-8% a year.42 Pesticides are applied to lawns at 5 times the application rate used on pesticide-treated agricultural lands.43 Even though general population exposures may be lower than those in occupational settings, a relative risk as small as 1.2 could explain 15% of current NHL risk, assuming that over 90% of the general population is exposed.8 Therefore, the role of agricultural and residential pesticides in the etiology of NHL warrants further evaluation.
Lifestyle factors
Little is known concerning the role of diet in the etiology of NHL.44 The risk of NHL has been linked to increased consumption of animal protein, fat, and meat,45–,47 while higher intakes of fruits, cruciferous vegetables, and vegetables high in carotene have shown an inverse association with NHL.31,45,48 Long-term regular use of vitamin supplements appears to have no association with the risk of NHL49 or mortality from NHL.50 The data on diet, however, are not conclusive. The most consistent findings to date are that diets high in animal proteins and fats appear to increase the risk of NHL, and fruit and vegetable consumption appears to decrease the risk.
Several epidemiologic studies have evaluated the role of alcohol consumption in the etiology of NHL, but the findings have been inconsistent. Alcohol consumption has been linked to an increased risk47 and a decreased risk,51 or had no relation.52
Cigarette smoking appears to have no or only a weak association with NHL. Most previous studies have shown no association53 or only modest increase in risk, often not statistically significant.54 However, smoking has been linked with high-grade NHL55 and with follicular NHL.56
Hair coloring products are widely used and contain compounds that are mutagenic and carcinogenic in animals. Several studies have suggested that the use of hair dyes, particularly long-term use of dark permanent dyes, may increase the risk of NHL,57 whereas others have reported no increased risk.58 A small increase in risk of follicular NHL with hair dye use has also been reported.57
Genetic susceptibility
Tumor suppressors and oncogenes.
Cytogenetic studies have shown that most NHL exhibit chromosomal abnormalities and that some of the reciprocal chromosomal translocations are closely correlated with specific histological and immunological types.59 These chromosomal abnormalities include the t(14;18) involving the BCL2 proto-oncogene in 85-90% of follicular lymphomas and 20-30% of diffuse large B-cell lymphomas;60 the t(3;14) and other translocations involving the BCL6 proto-oncogene in 10-15% of follicular lymphomas and 30-40% of diffuse large B-cell lymphomas;61 and the t(8;14) and other translocations involving the c-MYC proto-oncogene in 100% of Burkitt’s lymphoma and in 10%-15% of diffuse large B-cell lymphoma.62 It has also been suggested that microsatellite instability contributes to the development of lymphoid malignancies,63 but the relevance of microsatellite instability in hematological malignancies remains controversial.
Cancer susceptibility genes.
Suspected risk factors for NHL include environmental exposures (see above) and, therefore, genetically determined variation in the ability to metabolize such exposure may be important in determining NHL risk. These enzymes include the multigene families of cytochrome P450 (CYP) and glutathione S-transferase (GST). CYP1A1 is critical in the activation of polycyclic aromatic hydrocarbons and dioxin,64 and has been associated with childhood leukemia.65CYP3A4 is involved in the oxidation of parathion and probably other organophosphate insecticides,64 exposures that have been associated with NHL.66CYP2E1 metabolizes a number of carcinogens found in solvents.67 Occupations with exposures to solventsincluding chlorinated solvents, benzene, paint thinner, and mineral oilhave been linked to NHL risk.68
The GST-mediated glutathione conjugation is known to play a role in the detoxification of a broad array of chemicals, including pesticides, polycyclic aromatic hydrocarbons, heterocyclic amines, and solvents.64GSTM1 null genotype has been associated with an increased risk of acute myeloid leukemia.69 Also, GSTT1-null individuals were found to have a more than 4-fold increased risk of a myelodysplastic syndrome, which often progresses to acute myeloid leukemia.70 Two recent studies71,72 and preliminary analyses from our ongoing study in Nebraska suggested that GSTM1 and GSTT1 were not associated with the risk of NHL. However, none of these reports evaluated the association between suspected risk factors and NHL according to polymorphisms in these genes.
Other risk factors
Other environmental exposures that have been linked with NHL are generally either rare, only weakly associated, or based on inconsistent reports. Radiation exposure probably has little effect on NHL risk.8 Solvents have been associated with an increased risk of NHL, especially in occupational studies of rubber workers, aircraft maintenance workers, and dry cleaners.73 The effects of solvent exposure may be through their effect on the immune system. Benzene workers, chemists, farmers, grain handlers, petroleum refinery workers, anesthesiologists, pathologists, and woodworkers are in occupations that have also been linked to an increased risk of NHL.73 It was estimated that the portion of NHL that can be attributed to these occupational exposures varies from 4 to 11%.12
Conclusions
The effects of the HIV epidemic, NHL associated with immunosuppressive therapy, and improved diagnostic techniques account for only about one third of the increase in NHL incidence.12 The etiology of most of the remaining cases of NHL is essentially unexplained. There are, however, data concerning certain occupational and environmental risk factors that may provide a framework for a hypothesis explaining the increase in the incidence of NHL. It appears that common exposures with weak relative risks are more likely to explain the increases in NHL incidence rates compared to rare exposures with high relative risks.
Although the incidence of all NHL combined has increased in the past 30 years, the patterns of change in incidence vary across different subtypes. The subtype-specific NHL incidence patterns may reflect the influence of different etiologies as well as host factors. Evidence from several studies suggests that associations between risk factors and NHL subtypes may be stronger than associations between the same risk factors and NHL in aggregate. Future epidemiologic studies should focus on NHL risks and causal factors according to subtype,5 as defined by the new World Health Organization NHL classification, which is based on identifying specific disease entities and thus may be more useful for etiologic research. The Working Formulation does not incorporate immunophenotypic or molecular data, nor does it clearly delineate disease entities.
New techniques, such as cytogenetic and molecular analyses as well as gene expression arrays, are increasingly being used to characterize NHL and have made the identification of genetic abnormalities on frozen or routine paraffin-embedded tissue possible. It may be fruitful for future epidemiologic studies to incorporate the analysis of common genetic lesions to identify the specific etiologic factors most strongly associated with subtypes of NHL defined genetically. In addition, further study of genes that are associated with immune regulation, inflammatory cytokines, or genetic polymorphisms appears to be warranted.
II. Novel Agents for Non-Hodgkin’s Lymphomas
Bruce D. Cheson, MD,* Georgetown University Hospital, Lombardi Cancer Center, 3800 Reservoir Road NW, Washington, DC 20007
During the past few decades, results of clinical trials in the NHLs have failed to demonstrate a major impact on patient survival. One contributing factor is that many trials merely evaluated minor modifications of dose and schedule of commercially available, nonspecific agents. Optimism for progress in our current chemotherapy is based on the recent recognition that distinct molecular targets provide an opportunity to identify new drugs with unique mechanisms of action. Some of these drugs may be cytotoxic, others cytostatic, whereas others may work best by augmenting the sensitivity of tumor cells to other agents. This section will present information on several novel compounds as examples of the future for development of chemotherapy of NHL.
Depsipeptide
Depsipeptide (NSC 630176) is a bicyclic peptide originally isolated from Chromobacterium violaceum, strain 968, by Fujisawa Pharmaceutical Co., Ltd. In the initial studies, depsipeptide selectively decreased the mRNA expression of the c-myc oncogene and inhibited the growth of the Ha-ras-transformed NIH3T3 clonal cell line, Ras-1, but had no effect on Ha-ras mRNA expression.1 It did not affect DNA synthesis but caused cell cycle arrest at G0/G1. Recently, it has been shown to be a histone deacetylase inhibitor.2 The compound activates SV40 promoter transcription and inhibits histone acetylation. Other investigations of the effect of depsipeptide on G1 to S transition of the cell cycle showed that depsipeptide inhibits signal transduction through MAP kinase and causes p53-independent G1 arrest.3 Depsipeptide, either alone or in combination with hypomethylating agents, has been shown to induce a number of cellular proteins that may have critical effects on apoptosis, proliferation, and susceptibility to immunologic manipulation. The compound may also have antiangiogenic activity that contributes to antitumor efficacy. Further studies on transformed and tumor cells are needed to clarify the mechanism of action of depsipeptide.
Preclinical Studies
In vitro activity.
Potent antitumor effects of depsipeptide have been demonstrated both in vitro and in vivo.1 In vitro, depsipeptide exerted antiproliferative activity against 12 human tumor cell lines but was less potent against cultured normal cells. It was found that the longer the duration of depsipeptide exposure, the lower the concentration of drug necessary to induce the antiproliferative activity. In vivo, depsipeptide had moderate antitumor efficacy in various model systems; an intermittent administration schedule of depsipeptide resulted in less toxicity and greater efficacy than did a daily schedule.
Studies have demonstrated antiproliferative activity of depsipeptide against human B-cell chronic lymphocytic leukemic (B-CLL) cells4 and against B-cell prolymphocytic leukemia (B-PLL) cells.5 The mean LC50 after 96 hours of exposure in vitro to depsipeptide was 0.015 μM and 0.0002 μM for B-CLL and B-PLL, respectively. Preliminary evidence suggests that the cytotoxicity of depsipeptide is related to its ability to induce apoptosis,4 independent of functional p53.5 Depsipeptide was more cytotoxic to B-PLL cells, when compared to F-ara-A, gemcitabine, flavopiridol, and UCN-01, and had much more pronounced activity (1.6 × 103-fold greater) against B-PLL cells than normal peripheral blood mononuclear cells.5
In vivo activity.
In vivo studies demonstrated that higher doses of depsipeptide could be given and greater antitumor efficacy achieved when an intermittent administration schedule was used. The greater tumor activity observed with an intermittent schedule may be attributable to the higher individual doses that were administered because of the greater tolerance to depsipeptide. Intermittent administration of depsipeptide was better tolerated than daily treatment in mice implanted subcutaneously (SC) with LOX IMVI melanoma.
Phase I clinical experience.
The National Cancer Institute (NCI) sponsored three Phase I trials of a 4-hour intravenous (IV) infusion of depsipeptide. The drug was administered on a weekly × 3 every 4 weeks schedule, and a biweekly (days 1 and 5) every 3 weeks schedule. A starting dose of 1.0 mg/m2, defined as one third of the toxic dose low (TDL) in preclinical rat studies, was used in both studies. Evidence of clinical activity was seen at well-tolerated drug doses.
Nausea, vomiting, and fatigue have been major toxicities that have interfered with administration of drug. Asymptomatic, transient electrocardiogram (EKG) changes have been noted in most patients. Minor responses lasting several months were seen in a patient with an ACTH-producing islet cell tumor and one with a metastatic leiomyosarcoma.
Several patients experienced profound fatigue. Nausea and vomiting were also seen in most patients. The dose level of 17.8 mg/m2 appears to be well tolerated. A significant proportion of patients at all dose levels were delayed in receiving their second dose of chemotherapy (day 5) because of toxicities. An extensive cardiac evaluation has been performed in all patients because of preclinical toxicology data demonstrating cardiac injury in several animal species. Asymptomatic, transient EKG changes including ST-T wave flattening and inverted T waves have been noted. These changes are usually evident by 24 hours after completion of drug infusion and typically resolve before the day 5 drug administration. The EKG changes in several patients have been more pronounced following the day 5 infusion. One episode of atrial fibrillation developed in a patient who was experiencing severe nausea and vomiting from the investigational drug. There has not been any elevation of cardiac enzymes (CK-MB or troponin) following drug administration. MUGA scans obtained pretreatment and at various time points after initiation of therapy have not identified any significant decrement in cardiac functional capacity.
In a Phase I trial at the NCI, objective responses were reported in 11 patients; one complete response (CR) was reported in a peripheral T-cell lymphoma (PTCL) patient at the 12.7 mg/m2 dose level.6 In addition, 9 cutaneous T-cell lymphoma (CTCL) patients reported partial responses at the 17.8 mg/m2 dose level (S. Bates, personal communication). Toxicities attributed to depsipeptide included anemia, leukopenia, neutropenia, thrombocytopenia, fatigue, anorexia, nausea, vomiting, elevated AST/ALT, increased CPK, hypocalcemia, asymptomatic EKG changes (ST-T wave flattening and inverted T waves), and supraventricular arrhythmias (SVT/atrial fibrillation/flutter). A Phase II trial at the NCI is ongoing.
Further development of this agent is planned in patients with acute myelogenous leukemia, CLL, multiple myeloma, and NHL.
Rapamycin Analog
The macrolide rapamycin (sirolimus, Rapamune®; Wyeth-Ayerst, Princeton, NJ) and its derivatives inhibit the mammalian target of rapamycin (mTOR), downregulating translation of specific mRNAs required for cell cycle progression from G1 to S phase.7 Preclinically, mTOR inhibitors potently suppress growth and proliferation of lymphocytes and tumor cell lines.7 Today, rapamycin is approved as an immunosuppressive drug for renal transplant recipients. A related compound, CCI-779 (Wyeth-Ayerst), is being developed as a cancer therapeutic.
The phosphoprotein kinase, target of rapamycin, or TOR, was first described in the yeast S. cerevisiae as the functional target of rapamycin. In mammalian cells, mTOR is a large polypeptide kinase of 290 kDa.8 It is a downstream component in the phosphoinositol-3 kinase (PI3K)/Akt pathway (Figure 1, see Color Figures, page 522) and acts as a nutrient sensor and regulator of translation (reviewed in Rohde et al9 and Schmelzle and Hall10). In the presence of mitogen stimulation of the PI3K/Akt pathway and sufficient nutrients, mTOR participates in the activation of p70S6 kinase and the inactivation of 4E-binding protein-1 (4E-BP1). These events and, possibly, signals to other kinases result in the activation of the translation of specific mRNA subpopulations important for cell proliferation and survival. This results in the activation and proliferation of T and B cells as well as such nonimmune cells as fibroblasts, endothelial cells, hepatocytes, and smooth muscle cells (reviewed in Neuhaus et al11).
Similar to other natural immunosuppressants such as cyclosporin A and FK-506, rapamycin binds to a member of the ubiquitous immunophilin family of FK-506 binding proteins (FKBP), termed FKBP-12, to inhibit mTOR. Thus, rapamycin may be considered a “prodrug” for the active agent at the cellular level, the FKBP-12-rapamycin complex. The complex interacts with mTOR, inhibiting the activation of the phosphoprotein kinase, and, subsequently, the phosphorylation of downstream targets.
Although mutations of mTOR have not been reported in human cancers, mTOR is a component in the PI3K/Akt pathway (Figure 2, see Color Figures, page 522), which is of considerable interest to cancer therapeutics development because of the high frequency of abnormalities in components of the pathway seen in human malignancies. Given the sensitivity of lymphocytes to rapamycin, evaluating the activity of mTOR inhibitors against lymphoproliferative disorders is of interest. Certainly, the activation of the PI3K/Akt pathway appears to be important to T- and B-cell proliferation.12,13 Increased PI3K activity in a transgenic murine model induces a T-cell lymphoproliferative disorder and contributes to the early development of T-cell lymphomas.14 Constitutive activation of Akt pathway has been described in multiple myeloma cell lines, and persistent activation may be important in myeloma cell expansion.15–,17 While mutations in the tumor suppressor PTEN, a phosphatase that reverses the action of PI3K, are seen frequently in certain solid tumors, mutations are uncommon in NHL.18–,20 Thus, malignant lymphoid cells may depend on activation of the pathway through activation of PI3K or Akt or loss of PTEN, which would augment the activity of mTOR. The importance of this pathway to tumor cell survival may be a determinant of cell sensitivity to rapamycin compounds.21,22
The NCI originally evaluated rapamycin in the late 1970s. At that time, it was found to have antiproliferative activity in a variety of murine tumor systems. Rapamycin has since been shown to inhibit the growth of B-cell lymphoma cell lines23 as well as a variety of solid tumor cell lines. Rapamycin also augmented cisplatin-induced apoptosis in murine T-cell lines, the human promyelocytic cell line HL-60, and the human ovarian cancer cell line SKOV3.24 These data suggest that rapamycin has intrinsic antiproliferative activity and may enhance the efficacy of selected cytotoxic agents.
Preclinical studies of rapamycin showed that the compound is highly effective in preventing and treating acute allograft rejection in a variety of transplant models both as a single agent and in synergy with standard immunosuppressants (reviewed in Neuhaus et al11). The agent is orally administered, and the efficiency of absorption is modulated by p-glycoproteins. Rapamycin has a terminal half-life of 62 hours in stable renal transplant recipients treated with cyclosporine, and its steady state is usually reached within 7 to 14 days. Liver and intestinal cytochrome P-450 enzyme CYP3A4 metabolizes rapamycin, and metabolites are predominantly excreted through the gastrointestinal tract. Safety and efficacy of rapamycin for the prevention of renal transplantation graft rejection were evaluated in two studies with a total of 1295 patients. Rapamycin 2 or 5 mg/day was compared with 2 to 3 mg/kg/day of azathioprine25 or placebo.26 In all groups, the immunosuppressive regimen included cyclosporine and corticosteroids. In both studies, there was a significant reduction in graft failure at 6 months compared to control arms, and the degree of benefit favored the higher dose of rapamycin. Adverse events associated with rapamycin were infection, myelosuppression, and hyperlipidemia, though these were infrequent.
It is possible that rapamycin use may reduce the incidence of posttransplantation lymphoproliferative disorders (PTLD), in addition to the documented favorable reductions in graft rejection rates. In laboratory studies, rapamycin caused both profound growth inhibition and apoptosis in a series of B-cell lymphoma cell lines.23 A similar rapamycin derivative, RAD, had a profound inhibitory effect on in vitro growth of 6 different PTLD-like EBV+ lymphoblastoid B-cell lines and in vivo xenografts in mice.27 Thus, follow-up on the incidence of PTLD seen in patients treated with rapamycin will be of interest.
Although rapamycin induces therapeutic immunosuppression with chronic oral dosing, prolonged immunosuppression is not a desirable effect for a cancer therapeutic. Fortunately, in preclinical models, intermittent dosing schedules of rapamycin and CCI-779 were effective in inducing tumor growth delay without causing prolonged immunosuppression.28 However, rapamycin’s unfavorable pharmacological properties of variable intestinal absorption, prolonged terminal half-life, and poor aqueous solubility in intravenous formulations coupled with preference for an intermittent schedule to minimize immunosuppression compromised the evaluation of rapamycin as an antiproliferative agent in cancer patients.
CCI-779 is a soluble ester of rapamycin with impressive in vitro and in vivo cytostatic activity (Figure 3, see Color Figures, page 523). Results from the NCI human tumor cell line screen showed that CCI-779 and rapamycin share a mechanism of action that is distinct from other cancer therapeutics. The two agents are similar in activity. In vitro, human T-cell leukemia; prostate, breast, and small cell lung carcinoma; glioma; melanoma cell lines; and were among the most sensitive to CCI-779.29 In most in vivo human tumor xenograft studies, CCI-779 given on an intermittent schedule caused significant tumor growth inhibition rather than tumor regression.28,29 In addition, the immunosuppressive effects of CCI-779 resolve within 24 hours following the last dose. Given its proposed properties as a cytostatic agent, CCI-779 may be of value in delaying time to tumor progression and increasing survival in patients when used alone or in combination with other anticancer agents.
Preliminary results from two Phase I studies evaluating increasing doses of CCI-779 on different schedules have been reported.30–,33 The first study evaluated pharmacokinetics and biological effects of escalating doses of CCI-779 administered as a 30-minute intravenous infusion daily × 5 days every 2 weeks to patients with solid neoplasms.30,31 In this trial, 51 patients received 262 cycles at doses ranging from 0.75-19.1 mg/m2/d. Grade 3 toxicities included hypocalcemia, elevation in hepatic transaminases, vomiting, and thrombocytopenia. Other toxicities were generally mild to moderate and included neutropenia, rash, mucositis, diarrhea, asthenia, fever, and hyperlipidemia. Hypersensitivity phenomena including chest discomfort, dyspnea, flushing, and urticaria during CCI-779 infusions were also observed. In heavily pretreated patients, the recommended phase 2 dose was 15 mg/m2/day as thrombocytopenia limited repeated dosing at 19.1 mg/m2/d. The maximum tolerated dose (MTD) in minimally pretreated patients has not been reported. Pharmacokinetic data were reported on the initial 17 patients receiving doses of 0.75-3.12 mg/m2/day. In this limited data set, CCI-779 exhibited increasing peak concentrations with increasing dose, preferential red blood cell partitioning, and a median terminal half-life of 32.6 hours.
In the second study, CCI-779 was given as a weekly 30-minute infusion over a dose range of 7.5 to 220 mg/m2/week.32,33 Of note, the MTD of CCI-779 had not been defined at the time of this report. Mild to moderate toxicities included skin toxicity, variously described as dryness with mild pruritus, eczema-like lesions, urticaria, and aseptic folliculitis. Skin biopsies from some patients with folliculitis showed superficial pericapillary dermatitis. Although the frequency of infections was not noted to be high, 5 patients experienced reactivation of perioral herpes lesions. However, immunological analysis of blood cells did not show evidence of immunosuppression. Mild to moderate mucositis, nail changes, thrombocytopenia, leukopenia and anemia, and asymptomatic hyperlipidemia and a decrease in serum testosterone were also reported. Preliminary pharmacokinetic analysis of doses up to 60 mg/m2 indicated that peak plasma concentration, area under the curve, clearance, and volume of distribution at steady state of CCI-779 increased with dose. The mean clearance was 22 L/h and the mean half-life was about 20 hours. Three patients had partial responses (1 each with renal cell, neuro-endocrine, and breast carcinomas).
On the basis of these Phase I studies, it appears that CCI-779 is well tolerated and has antitumor activity over a broad dose range. The most common toxicities of CCI-779skin reactions and stomatitis, hyperlipidemia, and myelosuppressionare transient, generally mild to moderate in severity, and similar to those reported for rapamycin. Of note, rapamycin has been reported to cause pneumonitis, and this toxicity may be seen with CCI-779 treatment as the agent enters broader clinical development.34,35 NCI is currently sponsoring Phase II trials of CCI-779 250 mg/week in NHL and a variety of solid tumors.
Bcl-2 Antisense
Antisense oligonucleotides are chemically modified single-strand DNA molecules that have a nucleotide sequence that is complementary to the target mRNA and, therefore, capable of inhibiting expression of that target gene.36 The Bcl-2 gene is a potentially important target because it is overexpressed in most follicular B-cell NHL and chronic lymphocytic leukemias37,38 and in about a quarter of large B-cell NHL. Bcl-2 upregulation is thought to be responsible for maintaining the viability of tumor cells as well as inducing a form of multidrug resistance.39 Elevated Bcl-2 also correlates with poor response to therapy in NHL, AML, and possibly CLL.40– 44 These observations, and others, have stimulated interest in exploring an antisense against Bcl-2 and other genes important for tumor survival.
To inhibit the target mRNA, antisense oligonucleotides must first be incorporated into cells in order to be effective and this process appears to occur by endocytosis. The oligonucleotide then inhibits gene expression by hybridization with the mRNA, followed by cleavage of the mRNA by recruitment of RNAse-H. Critical to the function of these oligonucleotides is their resistance to nuclease digestion.
G3139 (oblimersen sodium; Genta Incorporated, Berkeley Heights, NJ) is the first antisense molecule to be widely tested in the clinic for the treatment of human tumors. G3139 is a phosphorothioate oligonucleotide consisting of 18 modified DNA bases (i.e., 18-mer) that targets the first 6 codons of Bcl-2 mRNA to form a DNA/RNA duplex. RNAse-H recognizes the DNA/RNA duplex, cleaves the Bcl-2 mRNA strand, and renders the message nontranslatable. Bcl-2 mRNA fragments are subsequently destroyed by ribonucleases.
Preclinical studies
After IV or subcutaneous (SC) injection to mice, G3139 distributes rapidly to highly perfused organs, especially lung and bone marrow. Oligonucleotides are generally excreted unchanged, predominantly by the kidney.45
Biodistribution studies of G3139 in vivo have demonstrated high tissue:plasma ratios, particularly in kidney and liver, but also significant distribution to the bone marrow and spleen.45 In addition, in vitro and in vivo studies showed both biologic and antitumor activity with submicromolar concentrations (e.g., ~170 nM).
A number of in vitro studies have shown synergistic enhancement of tumor cell killing when Bcl-2 antisense was used to reduce Bcl-2 protein content in combination with standard anticancer therapy (including antimetabolites, alkylators, corticosteroids, other cytotoxic chemotherapy, radiation, and monoclonal antibodies).46 B-cell lymphoid cell lines demonstrate significant enhancement of cytotoxicity after reduction of Bcl-2 produced by antisense treatment. In addition, fresh CLL cells obtained from patients and studied ex vivo have been shown to demonstrate downregulation of Bcl-2 protein by antisense exposure, leading also to increased susceptibility to killing by fludarabine.47
G3139 clinical studies in NHL
The first Phase I study of G3139 in patients with NHL was reported by Waters et al from the U.K.36 These investigators administered G3139 by SC infusion to 21 patients at doses that were escalated from 4.6 mg/m2/day to 195.8 mg/m2/day. The MTD was felt to be 147.2 mg/m2/day (5.3 mg/kg/day) with dose-limiting toxicities of thrombocytopenia, hypotension, fever, and asthenia. Toxicities were also noted to be schedule dependent, with thrombocytopenia as the dose-limiting event at 12 mg/kg for 5-7 days, fatigue with elevated liver chemistries at 7 mg/kg/day for 14-21 days, and fever and hypotension at 4 mg/kg/day for 14 days. However, the tolerance to treatment in this study may have been reduced because of the 14-day SC infusion schedule, whereas other studies have easily escalated the G3139 doses to 7 mg/kg/day even when they were given intravenously in combination with cytotoxic chemotherapy. Despite the low doses of drug used in this trial, Bcl-2 downregulation was achieved, and several major responses were observed. One patient with low-grade lymphoma who had progressive disease in nodes and bone marrow after 2 prior regimens attained a CR using G3139 alone, and it has been maintained for longer than 3 years. Of 14 patients entering the study with circulating lymphoma cells in peripheral blood, 10 demonstrated significant reductions after only 1 cycle of therapy. Subjective improvement was also noted in a majority of patients who entered the study with tumor-related symptoms. Bcl-2 content of lymphoma cells was assayed by serial biopsy and/or assay of circulating malignant cells in peripheral blood, and these studies demonstrated that G3139 infusions could downregulate Bcl-2 protein within 5 days of reaching steady-state plasma levels.
Pharmacokinetic data have suggested a linear relationship between dose and plasma levels up to 7 mg/kg/day. Multiple Phase I-II trials demonstrated that doses of G3139 as low as 3 mg/kg/day x 5-7 days provided biologically relevant plasma levels and downregulated Bcl-2 protein, and, for most diseases, doses of 5-7 mg/kg could be administered with an acceptable safety profile. Ongoing trials of G3139 in relapsed acute leukemia have administered doses of 7 mg/kg/day for up to 10 days with acceptable toxicity, and 7 mg/kg/day is undergoing evaluation in patients with multiple myeloma.
Multiple Phase I-II trials in other malignant disorders indicate that the most common toxicity associated with the agent is fatigue. Liver function abnormalities and thrombocytopenia have been observed but are usually transient and have not delayed drug dosing.
However, an interesting observation was encountered in a Phase I-II study of 14 patients with CLL.50 Patients had received a median of 5.5 prior drugs, and most had advanced-stage disease, including 2 with Richter’s transformation. Doses of G3139either 4, 5, or 7 mg/kg/day for 5-7 dayswere poorly tolerated with fever, hypotension, back pain, and thrombocytopenia. Other toxicities occurring in single cases included cold agglutinin disease, hemolytic anemia, atrial fibrillation, headache, disorientation, and intestinal obstruction. One patient also developed pneumonia. Nevertheless, antitumor activity was indicated by an episode of tumor lysis in 1 patient after 72 hours of therapy, a reduction in lymphadenopathy in 4 patients, a decrease in splenomegaly in 1 patient, and a transient decrease in circulating lymphocytes of at least 33% in 5 patients. This pattern of toxicity has not been observed in nonlymphoid diseases at doses of 7 mg/kg/day or higher. This Phase I trial established that the MTD for cycle 1 in CLL as monotherapy is 3 mg/kg/day, although patients could be safely escalated to 4 mg/kg/day in subsequent cycles. An ongoing Phase III trial is comparing fludarabine plus cyclophosphamide with or without G3139 in patients who have failed prior fludarabine therapy. This multicenter experience has thus far demonstrated adequate safety of a G3139 dose of 3 mg/kg/day.
In vitro study of NHL cell lines has shown marked synergy between rituximab and G3139.49 This observation has led to a series of clinical trials in CLL and NHL evaluating the combination of these two agents.
Conclusions
The availability of new monoclonal antibodies and radioimmunoconjugates has resulted in a marked shift in the therapeutic paradigm for patients with NHL. It is likely, however, at least for the foreseeable future, that combinations with chemotherapy may still be needed. Newer regimens need to be developed that replace nonspecific cytotoxic agents with drugs with specific biological and molecular targets, especially those that interact positively with antibodies and cytokines. Rational strategies that combine chemotherapy and biological agents based on a better understanding of mechanisms of drug action and resistance, interactions among agents, and tumor biology may eventually increase the cure rate for patients with NHL. However, to achieve this goal, patients should be entered into well-designed clinical trials so that newer and more effective strategies can be made available as quickly as possible.
III. Immunotherapy for Non-Hodgkin’s Lymphoma
Julie M. Vose, MD*
University of Nebraska Medical Center, 987680 Nebraska Medical Center, Omaha, NE 68198-7680
Monoclonal antibodies are the first example of successful therapy for lymphoma that was developed using our knowledge of the immune system. In 1975, Kohler and Milstein1 discovered that the antibody-producing cells from a mouse spleen could be immortalized by fusion with myeloma cells. These fused cells (hybridoma) were able to live indefinitely and produce antibodies to provide the reagents and systems for further experiments. The first attempt at using monoclonal antibodies in lymphoma therapy was reported by Nadler et al2 in 1980. Although a transient decrease in the white blood cell count was seen after each treatment, no significant antitumor effect was noted, perhaps due to soluble antigen in the serum that blocked the antibody.
Each lymphoma is a clone of identical cells, all of which have the same immunoglobulin on their surface. Each B cell’s immunoglobulin is unique. The unique portion of the immunoglobulin, the idiotype, is an ideal target for therapy. Early studies targeted the idiotype with individual monoclonal antibodies designed for a particular patient’s lymphoma. Many of these patients had direct antilymphoma responses that lasted for several years and were associated with very limited toxicity.3 In an attempt to make a more generic antilymphoma antibody, molecules found on many B-cell lymphoma cells became targets for antibodies. Clinical trials with antibodies have mostly targeted CD20, which is present on 95% of all B-cell lymphomas, as well as CD19 and CD22 (Figure 4 ).
Unconjugated Antibodies
Rituximab: For indolent non-Hodgkin’s lymphoma
The unconjugated monoclonal antibody that has been the most extensively studied and used clinically is the chimeric anti-CD20 monoclonal antibody rituximab (IDEC Pharmaceuticals, San Diego, CA, and Genentech, Inc, South San Francisco, CA). This antibody consists of the murine variable regions from the parent 2B8 murine anti-CD20 grafted onto a human IgG1 constant region.4 The CD20 antigen is an excellent target for immunotherapy since it is found only on mature B cells and not on precursor B cells, which would lead to long-term B cell depletion, and the antigen is not shed, internalized, or modulated to any great extent once antigen-antibody binding has occurred.5 Apoptosis also appears to be triggered by this antibody-antigen binding motif.6
The initial Phase I studies of the rituximab anti-CD20 antibody used a bolus of the antibody that was repeated weekly in patients with relapsed CD20+ non-Hodgkin’s lymphoma (NHL).7 Multiple Phase II studies enrolled many patients with relapsed indolent CD20+ NHL. The patients received a dose of 375 mg/m2 of rituximab weekly for 4 weeks. The pivotal trial showed a 6% complete response (CR) rate and a 42% partial response (PR) rate, for an overall response rate of 48% in this patient population with relapsed indolent NHL.8 The patients had received a median of 2 prior chemotherapy regimens before being treated with the rituximab. Patients who were tested before and after therapy with rituximab could clear a detectable bcl-2 rearrangement [polymerase chain reaction (PCR) for t(14;18)] in the blood and marrow compartments; however, some of these patients still had lymph nodes that were detectable.9
More recent studies have also looked at the use of rituximab as maintenance therapy following standard weekly × 4 therapy. In a study by Ghielmini et al,10 202 patients with newly diagnosed follicular lymphoma received rituximab 375 mg/m2 for 4 weeks and then were randomized to receive no maintenance or rituximab 375 mg/m2 for 1 dose at months 3, 5, 7, and 9. This study found a median event-free survival of 22.4 months for the maintenance arm versus 13.4 months for the observation arm (P < 0.05). Of the patients in remission at 12 weeks after the rituximab, 80% remained in remission at 12 months in the maintenance arm compared to 57% in the observation arm (P < 0.05).
Newer uses for rituximab include combination therapy with various chemotherapeutic agents, combination uses in transplantation regimens, use with other immunomodulatory agents, and uses for other histologic subtypes of lymphoma. Originally, combination therapy was based on the in vitro study that demonstrated synergism with the anti-CD20 antibody and chemotherapy in sensitizing previously chemotherapy-resistant cell lines.11 In the first combination study in indolent lymphoma patients, reported by Czuczman et al,12 38 patients with indolent lymphoma (31 previously untreated) were treated with rituximab plus CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) chemotherapy. The response rate was 100%, including a 66% CR rate. With a median follow-up of 6 years, 21 of these 40 patients remained in remission at 46.8+ to 86.3+ months.13 Alternative combinations of rituximab with fludarabine14 have also demonstrated an overall response rate of 90%. With a median follow-up of 15 months, 30 of 36 patients remained in remission.
A new area for the use of rituximab is in combination with other immune modulatory agents. Examples of this would include a Phase II clinical trial in which rituximab was combined with alpha interferon. In 38 patients with relapsed indolent lymphoma, rituximab at 375 mg/m2 weekly × 4 plus interferon alpha-2b at 2.5-5.0 million units subcutaneously 3 times per week for 12 weeks were administered. The overall response rate was 17 of 38 (45%), with 11% CR. Toxicities included asthenia, chills, fever, and headaches.15 In addition, a Phase I clinical trial of rituximab in combination with interleukin-12 (IL-12) has been reported by Ansell et al.16 In this trial, 43 patients with relapsed NHL received rituximab 375 mg/m2 for 4 weeks and IL-12 in a dose escalation schema subcutaneously twice weekly. Objective responses occurred in 29 of 43 (67%) of patients with 8 of 11 CRs at IL-12 doses of 300 mg/kg or greater.
Rituximab for Aggressive NHL
As a single agent, rituximab was evaluated by Coiffier et al17 in 54 patients with relapsed diffuse large cell or mantle cell lymphoma. These patients received rituximab for 8 weekly doses. A total of 5 CR and 12 PR were observed, for an overall response rate of 31%. Another study by Foran et al18 treated 120 patients with newly diagnosed or recurrent mantle cell lymphoma, immunocytoma, or small lymphocytic lymphoma with rituximab for 4 weekly infusions. The overall response rate in these patients was 30% (36 out of 120 patients). Ten patients, all with mantle cell lymphoma, achieved a CR with the therapy.
With respect to combination therapy for aggressive NHL, a Phase II trial in which 33 patients with newly diagnosed diffuse large B-cell NHL received rituximab at 375 mg/m2 on day 1 of each cycle followed by CHOP on day 3 of each cycle was published by Vose et al.19 The CR rate in this trial was 61%, with a PR rate of 33%, for an overall response rate of 94%. All patients were able to receive all the cycles of therapy, with a 27% neutropenia rate; < 1% of cycles had thrombocytopenia. With a minimum follow-up of 24 months, 29 of 33 patients (88%) were alive and free of disease at the time of the original publication. Subsequently, a large Phase III randomized trial by Coiffier et al20 evaluated 397 patients with newly diagnosed aggressive NHL age 60-80 years who were randomized to CHOP alone versus CHOP + rituximab. In this trial, the CR rate was superior for the combination (76% versus 63%, P = 0.0005). In addition, the event-free and overall survival rates were superior for the combination arm (P < 0.001 and P = 0.007, respectively). A second large randomized trial performed in the Eastern Cooperative Oncology Group is also testing this question in older patients with aggressive NHL.
The combination of CHOP + rituximab has also been evaluated in patients with newly diagnosed mantle cell lymphoma. A report from the Dana Farber Cancer Institute evaluated 40 patients who received this regimen.21 The CR rate was 33%, the CR(u) rate was 15%, and the PR rate was 49%, for an overall response rate of 98%. The median progression-free survival was 16 months, which was not different from that of similar historical control patients receiving CHOP alone. Although 11 of 23 patients with detectable bcl-1 rearrangements became PCR-negative, this did not correlate with the overall response to therapy. Rituximab has also been added to salvage chemotherapy such as EPOCH (etoposide, prednisone, vincristine, cytoxan, and adriamycin). In a study of 38 untreated or relapsed patients with aggressive NHL, complete remissions were achieved in 64% of previously treated patients.22 Synergism may be demonstrated with other chemotherapy or immunotherapy agents in the future.
Rituximab Combined with High-Dose Therapy and Autologous Stem Cell Transplantation
Because rituximab has been demonstrated to have an effect of “clearing” bcl-2 positive cells from the blood and marrow, it has been recently tested in the transplant setting as an additive therapy. A few preliminary studies have now been presented in abstract form. A study from Salles et al23 evaluated 26 patients treated with rituximab prior to hematopoietic stem cell harvest. The patients had follicular lymphoma (n = 17), mantle cell lymphoma (n = 4), and marginal zone and small lymphocytic lymphoma (n = 5). The patients received 4 doses of rituximab at 375 mg/m2. The mobilization was attempted with high-dose cyclophosphamide, etoposide, and granulocyte colony-stimulating factor (G-CSF) at 4 weeks after the rituximab. Three of the patients failed to mobilize, and the rest were transplanted. For those patients with adequate follow-up, 10 of 11 patients were alive in complete remission. The PCR analysis in the stem cell product demonstrated PCR negativity in 6 of 7 follicular lymphoma patients, while 2 mantle cell lymphoma patients and 1 small cell lymphocytic lymphoma patient had residual PCR-positive cells.
Another trial by Flinn et al24 evaluated 40 patients with follicular lymphoma (n = 19), mantle cell lymphoma (n = 9), or small lymphocytic, marginal zone, or lymphoplasmacytic lymphoma (n = 12) who were receiving autologous transplantation. The patients received rituximab 375 mg/m2 on day 1 of mobilization, followed by cyclophosphamide and G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF). The last 15 patients’ hematopoietic stem cells were also CD34+ selected. Preliminary results demonstrated that 7 of the patients with unmanipulated grafts had a PCR-positive product, while none of the CD34+ selected grafts did.
Epratuzumab
This humanized monoclonal antibody is directed against the CD22 determinant RFB4, which is present on 75% of B lymphocytes. In a Phase I/II trial, Leonard et al25 reported that the overall response rate was 43% and 27% at dose levels of 360 mg/m2 and 480 mg/m2, respectively, in patients with recurrent follicular lymphoma. The median time to progression for the responders was 23.7+ months.25 In addition, patients with diffuse large cell lymphoma had an overall response rate of 34%, 16%, and 25% at dose levels of 240 mg/m2, 360 mg/m2, and 600 mg/m2 with a time to progression of 8.1 months. Subsequently, a combination trial of epratuzumab 360 mg/m2 and rituximab 375 mg/m2 weekly × 4 was conducted.26 Of 15 patients with follicular lymphoma who were rituximab naïve, 10 (67%) demonstrated objective responses. Nine of the 15 (60%) had a complete remission, with none of the responders relapsing at a follow-up time of 15+ months. Further testing of this and other combinations of epratuzumab is ongoing.
Apolizumab/Hu1d10
Apolizumab, a humanized monoclonal antibody, is directed against a polymorphic determinant of HLA-DR expressed on normal and malignant B cells capable of inducing antibody-dependent cytotoxicity (ADCC) complement-mediated lysis and direct apoptosis of lymphoma cell lines. A Phase I dose escalation study in 20 B-cell lymphoma patients demonstrated that 4 of 8 follicular lymphoma patients achieved durable responses. Subsequently, a Phase II randomized study was performed in patients with relapsed or refractory follicular, small lymphocytic, or marginal zone/MALT B-cell lymphoma. Patients were randomized to receive Hu1D10 infusions at 1 of 2 dose levels once weekly for 4 consecutive weeks. At the time the abstract was presented, 21 patients had been treated.27 The adverse events were infusion related, primarily grade 1 and 2. Response data from this trial are pending.
Alemtuzumab/Campath-1H
Alemtuzumab (Campath-1H) is a humanized monoclonal antibody that targets CD52, a cell surface protein present at high density on most normal and malignant B and T lymphocytes. In a Phase II study, alemtuzumab was administered 3 times per week for 12 weeks to 29 patients with relapsed and refractory B-CLL. The overall response rate was 38%, with most patients achieving a PR.28 The results of the expanded access trial in 136 patients with pretreated B-CLL demonstrated an overall response rate of 39% (CR 7% and PR 32%). The median progression-free survival was 7 months for the 54 responders.29 Alemtuzumab has also been studied in further Phase I trials, in addition to rituximab.30 Of 9 patients with recurrent CLL treated in a Phase I trial, 8 (88%) had a hematologic response in the peripheral blood and 4 (45%) had significant recovery of their ANC (median 300% increase). Further studies with combination therapy are ongoing.
Radioimmunotherapy
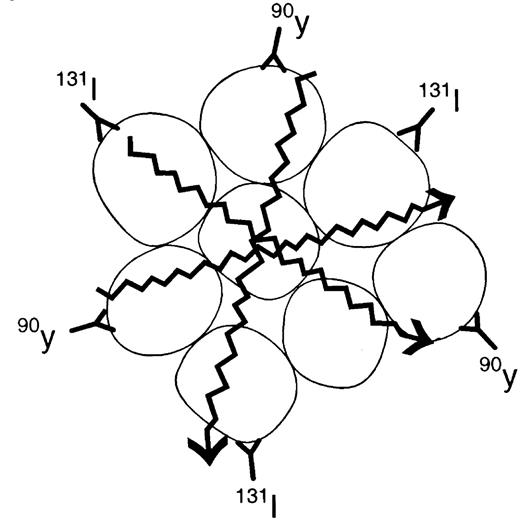
Radioimmunotherapy (RIT) is ideal treatment for NHL since the CD20 antibody has shown therapeutic efficacy as a solo agent and also because NHL is very radiosensitive. The major antilymphoma effect of RIT is the Iodine-131 or the Yttrium-90 that is conjugated to the antibody directed against CD20. Both radioconjugates emit beta-particles. This energy induces lethal DNA damage to the targeted cell and to nearby cells that do not necessarily express the target antigen (bystander effect) (Figure 4). The Yttrium-90 emits a particle that has higher energy with a longer path length than Iodine-131, which allows for deeper tissue penetration. However, Iodine-131 emits gamma emissions, which allows for imaging of the patient with localization of the radioimmunoconjugate. There are 2 anti-CD20 radioimmunoconjugates currently being evaluated for NHL therapy.
Iodine-131 anti-Cd20 (tositumomab [Bexxar])
Tositumomab is an IgG2a murine antibody (Corixa Pharmaceuticals, South San Francisco, CA). With the RIT administration, a dosimetric dose with an unlabeled antibody is given prior to the trace Iodine-131-labeled (5 mCi) dose administered to the patient. Over the next week, 3 whole-body gamma camera images are taken of the patient to calculate the exact elimination of the radioactivity in preparation for individualized dose administration. One week after the dosimetric dose is given, the therapeutic dose is administered. Once again, an unlabeled antibody predose is given, followed by the Iodine-131 anti-CD20 calculated to administer 75-cGy total body dose (65 cGy for patients with a platelet count 100,000-150,000/μL).
In the initial Phase I/II trial at the University of Michigan, 57 patients with indolent, transformed, or de novo aggressive lymphoma were treated in this manner with Bexxar. Twenty patients had a CR and 22 patients demonstrated a PR with this one-time therapy.31 Further long-term follow-up on these patients demonstrated a CR duration median of 20 months, with 7 patients remaining in CR from 3 to 5.7 years.32 This original study led to a Phase II dosimetry validation study in which 45 patients with indolent or transformed lymphoma were treated with a 75-cGy total body dose of Iodine-131-anti-CD20 as described. In this trial, the overall response rate was 57% and was similar in patients with indolent lymphoma (57%) or transformed lymphoma (60%). The CR rate was 32%, with a median duration of 20 months. The principal toxicity was hematologic, with 11% of the patients having a platelet nadir of less than 10,000 cells/μL and 4% of the patients having an absolute neutrophil count of less than 100/μL.33
The pivotal trial was performed in 60 patients at several centers throughout the United States and at 2 sites in England. The patients in this trial had received a median of 4 prior regimens (range 2-13) and had a median age of 60 years. The overall response to the Bexxar was 65% compared with 28% for the patients’ last prior chemotherapy (P < 0.001), and the CR rate was 17% for the antibody compared with 3% for the last chemotherapy (P = 0.011).34 Similar mild hematologic toxicities were seen in this trial as compared to the Phase II trial.
Kaminski et al35 also evaluated Bexxar in previously untreated follicular lymphoma patients. They evaluated 76 patients given 75-cGy total body dose of Bexxar, as previously outlined. The overall response rate was 97%, with 48 (63%) patients achieving a CR with this therapy. The 3-year progression-free survival was 68%. Of the 34 patients who were PCR positive in the blood or bone marrow prior to therapy, 79% were PCR– after B-cell recovery. There was mild hematologic toxicity, with the median absolute neutrophil nadir being 1300 cell/μL, hemoglobin 12.2 g/dL, and platelets 83,000/μL.
A recent randomized study of 78 patients with relapsed indolent lymphoma was performed with patients randomized to unlabeled tositumomab versus Iodine-131 tositumomab (Bexxar). The overall response rate for patients randomized to the Bexxar arm was 28 of 42 (67%) compared to 6 of 36 (17%) in the unlabeled tositumomab arm (P < 0.001). After disease progression, 19 patients crossed over to the Bexxar therapy from the unlabeled arm and 17 of 19 (89%) had a response to the Bexxar.36
Bexxar has also been evaluated at much higher doses in the setting of autologous stem cell transplantation. The Phase I/II study conduced by Press et al37 achieved the dose-limiting toxicity at 27 cGy to the healthy organs. In this study, the patients received the therapeutic dose only if they had a favorable biodistribution in the tracer dose. Tumor bulk and splenomegaly presented problems with biodistribution in some patients. In a follow-up of the original study, Lui et al38 found 45% of the patients progression-free and 60% alive at several years posttherapy.
More recently, Press et al39 have reported on the preliminary results of a Phase I/II study combining high-dose Iodine-131-anti-CD20 with high-dose cyclophosphamide and etoposide, followed by autologous hematopoietic stem cell transplantation. This study was an attempt to substitute the radioimmunoconjugate for total body irradiation in the NHL transplant regimen. This study treated 38 patients with relapsed NHL; after a median follow-up of 18 months, 78% of the patients were progression-free. However, with the addition of standard high-dose chemotherapy, transplant complications such as mucositis and opportunistic infections became more of an issue. A Phase III trial comparing this regimen to the standard cyclophosphamide, etoposide, and total body irradiation regimen will be initiated after the Phase II study is completed.
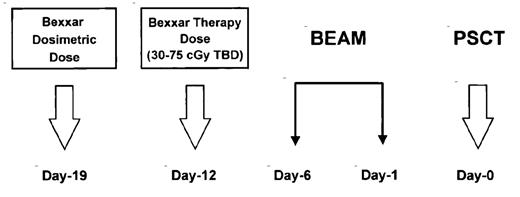
Another method of using radioimmunoconjugate therapy with a standard transplant regimen is to combine the lower dose of Bexxar (75-cGy total body dose), which can be administered on an outpatient basis, with a standard chemotherapy-only transplant regimen. An ongoing Phase I/II trial at the University of Nebraska Medical Center combines Bexxar in a dose escalation study at doses ranging from 3075cGy total body dose with the BEAM regimen (BCNU, etoposide, Ara-C, and melphalan) followed by autologous stem cell transplantation (Figure 5 ). This trial has had a CR rate of 60% in chemotherapy-refractory NHL patients. The 2-year event-free survival was 60%, and the 2-year survival was 70%. There appeared to be minimal additional toxicity created by adding the Bexxar to the BEAM regimen, other than a slight increase in the mucositis score.40 A larger Phase II trial is ongoing with the Bexxar administered at 75 cGy along with the BEAM regimen in patients with chemotherapy-sensitive aggressive NHL.
90-Yttrium anti-Cd20 (Y2b8) (ibritumomab tiuxetan [Zevalin])
The other radioimmunoconjugate that has had extensive clinical evaluation is Yttrium-90-2B8 (IDEC). The compound is composed of a murine immunoglobulin G1 kappa monoclonal antibody, ibritumomab (IDEC-2B8); the linker chelator tiuxetan (isothiocyanateobenzyl MX-DTPA); and the radioisotope Yttrium-90, which is securely chelated via the linker. This antibody was initially used with an indium-labeled tracer dose for dosimetry because yttrium does not release gamma emissions and cannot be imaged. Results from the Phase I/II trials showed the MTD to be 0.4 mCi/kg in most patients and 0.3 mCi/kg in patients with mild thrombocytopenia. The overall response rate was 67% (26% CR and 41% PR) in all histologies at all doses and 82% in the patients with indolent lymphoma.41 Ten percent of the patients developed a platelet count less than 10,000/μL, and 28% developed an ANC less than 500/μL.
Zevalin has also been studied in patients who have failed rituximab therapy. Fifty-seven patients with follicular NHL who were refractory to rituximab were treated on this study with Zevalin.42 The median age of the patients was 54 years, and 74% had bulky disease with ≥ 5 cm lymph nodes. The overall response rate was 74% (15% CR and 59% PR). The median time to treatment failure was 6.8 months. Grade IV neutropenia and thrombocytopenia occurred in 35% and 9% of patients, respectively. The median ANC nadir was 700/μL, and the median platelet nadir was 33,000/μL.
A Phase III randomized trial comparing rituximab to Zevalin was reported by Witzig et al.43 One hundred forty-three patients with recurrent indolent lymphoma were randomized to receive either rituximab or Zevalin. The overall response rate for the Zevalin-treated patients was 80%, versus 56% for the rituximab group (P = 0.002). The CR rate was 30% and 16% in the Zevalin and rituximab groups, respectively (P = 0.04). The median duration of response was 14.2 months in the Zevalin group and 12.1 months in the rituximab group (P = 0.6). Reversible myelosuppression was the primary toxicity. An additional Phase II study of Zevalin in patients with relapsed indolent NHL with mild thrombocytopenia has also recently been reported by Wiseman et al.44 The patients were treated with an attenuated Zevalin dose of 0.3 mCi/kg if they had a platelet count of 100,000-149,000/μL. The overall response rate was 83% (37% CR). The estimated median time to progression for all patients was 9.4 months. Zevalin has also been combined with the BEAM transplant protocol in a Phase I trial. Winter et al45 evaluated 12 patients in a dose escalation study with the Zevalin administered in 100, 300, or 500 cGy to critical organs along with BEAM and autologous stem cell transplantation. In this trial, 2 of 3 patients in the third cohort had a greater than 20% decline in the lung diffusion capacity, which was a dose-limiting criterion according to the protocol.
Vaccine Approaches
Most vaccine approaches have focused on using the idiotype as the active vaccination target. The idiotype is a unique determinant present on all B cells. Once clonal proliferation occurs, identical idiotype markers are present on all of the patient’s lymphoma cells. This idiotype can be used in the manufacture of a patient-specific vaccine. Active vaccination would potentially produce both humoral and cellular immune responses and be longer acting than passive immunotherapy.
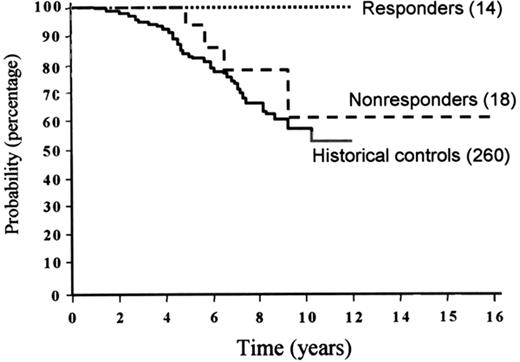
To make an idiotype vaccine, a lymph node biopsy must be performed to obtain an adequate sample of the patient’s lymphoma. In the original experiments, a hybridoma was made by fusion to a myeloma cell line. These hybridomas secrete the immunoglobulin-bearing idiotype made by the tumor cell. The idiotype is then purified and chemically coupled to a foreign protein to make it more recognizable to the patient’s immune system. The study performed at Stanford University involved 41 patients with follicular lymphoma who received CVP (cyclophosphamide, vincristine, prednisone) chemotherapy to remission.46 Subsequently, they received a series of vaccinations of their idiotype conjugated to KLH (keyhole limpet hemocyanin) and given with another adjuvant to improve their immune response. Fourteen patients generated a specific immune response against the idiotype expressed by their own lymphoma, and 18 patients did not. The freedom from progression for those patients who did mount an immune response was improved over those who did not mount an immune response. The overall survival for the cohort who did mount an immune response was 100% out as far as 12 years from diagnosis, which was significantly better than for those patients who did not mount an immune response and for historical control patients who received CVP alone (Figure 6 ). Patients who had a smaller tumor volume appeared to benefit from the vaccine strategy to a greater extent than those with some lymphoma still present.
A similar study reported by Bendandi et al47 evaluated 20 patients with previously untreated follicular lymphoma who were in first complete remission and received a series of custom-made idiotype vaccinations with GM-CSF as the adjuvant. Eleven of the patients had detectable t(14;18) by PCR both at diagnosis and after chemotherapy. However, 8 of 11 patients converted to a negative PCR following the vaccinations series. Tumor-specific cytotoxic CD8+ and CD4+ T cells were uniformly found in most patients; antibodies were found in the majority of patients as well. The anti-idiotype vaccine approach is currently being tested in two large Phase III randomized trials in first CR patients.
New vaccine approaches are also being evaluated in an attempt to improve the number of patients who can mount an immune response and to improve the quality of the immune response. A technology used in an attempt to improve outcomes is the use of a second generation “molecular rescue” idiotype protein for the vaccine. In this manufacturing process, NHL cells are harvested in the same way and have the tumor sequence identified by PCR. The gene is cloned, transfected into mammalian cells, purified, and produced for the vaccine (Figure 7 ). A Phase III clinical trial is also currently going on in the US with this vaccine preparation. Naked DNA is being used as a vaccine. As reported by Stevenson,48 the gene coding for the protein can be cloned into a mammalian expression vector, which can be injected directly into a muscle. The protein, the gene, or both find their way to the draining lymph nodes and to the antigen-presenting cells, which are dendritic cells that process the protein and interdigitate with the immune system. This method is currently undergoing pilot clinical trials in patients.
Another strategy is to use dendritic cells as part of the vaccine approach. A study by Timmerman et al49 evaluated 35 patients with follicular lymphoma who received idiotype pulsed dendritic cells. Ten patients with relapsed measurable lymphoma were enrolled. Anti-idiotype (Id) cellular proliferative responses were measured in 8 of 10 patients, while humoral anti-Id responses were not detected. Clinical responses included 2 CR (44 and 33+ months) and 1 PR (12 months). Sixteen additional patients were vaccinated in first remission following CVP chemotherapy. Eight of 16 patients mounted cellular anti-Id responses, but no serum anti-Id antibodies were detected. Fourteen of the 16 patients remain progression-free at a median follow-up time of 27 months (range 15-38 months). Additional studies are ongoing to evaluate this technique.50,51
Another methodology to enhance rapid production of protein-based vaccines has been tested using a plant-based transient expression system. In this method, a modified tobamoviral vector that encodes the idiotype-specific single-chain Fv fragment (scFv) of the immunoglobulin was used. Nicotiana benthamiana plants were then infected with the designed vector, which then contained high levels of secreted scFv protein in the extracellular compartment.52 This material was specific by anti-idiotype Western blot, ELISA, and affinity chromatography. A tumor model system using this vaccine protected the mice from a lethal challenge of syngeneic lymphoma. This method is now being taken forward into human clinical trials.
Other methods for improving vaccination would be with the use of alternative adjuvants to improve the immune response, such as GM-CSF (which is now in clinical trial), IL-12, IL-18, or CPG-oligonucleotides.53,54 Heat shock proteins are also being tested in an autologous lymphoma vaccine. Another strategy is to use CD40-activated lymphoma cells to prime a T-cell response. Data suggest that tumor cells require both adhesion and costimulatory molecules to induce clinically significant T-cell-mediated antitumor immunity. A clinical trial was initiated to evaluate patients using CD40-activated lymphoma to stimulate T cells for adoptive transfer.55
Conclusions
Although we have not been able to make significant advances in the area of cytotoxic therapy for lymphoma over the past decade, the development of immunotherapy has been advancing steadily. Passive immunotherapy with anti-B-cell antibodies, RIT, and now active vaccination protocols are adding new therapeutic options for patients with NHL. The optimal use of these therapies—alone or in combination with standard or high-dose chemotherapy and stem cell transplantation—awaits results of clinical trials.
Radioimmunoconjugates directed against a non-Hodgkin’s lymphoma cell demonstrating the cross-fire effect.
Radioimmunoconjugates directed against a non-Hodgkin’s lymphoma cell demonstrating the cross-fire effect.
Combination radioimmunoconjugate plus high-dose chemotherapy and autologous hematopoietic stem cell transplantation protocol.
Combination radioimmunoconjugate plus high-dose chemotherapy and autologous hematopoietic stem cell transplantation protocol.
Survival of patients with follicular lymphoma receiving anti-idiotype vaccine in first remission by immune response, compared to 260 historical controls treated at Stanford University.
Rerpinted with permission from Levy R. Karnofsky Lecture: Immunotherapy of lymphoma. J Clin Oncol. 1999;17:7-13.
Survival of patients with follicular lymphoma receiving anti-idiotype vaccine in first remission by immune response, compared to 260 historical controls treated at Stanford University.
Rerpinted with permission from Levy R. Karnofsky Lecture: Immunotherapy of lymphoma. J Clin Oncol. 1999;17:7-13.
Dr. Vose has received research support from Immunex, Amgen, Corixa, IDEC, Genentech, and Bristol Myers.