Abstract
Central nervous system involvement with malignant lymphoma whether primary or secondary is an uncommon but not rare complication observed in the management of patients with hematological malignancy. Its importance lies in the considerable morbidity and mortality with which it is associated and the inadequacy of therapy.
In Section I, Dr. Lauren Abrey addresses the totality of the problem of primary central nervous system lymphoma, with emphasis on strategies increasingly dependent on systemic chemotherapy.
In Section II, Dr. John Sandlund reviews the success of sequential clinical trials of overall therapy for acute lymphoblastic leukemia in childhood, identifying those patients at high risk of central nervous system leukemia and the development of a rational therapeutic strategy for prevention.
In Section III, Dr. Andrew Lister discusses the issue of secondary central nervous system involvement with lymphoma and the indications for prophylaxis.
I. Primary Central Nervous System Lymphoma
Lauren E. Abrey, MD*
Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021
Primary central nervous system lymphoma (PCNSL) is a rare form of non-Hodgkin’s lymphoma (NHL) arising within and confined to the CNS. It was first described by Bailey1 in 1929 as a perithelial sarcoma. Subsequent classifications have included reticulum cell sarcoma and microglioma. Improvements in histopathology and immunohistochemical techniques definitively established the lymphoid nature of PCNSL.
PCNSL is of particular interest for several reasons. First, this tumor has increased in incidence over the past several decades. Therefore, although it remains relatively rare, it is an increasingly important differential diagnosis of intracranial mass lesions. Second, unlike many primary brain tumors, PCNSL is very responsive to treatment, and aggressive management may lead to prolonged remission or cure. Finally, the long-term consequences of aggressive therapy may result in significant neurologic dysfunction.
Epidemiology
PCNSL accounts for approximately 1% of all primary brain tumors in large autopsy-based series. More recent data suggest that the incidence among immunocompetent patients in the United States is increasing. Data from the National Cancer Institute Surveillance, Epidemiology, and End Result (SEER) database found a threefold increase in PCNSL between 1973-1975 and 1982-1984. Further analysis found a tenfold or greater increase between 1973 and 1992.
The incidence of ocular lymphoma has similarly increased by 1.5-fold. There has been a parallel rise in the incidence of all extranodal lymphomas, but the increase has been disproportionate in the brain and eye. This increased incidence is not explained by advances in neuroimaging or tumor diagnosis. As PCNSL primarily affects individuals age 60 and older, one possible explanation would be the general aging of the population; however, the data indicate an increase across all age groups.
PCNSL is diagnosed in 1.6% to 9.0% of the human immunodeficiency virus (HIV)-infected population2,3 and is the second most common intracranial mass lesion. Prior to the introduction of highly active antiretroviral therapy (HAART), the incidence of PCNSL in the HIV-infected population was continuing to rise. However, the impact of these new drug regimens on the CD4 count may result in a decline in PCNSL, as the susceptibility to PCNSL is inversely proportional to the CD4 count.4
Pathology
Grossly, PCNSL is a soft, granular, ill-defined lesion. Associated necrosis, hemorrhage, and neovascularity are uncommon except in AIDS-related PCNSL. Microscopically, PCNSL is a diffuse lesion with an angiocentric growth pattern; some tumors may even invade the blood vessel wall. In addition to malignant lymphocytes, there are varying numbers of small, benign, reactive T lymphocytes infiltrating the tumor, and reactive astrocytes are common. Malignant lymphocytes freely invade normal surrounding brain, and autopsy studies have demonstrated widespread infiltration of normal brain.
Immunohistochemical stains are extremely useful in differentiating PCNSL from high-grade glioma and metastatic carcinoma. Leukocyte common antigen clearly identifies the malignant cells as white blood cells but may be negative in a small number of PCNSLs. Ninety percent or more are B-cell lymphomas (CD20+), usually of diffuse large-cell, large-cell immunoblastic, or lymphoblastic subtype. These tumors can be identified by the immunohistochemical B-cell marker L26. The reactive infiltrating cells are typically T lymphocytes, although primary T-cell lymphomas (CD3+, CD45RO+) are reported.
Histologically, PCNSL is indistinguishable from systemic NHL. Biologically, PCNSL behaves in an aggressive fashion, and it should be considered a high-grade tumor. Genetically, PCNSL has been found to demonstrate clonal abnormalities of chromosomes 1, 6, 7, and 14, identical to those detected in systemic NHL.5 Analysis of cell surface markers including NCAM and integrins is also identical to that of systemic lymphoma. Kumanishi et al found p15 and p16 deletions in 4 out of 5 PCNSL tumors.6
Pathogenesis
The pathogenesis of PCNSL in immunocompetent patients is unknown. T lymphocytes normally traffic in and out of the CNS; however, there is no normal traffic of B lymphocytes. Therefore, several different hypotheses have been proposed. There are no data to support or disprove any of these potential mechanisms.
PCNSL may arise from a systemic lymphoma that seeds multiple organs, including the brain. The immune system has the capacity to find and eliminate the systemic tumor, but the brain, an immunologically privileged site, gives sanctuary to the malignant lymphocytes, thereby allowing tumor development. This seems unlikely, as there is no evidence of concomitant lymphoma in other immunologically privileged sites, such as the testes, concomitant with PCNSL.
Another theory is that lymphocytes become trapped in the CNS after an inflammatory process and then undergo malignant transformation. However, inflammatory diseases almost exclusively attract T lymphocytes, and PCNSL is usually of B-cell origin. Also, the incidence of PCNSL is not increased in patients with inflammatory CNS diseases.
Clinical Features and Evaluation (Table 1)
The typical patient is between 55 and 70 years old; most have had symptoms for only a few weeks prior to seeking medical attention. Cognitive and personality changes are the most common initial symptoms, reflecting the predilection of PCNSL to involve the frontal lobes, corpus callosum, and deep periventricular structures. PCNSL is multifocal in approximately one third of patients and may present with any focal neurologic finding, such as hemiparesis or aphasia. Seizures are a presenting complaint in about 10% of patients, less frequent than glioma or brain metastasis. Age less than 60 and an excellent performance status are the most important prognostic factors.
In AIDS-related PCNSL, the typical patient is younger (30-40 years old), and seizures are more common (25%). The median latency from HIV diagnosis is approximately 5 years. Some studies have found a higher incidence of multiple lesions in AIDS-related PCNSL; however, multifocal lesions in AIDS patients may have different etiologies.
More than 40% of patients have evidence of leptomeningeal dissemination, but concomitant clinical findings are uncommon. Primary leptomeningeal lymphoma is rare and typically presents increased intracranial pressure, multifocal cranial neuropathies, or multilevel root involvement. Cerebrospinal fluid (CSF) should be obtained in all newly diagnosed patients. CSF evidence of PCNSL may also be a poor prognostic indicator. Tumor markers, including lactate dehydrogenase isoenzymes, β-glucuronidase, and β2-microglobulin, may provide circumstantial evidence of leptomeningeal lymphoma. Immunocytochemical analysis and detection of immunoglobulin gene rearrangements by polymerase chain reaction have been used in the diagnosis of lymphomatous meningitis when routine cytologic evaluation is inconclusive.
About 15% of patients with PCNSL have ocular disease at presentation, while 50% to 80% of patients with isolated ocular lymphoma go on to develop parenchymal brain lymphoma. Ocular symptoms include blurred, cloudy vision, decreased visual acuity, or “floaters,” but as many as half of affected patients are asymptomatic. Complete ophthalmologic evaluation, including slit lamp examination, is recommended in all patients. Diagnosis is often delayed in patients with isolated ocular lymphoma because of misdiagnosis as chronic vitreitis or uveitis.
Systemic lymphoma is an uncommon finding in PCNSL, and there is disagreement as to whether a comprehensive systemic extent of disease evaluation is needed. In a series from the Mayo Clinic, approximately 2% to 3% of PCNSL patients were found to have systemic lymphoma on an abdominopelvic computed tomography (CT) scan or bone marrow biopsy.
The optimal neuroimaging of PCNSL is gadolinium-enhanced magnetic resonance (MR) scanning. Most lesions are supratentorial and periventricular, often involving deep structures such as the corpus callosum and basal ganglia. Lesions may be hypo- or hyperintense on precontrast T1 imaging. Dense, homogeneous contrast enhancement is seen in immunocompetent patients but may be irregular and heterogeneous in AIDS-related PCNSL. Peritumoral edema and local mass effect are often less than expected with intracranial lesions of other etiologies. Calcification, hemorrhage, or cyst formation is rare.
Treatment
Surgery
The role of surgery is to establish a histopathologic diagnosis; therefore, a stereotactic needle biopsy is the procedure of choice. Aggressive resection does not improve survival and may result in neurologic deterioration.
Corticosteroids
Corticosteroids are used empirically in the treatment of vasogenic edema caused by any intracranial mass. In PCNSL, corticosteroids also have a potent oncolytic effect, causing tumor cell lysis and radiographic regression in up to 40% of patients.7 The onset of action is quite rapid, with resolution of symptoms and marked reduction in tumor size within 24 to 48 hours. This can be problematic if a tissue diagnosis has not been obtained. Therefore, steroids should be withheld in any patient with a presumptive diagnosis of PCNSL until stereotactic biopsy has been performed.
Radiotherapy
PCNSL is a radiosensitive tumor, and whole-brain radiotherapy (RT) was the standard treatment for many years. Whole-brain RT is necessary because of the diffuse infiltrative nature of this neoplasm and results in median survivals ranging from 10 to 18 months.8,9 Craniospinal RT does not confer any additional survival benefit and is associated with significant morbidity, limiting the administration of subsequent chemotherapy.
The optimal dose of whole-brain RT remains controversial, but the results of several studies suggest a dose between 40-50 Gy. The addition of a boost does not improve local tumor control or survival. In patients with evidence of ocular lymphoma, the posterior two thirds of the globe should be radiated to a dose of 36-40 Gy. Treatment planning should take into account both intracranial and ocular disease to eliminate overlapping fields and to minimize any toxicity to the optic nerve and retina.
Chemotherapy
The use of chemotherapy has significantly improved the treatment of PCNSL. However, the standard regimens (CHOP, MACOP-B) used in the treatment of systemic lymphoma are not effective in PCNSL because of their inability to penetrate the blood-brain barrier.
High-dose methotrexate (MTX) is the single most active agent in the treatment of PCNSL. While standard-dose MTX does not cross the blood-brain barrier, doses ≥ 1 g/m2 result in tumoricidal levels in the brain and doses ≥ 3.5 g/m2 yield tumoricidal levels in the CSF. Therefore, most treatment regimens now incorporate high-dose MTX (1 to 8 g/m2) alone or in combination with other chemotherapeutic agents followed by whole-brain RT. This combined-modality approach has resulted in response rates approaching 100% and median survivals ranging between 30 and 60 months (Table 2 ).10– 14
There has been increasing interest in using chemotherapy alone in order to minimize long-term effects of treatment. One approach has been to employ hyperosmolar agents to disrupt the blood-brain barrier, followed by intra-arterial MTX.13 This technique results in similar overall response and survival rates as the combined-modality approach; however, the acute toxicities are more significant and include focal seizures, cerebral ischemia, cerebral edema, and local arterial trauma. Careful neuropsychological testing of this patient cohort has been performed and indicates that patients who remain in remission are not at increased risk for delayed neurotoxicity.
In our experience, it is possible to treat older patients with MTX-based chemotherapy alone and achieve similar results as those achieved using combinedmodality treatment in older patients. Both groups have a median survival of 32-33 months;14 the difference is that patients treated with chemotherapy alone are more likely to relapse early, while patients treated with combined-modality therapy are more likely to develop delayed neurotoxicity. Importantly, older patients are able to tolerate aggressive chemotherapy without any excess acute morbidity.
High-dose chemotherapy with autologous peripheral blood stem cell support has been used a strategy to dose intensify chemotherapy given to patients with primary CNS lymphoma. Theoretically the administration of high dose consolidation chemotherapy can be used in place of standard cranial radiotherapy in an effort to avoid treatment-related neurotoxicity. There have been two small trials for newly diagnosed patients and the preliminary results indicate that this strategy is feasible.1,2 Further studies will be needed to identify the optimal induction and high dose chemotherapy regimens.15,16
AIDS-related
The treatment of AIDS-related PCNSL is dictated in large part by the clinical condition of the patient. One of the most critical factors is making a definitive diagnosis early, as delay may result in significant neurologic deterioration, precluding the ability to tolerate aggressive treatment.
Ocular lymphoma
There is no standard approach to isolated ocular lymphoma. Ocular lymphoma is exquisitely sensitive to corticosteroids (including topical ophthalmic preparations) and focal RT. Unfortunately, in most patients the disease will recur either in the eyes or in the brain, at which time the disease may be more refractory to therapeutic intervention. Systemic administration of MTX and cytarabine can yield therapeutic levels of drug in the intraocular fluids, and clinical responses have been documented; however, relapse is common.22–,24 Therefore, our current approach is to treat isolated ocular lymphoma with combined-modality therapy.25,26 Direct intravitreal administration of chemotherapy is being explored as a therapeutic alternative.
Relapse
The risk of relapse for patients treated with combined-modality therapy is about 50%. Most recurrences are observed within 2 years of completing initial therapy, but relapses have been seen as late as 5 years. Patients with ocular or leptomeningeal disease at diagnosis have a higher likelihood of recurrence. Relapse primarily occurs in the brain at either the original or distant sites; however, leptomeningeal and ocular relapses are seen, and systemic relapse has been reported to account for as much as 10%.
The prognosis at relapse is generally poor, but further treatment often results in transient remission. Prolonged survival is possible, and some patients continue to be sensitive to salvage therapy despite multiple relapses. Success has been reported using high-dose MTX (even in patients previously treated with MTX), high-dose cytarabine, PCV (procarbazine, lomustine, and vincristine), and high-dose cyclophosphamide. RT is particularly effective for ocular relapse. Intensive chemotherapy with autologous peripheral blood stem cell support is standard therapy for patients with relapsed, chemosensitive, systemic NHL; this strategy has been used with some success for relapsed PCNSL.3 However, patients previously treated with whole brain radiotherapy have a higher risk of neurologic toxicity.27
Treatment-Related Neurotoxicity
The most significant consequence of aggressive combined-modality therapy utilizing MTX followed by cranial RT is delayed neurologic toxicity. Older patients are at particularly high risk of developing a progressive neurological syndrome characterized by dementia, gait ataxia, and urinary dysfunction. Up to 90% of patients over 60 who survive 1 year after completion of treatment will be affected.28 Patients usually become symptomatic within 1 year of treatment, with a significant decline in their performance status necessitating constant supervision and custodial care. Attempts to treat delayed neurotoxicity have been generally unrewarding, although a subset of patients may have transient improvement following placement of a ventriculoperitoneal shunt.29,30 Other agents, such as methylphenidate, have been utilized with success in individual patients.
Delayed treatment-related cerebrovascular disease has been observed in younger patients 7-10 years after completion of therapy.31,32 This has been observed in isolation or in conjunction with a progressive leukoencephalopathy. Accelerated atherosclerosis is a known complication of cranial RT that typically develops 10 to 20 years after treatment.33,34 Stroke-like episodes have been reported acutely in children receiving high-dose MTX, but these typically occur days to weeks after chemotherapy and resolve spontaneously. It is also possible that PCNSL may predispose patients to cerebrovascular damage if lysis of angiocentric tumor cells damages neighboring endothelium.
II. Lymphomatous Meningitis: The Acute Lymphoblastic Leukemia Model
John T. Sandlund, MD*
St. Jude Children’s Research Hospital, 332 N. Lauderdale, Box 318, Memphis, TN 38101-0318
Supported by grant CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC)
CNS involvement among children with acute lymphoblastic leukemia (ALL) has historically been defined at most institutions by either the presence of at least 5 leukocytes per microliter of cerebrospinal fluid (CSF) associated with the presence of leukemic blasts (identified on a cytocentrifuged preparation) or the presence of a cranial nerve palsy on physical examination.1 Therapeutic approaches for both CNS prophylaxis and therapy have included the following: (1) intrathecal administration of chemotherapy ranging from single-agent MTX to triple-agent intrathecal therapy consisting of MTX, hydrocortisone, and cytarabine; (2) cranial irradiation; and (3) the systemic administration of chemotherapeutic agents with good CNS penetration (e.g., high-dose MTX, high-dose cytarabine, and dexamethasone). Despite these measures, there are patients who have been shown to still be at increased risk for CNS treatment failure.
CNS Status Refinement
Mahmoud et al2 challenged the conventional definition by showing that the presence of leukemic blast cells in the CSF, regardless of cell count, increased the risk of CNS relapse. In that study, all 351 children with newly diagnosed ALL were entered on a randomized trial in which each patient received intrathecal therapy throughout the first year. Patients who were considered at increased risk for treatment failure because of their clinical or cytogenetic features also received 18-Gy cranial irradiation and intrathecal chemotherapy 1 year from the remission date. Those with CNS disease at diagnosis (as defined by at least 5 leukocytes/microliter of CSF with leukemic blasts on a cytocentrifuged prep or by the presence of cranial nerve palsy on physical examination) received 24-Gy cranial irradiation and additional intrathecal chemotherapy. Patients were classified retrospectively into 3 CNS groups based on the CSF findings: 291 patients had CNS-1 status (no blasts in the CSF), 42 had CNS-2 status (blasts present with fewer than 5 leukocytes/microliter), and 18 had CNS-3 status (5 or more leukocytes/microliter of CSF with leukemic blasts on a cytospin sample or cranial nerve palsy). The probability of an isolated CNS relapse in patients with CNS-2 status was higher than in those with CNS-1 status but was not different from that of patients with CNS-3 status. All CNS relapses occurred during the first year of treatment, before scheduled cranial irradiation. In a multivariate analysis, CNS-2 status was independently related to the risk of an isolated CNS relapse, suggesting that these patients require intensification of CNS-directed treatment early in the course of therapy. While a study of the former Pediatric Oncology Group confirmed this result,3 studies by the former Children’s Cancer Group and the Dutch Childhood Leukemia Study Group did not find a significant difference in outcome between patients with or without a lower number of blasts in the CSF.4,5 These seemingly conflicting results may reflect differences in therapy.
Traumatic Lumbar Punctures
Gajjar et al6 performed a single-institution retrospective study of children with newly diagnosed ALL in which they demonstrated that a traumatic lumbar puncture (LP) at diagnosis adversely affected outcome. In this study, 546 children were treated on 2 consecutive St. Jude trials in which 2 sequential LPs were performed at presentation—the first for diagnosis and the second for instillation of the first intrathecal chemotherapy treatment, generally 1 to 2 days later. It was demonstrated that patients with 1 CSF sample contaminated with blast cells had an inferior event-free survival compared to those with CNS-1 status (P = 0.026). The prognosis for those with 2 consecutive contaminated CSF samples had a particularly poor treatment result (5-year event-free survival = 46% ± 9%); this feature was shown to be the strongest prognostic indicator in a Cox multiple regression analysis, with a hazard ratio of 2.39 (95% confidence interval, 1.36-4.20). It was concluded from this study that contamination of the CSF with circulating leukemic blasts adversely influences treatment outcome and is an indication for early intensification of intrathecal chemotherapy administration. This result was recently confirmed by the investigators of the Berlin-Frankfurt-Münster group (BFM; M Schrappe, personal communication).
A recent study by Howard et al (unpublished data) examined risk factors associated with the occurrence of traumatic (at least 10 red blood cells per microliter) and/or bloody (at least 500 red blood cells per microliter) LPs. Risk factors associated with traumatic or bloody taps included the following: (1) age less than 1 year; (2) black race; (3) early treatment era during which sedation was used very seldom; (4) a platelet count less than 100 × 109/L; (5) a short (1 day) time interval since the previous LP; and (6) a less experienced practitioner. On the basis of these findings, the investigators recommended that diagnostic LPs in newly diagnosed patients with ALL should be performed by an experienced practitioner, in a dedicated procedure area with general anesthesia, following platelet transfusion if the platelet count is less than 100 × 109/L and circulating blasts are present. Using this approach, we have already substantially reduced the rate of traumatic LP with blasts from 11% to 4% to date.
Impact of Intensified CNS Therapy/Prophylaxis
In a St. Jude Children’s Research Hospital study performed by Pui et al,7 it was demonstrated that early intensification of intrathecal chemotherapy used in the context of the Total Therapy Study XIII virtually eliminates CNS relapse in children with ALL. Children with any amount of leukemic blasts in the CSF, whether or not the CSF blasts were introduced iatrogenically to the CSF because of a traumatic LP and regardless of the presence or absence of other high-risk clinical features, received additional doses of intrathecal chemotherapy (MTX, hydrocortisone, and cytarabine) during induction and throughout the first year of continuation therapy. Cranial irradiation at 18 Gy, given during weeks 56 to 59 of the continuation phase, was reserved for only those with certain high-risk features: B-cell progenitor phenotype with a leukocyte count of at least 100 × 109/L, T-cell phenotype with a leukocyte count of at least 50 × 109/L, or a karyotype with the Philadelphia chromosome. The 5-year cumulative risk of an isolated CNS relapse among the 165 patients studied was 1.2% (95% confidence interval, 0%-2.9%), whereas the risk of any CNS relapse was 3.2% (95% confidence interval, 0.4%-6.0%). It appears from this study that early intensification of intrathecal chemotherapy administration may reduce or eliminate the occurrence of CNS relapse associated with the above-mentioned risk factors (i.e., CNS-2 status at diagnosis, and traumatic or bloody LP at diagnosis). A similar result was obtained in a subsequent St. Jude clinical trial (XIIIB) (unpublished data).
Trend Toward Reducing Use of Radiotherapy for CNS Disease/Prophylaxis
Most clinical trials limit the use of cranial irradiation to 5% to 10% of patients at high risk of CNS relapse, in large part because of the concern of late sequelae such as second cancer, endocrinopathy, and neuropsychologic defects. Moreover, in some protocols, cranial irradiation is given at a reduced dose. For example, the BFM has reduced the dose of prophylactic cranial irradiation to 12 Gy and the dose of therapeutic cranial irradiation for those with overt CNS disease to 18 Gy.8 Other trials, which have eliminated cranial irradiation in all patients, have not observed an excessive rate of relapse.9– 11 The elimination of cranial irradiation is also being studied in our current St. Jude trial. Thus far, no CNS relapse has been observed among 150 patients treated with median follow-up of 2 years (unpublished data).
CNS Disease in Pediatric NHL
Children with NHL are considered to have CNS involvement if lymphoma cells are identified in the CSF on a cytocentrifuged preparation or if a cranial nerve palsy is identified in a physical exam.12 These criteria are similar to those used for children with ALL, although there are some differences. For example, children with Burkitt’s lymphoma who have any classic L3 blasts in the CSF would be considered to have CNS disease, even if there were fewer than 5 white cells per microliter in the unspun CSF.
In a single-institution study of 445 children with newly diagnosed NHL, 36 (8%) were found to have CNS disease.13 Among these, 23 had morphologically identifiable lymphoma cells in the CSF, 9 had cranial nerve palsies, and 4 had both features. CNS disease at diagnosis was identified in 13%, 7%, and 1% of Burkitt’s, lymphoblastic, and large-cell lymphoma cases, respectively. In a multivariate analysis of various risk factors, including CNS disease, stage, and LDH, only stage and serum LDH had prognostic significance. Among patients with Burkitt’s lymphoma, a multivariate analysis demonstrated that only serum LDH had independent prognostic significance. This review therefore suggested that CNS disease per se was not an independent risk factor. Other studies have made similar observations.14,15 However, in a retrospective study performed by the CCG,16 it was concluded that among patients with Burkitt’s lymphoma, the presence of meningeal disease or CNS parenchymal masses at diagnosis was associated with a nominally worse outcome independent of initial bone marrow status and LDH level, although this effect was not statistically significant. In the recently published result of the French LMB-89 study for children with B-cell lymphoma and L3 leukemia, CNS involvement was the only adverse prognostic factor identified among group C patients.17
The modalities used for both CNS prophylaxis and treatment of overt CNS disease are similar to those used for children with ALL. They include high-dose systemic chemotherapy (e.g., MTX, cytarabine), intrathecal instillation of chemotherapy (e.g., single-agent MTX, triple-agent therapy [MTX, hydrocortisone, and cytarabine]), and, less frequently, cranial irradiation. The implementation of these approaches does vary with respect to histologic subtype.
Burkitt’s lymphoma. Most centers currently use systemic high-dose MTX, and cytarabine and intrathecal MTX, hydrocortisone, and cytarabine for both CNS prophylaxis and treatment. Two of the most successful treatment regimens are the French LMB-8917 regimen and the German BFM-90 protocol.18 The LMB-89 regimen incorporated cranial irradiation for those with overt CNS disease at diagnosis; however, most current regimens have excluded cranial irradiation. In fact, the current international collaborative French study has excluded cranial irradiation. In the BFM-90 regimen,18 cranial irradiation was not incorporated; however, an intraventricular access device was used for drug delivery to the spinal fluid. In this regard, St. Jude is currently piloting a regimen in which an intraventricular access device is used in the context of LMB-89 directed systemic therapy.
Lymphoblastic lymphoma. Systemic and intrathecal chemotherapy is used for CNS prophylaxis and treatment. For patients with overt CNS involvement at diagnosis, many centers would consider incorporating cranial irradiation. The role of cranial irradiation for CNS prophylaxis is more controversial, although, as in the case for ALL, there is a distinct trend to move away from it. For example, in the highly effective BFM-90 regimen,19 patients with stage III or IV disease receive 12-Gy cranial irradiation as prophylaxis; however, a subsequent study is determining the safety of its omission. Among patients with CNS-2 status at diagnosis, a current St. Jude study incorporates intensified intrathecal treatment without cranial irradiation.
Large-cell lymphoma. Determining the optimal approach to CNS prophylaxis and treatment for this group is somewhat problematic, in part because the large-cell lymphomas are a more heterogeneous group. Those with a B-cell immunophenotype are often treated with the same regimen used for Burkitt’s lymphoma, as described above. The majority of non-B-cell cases are anaplastic large-cell lymphomas for which a spectrum of therapeutic approaches has been reported. The BFM has had great success using a regimen derived from a Burkitt’s lymphoma strategy.20 In the United States, the APO regimen has also been shown to be effective; with this approach, CNS prophylaxis includes single-agent intrathecal MTX.21 The optimal approach to managing overt CNS disease at diagnosis is controversial, primarily because of the low frequency of this clinical presentation.
Primary CNS lymphoma. PCNSLs in children are very rare. Also, there is little information with respect to clinical trial data in children to guide treatment. For children who are HIV negative, most pediatric oncologists would consider intensive systemic multiagent chemotherapy, featuring agents with good CNS penetration (e.g., high-dose MTX/cytarabine, dexamethasone); cranial radiotherapy would also be a consideration in some cases. Strategies that have been shown to be effective in adults are often used on an individual basis in children.
Patients who are HIV positive and develop a PCNSL are considered to have an extremely poor prognosis. In an attempt to provide a novel curative approach, Slobod et al22 treated 2 HIV-positive patients who presented with primary EBV-positive CNS lymphomas with hydroxyurea. This strategy was used based on in vitro studies of an EBV-positive Burkitt’s lymphoma cell line, in which exposure to hydroxyurea resulted in loss of cytoplasmic EBV episomes and subsequent loss of malignant phenotype. On the basis of this observation, hydroxyurea was given to HIV-positive patients who had EBV-positive PCNSLs with objective clinical and radiographic responses, suggesting that antiviral approaches may have a role in these malignancies.
CNS Prophylaxis in Adult ALL
The approaches most commonly used for CNS prophylaxis in adults are similar to those that have been used in children: (1) intrathecal therapy (e.g., MTX, cytarabine, hydrocortisone); (2) high dose systemic therapy; and (3) cranial irradiation.23 These measures have reduced the rate of CNS relapse to < 5-10% from the > 30% rate reported when no prophylaxis is provided.23 Gökbuget and Hoelzer reviewed the published data on CNS prophylaxis and found that a combination of all three of the above mentioned approaches resulted in the lowest incidence of isolated or combined CNS relapses (5%, range of 1-12%).23,24 Nevertheless, the use of cranial irradiation remains controversial. In the GMALL studies, a higher rate of CNS relapses was observed when cranial irradiation was either omitted or delayed.23,24 However, in Kantarjian et al’s study of the Hyper-CVAD regimen, which features high-dose systemic (MTX and cytarabine) and intrathecal therapy (no cranial irradiation) for CNS prophylaxis, the CNS relapse rate was very low (4%).26
III. Secondary Central Nervous System Lymphoma: The Case for Prophylaxis
Andrew Lister, MD*
St. Bartholomew’s Hospital, 45 Little Britain, West Smithfield, EC1A 7BE London, United Kingdom
“Secondary” lymphomatous involvement of the CNS was first recognized in the 19th century when Murchison1 described a tumor encroaching on the foramen magnum infiltrating the dura mater at autopsy. The problem of extradural deposits was recognized later.2–,4 By the middle of the 20th century, secondary central nervous system lymphoma (SCNSL) had been the subject of many manuscripts,5–,7 representing as closely as possible the natural history, with Sparling et al6 in 1947 reporting an autopsy incidence of only 1 in 118 cases. As the natural history of the lymphomas has been superseded by the clinical course (induced by partially successful therapy not targeting the CNS), survival of some subtypes has been prolonged. In the 1970s, incidence of SCNSL increased to approximately 10%.8,9
A clear clinical picture, reflecting the outcome of therapy introduced in the late 1960s and early 1970s, emerges from a number of retrospective analyses from both single institutions and groups,10– 22 in which symptomatic disease occurred in 4-29%, depending on histology and extent of disease. The commonest features were headache, cranial nerve palsies, spinal cord compression, and altered mental state and affect. These problems usually arose within the context of poorly controlled lymphoma elsewhere, although the nervous system was occasionally an isolated site of recurrence. In the large majority of cases, the diagnosis was based on the history and the finding of abnormal cells on a cytospin of CSF. There was a strong association with bone marrow involvement; a correlation was also drawn between central nervous system lymphoma (CNSL) and involvement of the testis or paranasal sinuses. Likewise, close correlation was found between histological subtype and probability of the occurrence of CNSL; it was common with lymphoblastic lymphoma and Burkitt’s and “Burkitt’s-like” lymphoma, to the extent that the next generation of treatment included CNS-targeted therapy.
The Problem Today: Incidence, Risk Factors
Twenty years on, the demonstration of new prognostic factors and the introduction of the International Prognostic Index (IPI) have made it possible to identify more closely those patients for whom SCNSL is a high enough risk to warrant specific prophylactic therapy.
At the M.D. Anderson Hospital,23 24 of 605 patients with ‘large-cell’ or immunoblastic lymphoma developed CNS recurrence, with an actuarial risk at 1 year of 4.5%. In 5 cases, the recurrence was concurrent with systemic progression (within 40 days); in 7 others, it preceded systemic progression up to 6 months later. Involvement of more than 1 extranodal site and elevated LDH at presentation were both independently predictive of CNS recurrence on multivariate analysis: if both were present, the actuarial risk was almost 20% at 1 year (Figure 1 ). However, despite intervention, with some apparent early benefit, only 1 of 24 patients was alive a year after recurrence.
The Hovon multicenter group24 reviewed the risk of CNS recurrence in a trial testing the role of high-dose therapy with hematopoietic stem cell rescue, in patients responding “slowly” to 3 cycles of CHOP. One hundred ninety-three of 267 patients entered complete remission (CR). Ten patients (5%) developed SCNSL, 8 of them simultaneously with systemic progression. The risk was highest for patients with a high IPI score, but CNS recurrence occurred in all the risk groups. Survival data were not presented.
Zinzani et al25 reported an apparently higher incidence of isolated CNS recurrence in an unselected series (excluding Burkitt’s and lymphoblastic lymphoma) of patients with high-grade NHL (Kiel classification). One hundred seventy-five patients entered CR following therapy with MACOP-B or F MACHOP, both of which include modest doses of MTX intravenously but exclude intrathecal therapy. None had clinical evidence of CNS involvement at presentation. The minimum follow-up at the time of analysis was 3 years. Nine of 175 developed isolated CNS recurrence at a median of 3 months after CR had been documented. Multivariate analysis revealed advanced stage (III and IV) to be the only independent predictor of the likelihood of isolated CNS recurrence, although B symptoms, elevated LDH, and bone marrow involvement were all significant on univariate analysis. The outcome, whether the recurrence was leptomeningeal or parenchymal, was appalling, with all patients having died within 2 years because of CNS progression.
In contrast, Haioun et al26 reported the outcome for 1373 patients treated in a GELA study for patients with ‘aggressive’ NHL; lymphoblastic lymphoma and Burkitt’s lymphoma were excluded. CNS prophylaxis included intrathecal MTX with each cycle of systemic chemotherapy and 2 pulses of MTX 2 g/m2 with folinic acid rescue. There were 16 isolated CNS recurrences and a further 6 with progression at other sites. Initial multivariate analysis confirmed more than one extranodal site and elevated LDH to be independent risk factors predictive of CNS recurrence, each with a relative risk (RR) of 5. A further multivariate analysis (incorporating IPI score as a unique parameter, male gender, and B symptoms) was subsequently performed. IPI score remained the only parameter significantly associated with increased risk (low and low-intermediate versus high-intermediate and high, RR 7). Once again, the prognosis overall was poor, the median survival being 5 months and progressive disease being the predominant cause of death.
A further study from the GELA27 adds support for the benefit of CNS prophylaxis for this group of patients. Seven hundred eight adults aged 61-69 years with at least 1 adverse prognostic factor (IPI) were entered onto a trial comparing a relatively intensive chemotherapy program incorporating both intrathecal MTX and consolidation with systemic MTX, ifosphamide, and cytosine arabinoside, with standard CHOP. The CR rates were the same, despite a higher treatment-related mortality in the trial arm; overall survival, however, was better in the latter (P = .002). The frequency of CNS recurrence was also significantly lower in the trial arm (8 versus 25; P = .003). These results have been published in abstract form only to date. They are, however, supported by an earlier analysis from the M.D. Anderson Hospital in which outcome of patients receiving CNS prophylaxis in the form of intrathecal and intravenous MTX was better than that of matched historical controls.16
The largest body of data defining the extent of the problem at the end of the 20th century comes from the Norwegian Radium Hospital, Oslo.28 Twenty-five hundred fourteen adults were treated for NHL according to protocols of the day, based on the histological subtype (Kiel) and the extent of disease at presentation. CNS prophylaxis was given to < 1%, 11%, and 83% of patients with low-grade, high-grade, and Burkitt’s or lymphoblastic lymphoma, respectively. The analysis addressed only the question of CNS progression, so 30 patients presenting with CNS involvement were excluded.
Overall, the incidence reported for the histological groupings was very similar to that of other series. Less than 3% of those with “low-grade histology” developed SCNSL. Multivariate analysis confirmed B symptoms and involvement of bone marrow and skin as significant prognostic factors, with relative risks of 2.8, 2.8, and 3.7, respectively. The incidence for patients with Burkitt’s or lymphoblastic lymphoma was, in contrast, very high, being 24% overall, 78% in those not receiving prophylaxis, and 19% at 5 years in those that did.
As in several other series, the SCNSL rate in ‘high-grade’ lymphoma was about 4%, the minority having received prophylaxis with intrathecal methotrexate about which no conclusions were drawn.
Univariate analysis revealed a multitude of factors, including IPI and age-adjusted IPI, to predict for CNS recurrence. Testicular involvement in itself was not significant. Further analysis confirmed 5 factors to have an independent impact on CNS involvement: age, LDH, albumin, retroperitoneal nodes, and number of extranodal sites (Table 3 ).
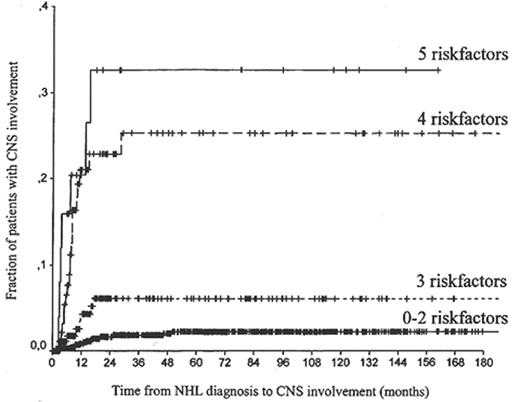
Although the hazard ratios are not identical, a general picture may be created by adding the risk factors and correlating increasing numbers with time-to-CNS involvement (Figure 2 ).
The Challenge Today
The elimination of CNS involvement with lymphoma is a very important goal, even if it affects only a relatively small proportion of patients, most of whose overall survival will be dictated by uncontrolled disease elsewhere. It is a highly distressing complication, with potentially extensive morbidity which, when established, is very difficult to eliminate. Theoretically, therefore, a prophylactic strategy, analogous to that employed so successfully for ALL, is indicated. The risk of meningeal involvement in childhood lymphoblastic leukemia has been reduced from more than 50% to very low levels, after painstaking observations, identification of groups with different degrees of risk, and clinical trials to determine the most effective therapy with the lowest acceptable toxicity for each category. Most children now do not develop CNSL, nor do most have excessive long-term morbidity from the therapy.
The first part of the process has been achieved for NHL. Follicular lymphoma and the other lymphocytic lymphomas have been shown to have a less than 1% probability of CNS infiltration, except when transformation has occurred: there can thus be no justification for prophylaxis. Burkitt’s lymphoma and lymphoblastic lymphoma (T and B) both have a high incidence of SCNSL: patients therefore now receive both intrathecal chemotherapy and high doses of MTX (and cytosine arabinoside in some instances) or cranial irradiation. As a consequence of this strategy, the incidence of CNS involvement is much reduced.
For the remainder of the lymphomas, predominantly diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), there is still no uniformity of practice, which reflects the complexity of the situation and the fact that the data are open to differing interpretation. However, the picture may be clearer than it was 20 years ago.
There is a recurring theme throughout the recent publications. CNS lymphoma is uncommon but not rare, and when it occurs, devastating. Patients presenting with a high IPI score, particularly reflecting the presence of a high LDH or involvement of more than one extranodal site, are at much higher risk of CNS involvement than the rest. Notwithstanding less impressive statistical proof of their individual significance, patients with testicular and sinus involvement are also at high risk. Some of the data reported above suggest that prophylaxis, with intrathecal therapy and systemic MTX, may reduce the risk.
It could therefore be concluded that all patients with these histological subtypes of lymphoma (DLBCL and PTCL-NOS) should have the CNS evaluated by history, examination, and LP, and that those with a high IPI score, or high LDH and more than one extranodal site, should proceed to prophylaxis. There is a superficial attraction to designing a randomized trial to test the hypothesis. It might be difficult to execute.
If it is difficult to select the appropriate group to receive CNS prophylaxis, it is equally difficult to determine what constitutes the best prophylaxis. Before the introduction of ‘high-dose’ MTX29 into combination chemotherapy, the only modalities available were intrathecal chemotherapy and irradiation. It may be clear from the above that intrathecal chemotherapy of short duration, while probably reducing the risk, does not eliminate it. Extrapolation from ALL makes this unsurprising: all treatments relying on intrathecal therapy alone demand much more prolonged treatment. Vital information about the efficacy of systemic MTX and the dose required in the absence of intrathecal therapy will come from the long follow-up analysis of the Southwestern Oncology Group-Eastern Oncology Group (SWOG-ECOG) study comparing CHOP with M-BACOD, MACOP-B, and PROMACE-CYTABOM, the trial arms including MTX and folinic acid rescue at a dose of 200 mg/m2, 400 mg/m2, and 1500 mg/m2, respectively. It may be anticipated that only the last dose might be effective. Further information accrued from clinical trials incorporating high-dose cytosine arabinoside may be helpful. Given at a dose of 2 g/m2, daily for 5 days, as part of the therapy for adults with ALL, cytosine arabinoside was as effective (compared with historical controls) as cranial irradiation in a small study.30
It would be foolhardy in the extreme to make didactic statements about optimal CNS prophylaxis: in the light of all that has gone before, recommendations can be made only on the basis of circumstantial evidence and must be seen as part of the best treatment of the disease overall. While none of the third-generation treatments above compared favorably with CHOP, perhaps a treatment for those with a high IPI score incorporating high-dose MTX (> 3 g/m2) and cytosine arabinoside (> 1 g/m2) might improve outcome. Were that perceived to be the case, a prospective evaluation of the strategy, particularly including long-term toxicity, would be required.
Attention has been focused on reasons in favor of prophylaxis as opposed to against it. Emphasis has been placed on the unpleasant nature of the complication and the difficulty of eliminating it, once established. There are powerful clinical and economic reasons for not giving CNS-directed treatment if it can be avoided. The long-term toxicity of irradiation given for PCNSL has been reviewed above. Even though the long-term sequelae of prophylactic cranial irradiation are less worrying, there are enough data to suggest that high-dose systemic chemotherapy may be as effective and less toxic. It is, however, not without morbidity and mortality, which increase with the dose. Conversely, intrathecal therapy is inconvenient and not to be desired, has well-known toxicity, and is costly for both the patient and the hospital. All this must be taken into account in devising the best way to improve therapy, and demonstrate the improvement, while offering the individual the best advice.
For future consideration: What emphasis should be given to the risk at the time of recurrent or progressive lymphoma? Do the same risk factors apply? Should more or less attention be directed to the problem? Should it be considered for only those still being treated with curative intent?
Initial evaluation for primary central nervous system lymphoma (PCNSL).
| Abbreviations: MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; CT, computed tomography. |
| Gadolinium-enhanced cranial MRI scan |
| CSF cytology |
| Ophthalmologic examination, including slit lamp |
| HIV serology |
| CT scan of chest, abdomen, and pelvis |
| Bone marrow biopsy |
| Gadolinium-enhanced spinal MRI, if spinal symptoms are present |
| Abbreviations: MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; CT, computed tomography. |
| Gadolinium-enhanced cranial MRI scan |
| CSF cytology |
| Ophthalmologic examination, including slit lamp |
| HIV serology |
| CT scan of chest, abdomen, and pelvis |
| Bone marrow biopsy |
| Gadolinium-enhanced spinal MRI, if spinal symptoms are present |
Chemotherapy regimens for PCNSL.
| Ref . | Type . | N . | Regimen . | RT . | Result . | Other . |
|---|---|---|---|---|---|---|
| Abbreviations: PCNSL, primary central nervous system lymphoma; DHAP, dexamethasone, high-dose cytarabine, and cisplatin; PCV, procarbazine, CCNU, and vincristine; MTX, methotrexate; BBBD, blood-brain-barrier disruption; MTV, methotrexate, thiotepa, and vincristine; IT, intrathecal; Ara-C, cytarabine; BOMES, BCNU, vincristine, methotrexate, etoposide, and methylprednisolone; MPV, methotrexate, procarbazine, and vincristine; RT, whole-brain radiotherapy; mo, months; PFS, progression-free survival; pt, patient. | ||||||
| Adapted with permission from Abrey LE, Primary central nervous system lymphoma. The Neurologist. 2000;6:245-254. | ||||||
| 35 | Series | 10 | DHAP | +/– | 70% response 40% prolonged remission | 4 newly diagnosed, 6 recurrent Several did not receive RT |
| 36 | Series | 10 | PCV | + | 100% response 30-mo median survival | PCV given post-RT 1 pt received carmustine |
| 11 | Series | 13 | MTX 3.5 g/m2 | + | 92% response 9+- mo median survival | Survival up to 54+ mos |
| 10 | Series | 25 | MTX 3.5 g/m2 | + | 88% response 33-mo median survival | 59% relapse rate |
| 37 | Series | 74 | MTX BBBD | – | 65% complete response 40.7-mo median survival | |
| 38 | Series | 31 | MTX 1 g/m2 | + | 64% response 41-mo median survival | |
| 23 | Phase II | 14 | MTV IT Ara-C | – | 100% response 16.5-mo median PFS | 68.8% alive at 54 mos 2 pts with severe leukoencephalopathy |
| 39 | Prospective | 19 | BOMES | + | 84% response rate 6-mo median PFS | 5 pts with concurrent systemic lymphoma |
| 40 | Series | 19 | MTX-based 3.5-8 g/m2 | – | 94% response rate | |
| 41 | Phase II | 102 | MPV Ara-C | + | 94% response rate 30+-mo median survival | |
| 14 | Prospective | 52 | MPV Ara-C | +/– | 60-mo median survival | 22 older pts did not receive RT |
| Ref . | Type . | N . | Regimen . | RT . | Result . | Other . |
|---|---|---|---|---|---|---|
| Abbreviations: PCNSL, primary central nervous system lymphoma; DHAP, dexamethasone, high-dose cytarabine, and cisplatin; PCV, procarbazine, CCNU, and vincristine; MTX, methotrexate; BBBD, blood-brain-barrier disruption; MTV, methotrexate, thiotepa, and vincristine; IT, intrathecal; Ara-C, cytarabine; BOMES, BCNU, vincristine, methotrexate, etoposide, and methylprednisolone; MPV, methotrexate, procarbazine, and vincristine; RT, whole-brain radiotherapy; mo, months; PFS, progression-free survival; pt, patient. | ||||||
| Adapted with permission from Abrey LE, Primary central nervous system lymphoma. The Neurologist. 2000;6:245-254. | ||||||
| 35 | Series | 10 | DHAP | +/– | 70% response 40% prolonged remission | 4 newly diagnosed, 6 recurrent Several did not receive RT |
| 36 | Series | 10 | PCV | + | 100% response 30-mo median survival | PCV given post-RT 1 pt received carmustine |
| 11 | Series | 13 | MTX 3.5 g/m2 | + | 92% response 9+- mo median survival | Survival up to 54+ mos |
| 10 | Series | 25 | MTX 3.5 g/m2 | + | 88% response 33-mo median survival | 59% relapse rate |
| 37 | Series | 74 | MTX BBBD | – | 65% complete response 40.7-mo median survival | |
| 38 | Series | 31 | MTX 1 g/m2 | + | 64% response 41-mo median survival | |
| 23 | Phase II | 14 | MTV IT Ara-C | – | 100% response 16.5-mo median PFS | 68.8% alive at 54 mos 2 pts with severe leukoencephalopathy |
| 39 | Prospective | 19 | BOMES | + | 84% response rate 6-mo median PFS | 5 pts with concurrent systemic lymphoma |
| 40 | Series | 19 | MTX-based 3.5-8 g/m2 | – | 94% response rate | |
| 41 | Phase II | 102 | MPV Ara-C | + | 94% response rate 30+-mo median survival | |
| 14 | Prospective | 52 | MPV Ara-C | +/– | 60-mo median survival | 22 older pts did not receive RT |
Risk of central nervous system involvement.
| Variable . | Relative Risk (95% Confidence Interval) . | P Value . |
|---|---|---|
| No. of extranodal sites (>1 vs ≤ 1) | 3.0 (1.7-5.4) | < 0.001 |
| Age > 60 vs ≤ 60 yrs | 2.8 (1.5-5.4) | 0.002 |
| Albumin < 3.5 g/L vs > 3.5 g/L | 2.5 (1.3-4.6) | 0.005 |
| LDH ≥ 450 m/L vs < 450 m/L | 2.1 (1.0-4.4) | 0.049 |
| Retroperitoneal glands: Yes vs no | 1.9 (1.0-3.5) | 0.037 |
| Variable . | Relative Risk (95% Confidence Interval) . | P Value . |
|---|---|---|
| No. of extranodal sites (>1 vs ≤ 1) | 3.0 (1.7-5.4) | < 0.001 |
| Age > 60 vs ≤ 60 yrs | 2.8 (1.5-5.4) | 0.002 |
| Albumin < 3.5 g/L vs > 3.5 g/L | 2.5 (1.3-4.6) | 0.005 |
| LDH ≥ 450 m/L vs < 450 m/L | 2.1 (1.0-4.4) | 0.049 |
| Retroperitoneal glands: Yes vs no | 1.9 (1.0-3.5) | 0.037 |
The risk of central nervous system (CNS) recurrence according to the number of risk factors (age, lactate dehydrogenase, albumin, number of extranodal sites, retroperitoneal involvement) in 1220 patients with high-grade non-Hodgkin’s lymphoma (NHL).
Reprinted with permission from Van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91:1178-1184.
The risk of central nervous system (CNS) recurrence according to the number of risk factors (age, lactate dehydrogenase, albumin, number of extranodal sites, retroperitoneal involvement) in 1220 patients with high-grade non-Hodgkin’s lymphoma (NHL).
Reprinted with permission from Van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91:1178-1184.
Incidence of central nervous system (CNS) recurrence in patients with increased lactate dehydrogenase (LDH) and involvement of more than 1 extranodal site (n = 93; dotted line) versus all other patients (n = 512; solid line).
Reprinted with permission from Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13:1099-1107.
Incidence of central nervous system (CNS) recurrence in patients with increased lactate dehydrogenase (LDH) and involvement of more than 1 extranodal site (n = 93; dotted line) versus all other patients (n = 512; solid line).
Reprinted with permission from Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13:1099-1107.