Abstract
Abnormalities of plasma von Willebrand factor (VWF) have been recognized to be associated with thrombotic thrombocytopenic purpura (TTP) for over 20 years. Patients with chronic, relapsing TTP have VWF multimers that are larger than normal, similar in size to those secreted by cultured endothelial cells. Recent observations have documented that a deficiency of a VWF-cleaving protease (termed ADAMTS13) may be responsible for the presence of these unusually large VWF multimers. Multiple mutations of the ADAMTS13 gene can result in ADAMTS13 deficiency and cause congenital TTP; autoantibodies neutralizing ADAMTS13 protease activity have been associated with acquired TTP.
In Section I, Dr. Evan Sadler reviews the structure, biosynthesis, and function of the ADAMTS13 protease. He describes the mutations that have been identified in congenital TTP and describes the relationship of ADAMTS13 deficiency to the development of both congenital and acquired TTP. Dr. Sadler postulates that the development of TTP may be favored by conditions that combine increased VWF secretion, such as during the later stages of pregnancy, and decreased ADAMTS13 activity.
In Section II, Dr. Bernhard Lämmle describes the assay methods for determining ADAMTS13 activity. Understanding the complexity of these methods is essential for understanding the difficulty of assay performance and the interpretation of assay data. Dr. Lämmle describes his extensive experience measuring ADAMTS13 activity in patients with TTP as well as patients with acute thrombocytopenia and severe illnesses not diagnosed as TTP. His data suggest that a severe deficiency of ADAMTS13 activity (< 5%) is a specific feature of TTP. However, he emphasizes that, although severe ADAMTS13 deficiency may be specific for TTP, it may not be sensitive enough to identify all patients who may be appropriately diagnosed as TTP and who may respond to plasma exchange treatment.
In Section III, Dr. James George describes the evaluation and management of patients with clinically suspected TTP, as well as adults who may be described as having hemolytic-uremic syndrome (HUS). Dr. George presents a classification of TTP and HUS in children and adults. Appropriate evaluation and management are related to the clinical setting in which the diagnosis is considered. A clinical approach is described for patients in whom the diagnosis of TTP or HUS is considered (1) following bone marrow transplantation, (2) during pregnancy or the postpartum period, (3) in association with drugs which may cause TTP either by an acute immune-mediated toxicity or a dose-related toxicity, (4) following a prodrome of bloody diarrhea, (5) in patients with autoimmune disorders, and (6) in patients with no apparent associated condition who may be considered to have idiopathic TTP. Patients with idiopathic TTP appear to have the greatest frequency of ADAMTS13 deficiency and appear to be at greatest risk for a prolonged clinical course and subsequent relapse. Management with plasma exchange has a high risk of complications. Indications for additional immunosuppressive therapy are described.
I. ADAMTS13, Von Willebrand Factor, and the Pathophysiology of Thrombotic Thrombocytopenic Purpura
J. Evan Sadler, MD, PhD*
Departments of Medicine and Biochemistry and Molecular Biophysics, and Howard Hughes Medical Institute, Washington University School of Medicine, 660 South Euclid Avenue, Box 8022, St Louis, MO 63110.
Thrombotic thrombocytopenic purpura (TTP) is characterized by microangiopathic hemolytic anemia and thrombocytopenia, often accompanied by fever, renal failure, and neurological deficits. Platelet-rich microvascular thrombi appear to be responsible for the renal and cerebral lesions, and often damage other organ systems as well. If untreated, TTP is almost always fatal. However, intensive plasma exchange therapy has reduced the mortality to approximately 25%.1,2
The pathophysiology of TTP has been mysterious until recently, although several clues have implicated a plasma protein defect. For example, from the first description of the disease,3 transfusions of blood were known to cause remissions in occasional patients, and the striking efficacy of plasma therapy suggests that a plasma protein deficiency or a circulating toxic factor is responsible. Twenty years ago, Moake et al found that some patients with chronic, relapsing TTP had plasma von Willebrand factor (VWF) multimers that were larger than normal, similar in size to those secreted by cultured endothelial cells. They proposed that “unusually large” VWF multimers (ULVWF) persisted after secretion in vivo because the patients lacked a protease or a disulfide reductase activity that reduces the size of VWF multimers and suggested that ULVWF could cause TTP by promoting uncontrolled platelet agglutination, thrombosis, and ischemia. Thus, the efficacy of plasma exchange could be due to the replacement of the missing “depolymerase” or the removal of an inhibitor.4
During the past decade, the proposed role of ULVWF in TTP has received considerable further support, starting with the independent discovery by Furlan et al5,6 and Tsai and Lian7 that most patients with TTP are deficient in a plasma metalloprotease that cleaves a specific peptide bond in the VWF subunit,8,9 thereby decreasing the size of VWF multimers. Congenital TTP is associated with the constitutional absence of the protease, whereas adults with acquired TTP usually have IgG autoantibodies that inhibit protease activity. Last year, this protease was purified to homogeneity and partially sequenced,10,11 which showed that it belongs to the ADAMTS family of metalloproteases.10 The corresponding gene and cDNA were cloned quickly, and the VWF cleaving protease was designated ADAMTS13.12,13 In a remarkable convergence of distinct experimental strategies, the ADAMTS13 gene was identified simultaneously by the positional cloning of mutations in families affected by autosomal recessive inherited TTP.14 These discoveries have provided a new molecular focus for investigations of TTP.
Structure of ADAMTS13
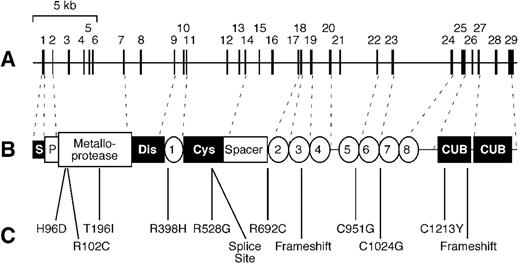
ADAMTS13 is one of 19 ADAMTS proteases described to date (http://www.gene.ucl.ac.uk/nomenclature/genefamily/adamts.html). The family name “ADAMTS” is an acronym for “a disintegrin-like and metalloprotease with thrombospondin type 1 repeats,”15,16 and ADAMTS13 has all of the structural motifs that define the family (Figure 1 ). The ADAMTS13 precursor consists of 1427 amino acid residues and contains a signal peptide, a relatively short propeptide, a metalloprotease domain of the reprolysin/adamalysin type, a disintegrin domain, a thrombospondin type 1 repeat (TSP1), a characteristic Cys-rich and spacer domain, and 7 additional TSP1 repeats. ADAMTS13 is the most divergent member of the family and, unlike any other ADAMTS protease, ADAMTS13 also has 2 CUB domains at its C-terminus. CUB domains are named for complement components C1r/C1s, urinary epidermal growth factor, and bone morphogenetic protein-1 (a synonym for procollagen C-proteinase), each of which has 1 or more CUB domains.17
The protease domain is a typical reprolysin-like or adamalysin-like metalloprotease, with a conserved active site sequence of HEXXHXXGXXHD in which the His residues coordinate a catalytic Zn2+ ion. Molecular modeling based on the structure of adamalysin II suggests that several other residues coordinate a structural Ca2+ ion and predicts the locations of 3 intrachain disulfide bonds.12 Chelation of either Zn2+ or Ca2+ inhibits the protease,8,9 which is consistent with the proposed presence of both metal ions in the metalloprotease domain. The functions of the various other domains are not established, although comparisons with related ADAMTS proteases suggest they may interact with other proteins or glycosaminoglycans. Disintegrin domains are found in many snake venom proteins and are so named because they can bind integrins and disrupt cellular interactions. However, the disintegrin domains of ADAMTS proteases are not known to have this activity, and other domains may be better candidates for intermolecular interactions. The Cys-rich domain of ADAMTS13 does contain an Arg-Gly-Asp sequence that might bind certain integrins.12,14 An Arg-Gly-Asp sequence is also present in the Cys-rich domain of ADAMTS2, but not in other family members.18 The potential integrin-binding activity of these Arg-Gly-Asp sequences has not been tested.
Several ADAMTS domains have been implicated in binding to other macromolecules. Recombinant ADAMTS1 binds to extracellular matrix, and binding is inhibited by heparin, suggesting that the ligand is a glycosaminoglycan. Matrix binding appears to depend on the ADAMTS1 spacer region and certain TSP1 repeats.19 The TSP1 repeats in platelet thrombospondin interact with a number of ligands, including fibrinogen, CD36, and several glycosaminoglycans.20,21
The CUB domains of ADAMTS13 are unique within the ADAMTS family but are common in the closely related astacin-like family and can determine substrate specificity. For example, procollagen C-proteinase is a metalloprotease with a C-terminal extension that contains 5 CUB domains. This protease removes the C-terminal propeptide from certain procollagens, and at least 3 CUB domains are required to recognize the procollagen substrate.22 Furthermore, procollagen cleavage is enhanced by a cofactor protein, POLCE, that also contains 2 essential CUB domains.23
At the present early stage of the field, one can only speculate about the purpose of TSP1, spacer, and CUB domains in ADAMTS13. These domains are completely conserved among ADAMTS13 from human, mouse, and the Japanese puffer fish (J.E. Sadler, unpublished data), suggesting they are likely to be important for function. Based on analogies developed in the preceding paragraphs, these domains are potential sites for binding to substrates, cofactors, cell surfaces, or the extracellular matrix.
Biosynthesis and Catabolism
By Northern blotting, full-length 4.6-kb ADAMTS13 mRNA is found only in the liver, suggesting that the liver is the principal source of plasma ADAMTS13.12–,14 Alternatively spliced mRNA forms occur in many tissues,12–,14,18 but whether they give rise to functional protein is unknown.
ADAMTS13 undergoes extensive posttranslational processing, involving proteolysis as well as glycosylation. ADAMTS13 appears to be synthesized as a zymogen that is activated by propeptide cleavage. The propeptide ends in a typical furin-like recognition sequence, Arg-Gln-Arg-Arg, suggesting that activation could occur intracellularly. The propeptide has been cleaved from ADAMTS13 that is purified from plasma.10,11 Therefore, some ADAMTS13 circulates as an active enzyme, but the existence of a significant pool of ADAMTS13 zymogen has not been excluded. The plasma protein is extensively glycosylated, which is consistent with the presence of 10 potential N-glycosylation sites throughout the protein and 7 potential O-glycosylation sites, one for each TSP1 repeat except the fourth.12 Glycosylation probably accounts for much of the discrepancy between the apparent mass of purified ADAMTS13 on gel electrophoresis (190 kDa) and the calculated mass for the ADAMTS13 polypeptide (145 kDa).
The plasma concentration of ADAMTS13 is not known precisely but is estimated to be 1 μg/mL.11 Its half-life in the circulation is approximately 2 to 3 days,24 and this exceptional stability allows patients with congenital deficiency to be treated with plasma infusions every 2 to 3 weeks to prevent the recurrence of thrombotic microangiopathy.25,26
Enzymology
The activity of ADAMTS13 depends on both Zn2+ and Ca2+; the required calcium ion can be replaced by Ba2+ or Sr2+ but not by Mg2+.8 The enzyme is active between pH 7 and pH 11, with optimal activity at pH 8, and activity is increased substantially at an ionic strength much lower than that of plasma.8 Aside from metal chelators, none of the usual protease inhibitors inhibit ADAMTS13.8,9 The resistance of ADAMTS13 to all plasma protease inhibitors is relatively unusual but is consistent with its long circulatory half-life.
The only known substrate for ADAMTS13 is VWF, which is cleaved between Tyr1605-Met1606 (Tyr842-Met843 in mature subunit numbering) in the second of the 3 consecutive A domains in the center of the VWF subunit.8,9 This cleavage produces fragments of 176 kDa and 140 kDa that are found in normal plasma VWF, suggesting that ADAMTS13 may be responsible for most of the proteolytic degradation of VWF subunits in vivo.27 The rate of VWF cleavage by ADAMTS13 is increased markedly by mild denaturation with low concentrations of urea8 or guanidine hydrochloride,9 or by fluid shear stress.9
Increased susceptibility of VWF to digestion persists after the removal of denaturants or shear stress,9 suggesting that these treatments unfold the VWF substrate and expose the cleavage site. This conclusion is supported by studies of mutant VWF. The common von Willebrand disease (VWD) type 2A mutation Arg1597Gln (Arg834Gln in mature subunit numbering) is within VWF domain A2, only 8 residues from the ADAMTS13-sensitive Tyr-Met bond. The mutation does not impair multimer assembly but increases the sensitivity of VWF to proteolytic degradation in the circulation, and recombinant VWF with this mutation is digested readily by ADAMTS13 in the absence of shear stress or denaturants.28 Therefore, a conformational change in VWF that promotes proteolytic cleavage may be localized to the A2 domain.28 For many VWD type 2A mutations, cleavage of the mutant subunits by ADAMTS13 probably causes the deficit in large multimers, which in turn causes a hemostatic defect.
Mutations in Congenital TTP
Soon after the development of an assay for plasma VWF cleaving protease activity, severe deficiency was found to be associated with autosomal recessive inherited TTP,5,29 or Upshaw-Schulman syndrome.30,31 Last year, genome-wide linkage analysis in 4 affected families mapped the responsible locus to chromosome 9q34 and showed that it encodes ADAMTS13.14 The ADAMTS13 gene spans 37 kb and contains 29 exons. Among 15 affected ADAMTS13 alleles, 12 different mutations were identified (Figure 1): 9 were single amino acid substitutions at residues conserved between human and mouse, 2 were frameshift mutations, and 1 was a splice site mutation. No patient had obvious null mutations on both alleles, suggesting that total ADAMTS13 deficiency could be lethal and, in fact, ADAMTS13 activity levels were extremely low but detectable (2%-7% of normal) in all patients. This landmark study demonstrates conclusively that ADAMTS13 deficiency causes inherited TTP, rather than being a secondary consequence of another molecular defect.14
ADAMTS13 and the Mechanism of Thrombotic Microangiopathy
With the benefit of recent knowledge concerning ADAMTS13, the ULVWF model of TTP4 can be adapted readily to account for microvascular thrombosis, the most dangerous aspect of TTP (Figure 2, see Color Figures, 520). Platelets in flowing blood adhere transiently to exposed, immobilized VWF. Transient adhesion is followed by the engagement of other adhesive and signaling receptors, which causes platelet activation, immobilization, and spreading. This platelet surface can recruit more VWF and more platelets by the same mechanism. Under the influence of fluid shear stress, the VWF is recognized by ADAMTS13, which cleaves VWF multimers, releases the platelets, and limits the growth of the thrombus. Without ADAMTS13, this feedback inhibitory mechanism fails, and microvascular thrombi continue to grow, causing tissue ischemia and infarction.
This model accounts for several details of the clinical picture of TTP and the behavior of VWF. Decreased ADAMTS13 activity would be expected to cause accumulation of ULVWF, as is observed in patients with inherited TTP due to ADAMTS13 deficiency.5 The administration of desmopressin releases ULVWF into the circulation and might be expected to exacerbate TTP, and this phenomenon has been reported.32 When subjected to fluid shear stress, the platelets in blood aggregate in a process that depends on VWF, and the threshold for shear-induced platelet aggregation is shifted to lower values of shear stress when ADAMTS13 activity is decreased.33,34 Certain inherited disorders are associated with increased VWF-platelet binding. In VWD type 2B, mutant VWF binds platelet GPIb with increased affinity.35,36 In platelet-type pseudo-VWD, mutant GPIbα binds VWF with increased affinity.37 Both of these conditions are characterized by bleeding rather than thrombosis, possibly because ADAMTS13 cleaves VWF in any growing platelet aggregate and prevents microvascular thrombosis.
The ULVWF model is consistent with several biochemical and clinical observations but raises many important questions. For example, according to the model, platelet thrombi form on immobilized VWF that is exposed to the blood, but the origin and properties of this VWF are not fully understood. When the vasculature is disrupted, VWF bound to connective tissue mediates platelet adhesion. However, it seems unlikely that enough deendothelialized vessels could be diffusely distributed to account for the disseminated microvascular thrombosis caused by ADAMTS13 deficiency. A more plausible source of exposed VWF may be the ULVWF multimers secreted by a relatively intact endothelium. In the absence of ADAMTS13, long strings of secreted ULVWF remain bound to endothelial cells and become decorated with adherent platelets. In the presence of ADAMTS13, these VWF strings are cleaved rapidly, and the platelets are released.38 Thus, TTP may be favored by conditions that combine increased VWF secretion and decreased ADAMTS13 activity. Such a “two-hit” model could explain the substantial variation in the age at which patients with inherited TTP develop symptoms. Deficiency of ADAMTS13 may set the stage, so that thrombotic microangiopathy supervenes after a triggering event injures or activates microvascular endothelial cells and causes the secretion of ULVWF.39 For example, the increase in VWF secretion during the third trimester of pregnancy may contribute to the tendency of TTP, whether inherited or acquired, to present during late pregnancy.39,40
Other Potential Factors in Thrombotic Microangiopathy
The developing conceptual framework involving VWF and ADAMTS13 not only has wonderful explanatory power but also highlights issues that need further study. Perhaps the most relevant for clinical practice is the observation that some patients with thrombotic microangiopathy have normal ADAMTS13 activity, and others have ADAMTS13 deficiency but no demonstrable inhibitor.41,42 These cases suggest there may be other lesions in the ADAMTS13 pathway, or mechanisms independent of ADAMTS13, that can cause TTP. If sufficiently common, alternative pathophysiologic mechanisms may reduce the utility of ADAMTS13 assays for the diagnosis and management of TTP. Suitable clinical trials are needed to address this important point.
Additional factors in the ADAMTS13 pathway have not been identified, but recent case reports suggest that a search for them may be appropriate. Two unrelated families have been described in which children born of a consanguineous marriage developed TTP that was characterized by ULVWF multimers and a good clinical response to prophylactic plasma transfusions; however, these patients had normal endogenous ADAMTS13 activity.43,44 Such anomalous cases would be consistent with a mutation in an unknown ADAMTS13 cofactor, or potentially with a mutation in ADAMTS13 that affected function in vivo but not in vitro.
Cleavage by ADAMTS13 is not the only mechanism by which VWF multimers can be decreased in size. Many other proteases can cleave VWF, and cleavage of VWF subunits at several sites has been observed in vivo.45 Plasma thrombospondin-1 recently was shown to have disulfide reductase activity toward VWF, with the ability to reduce intersubunit disulfide bonds and decrease VWF multimer size.46 It is not known whether deficiencies in these processes could cause TTP, or whether they may sometimes complement ADAMTS13 deficiency and prevent TTP. One possibly critical parameter may be whether shear stress can increase the efficiency of VWF multimer scission by other proteases or by thrombospondin-1. If not, these mechanisms may be unable to inhibit platelet thrombus growth.
A study of TTP patients with normal ADAMTS13 levels has uncovered an unexpected potential risk factor. The current model for the pathogenesis of TTP emphasizes the proposed role of VWF in the development of platelet-rich thrombi at sites of high fluid shear stress and is consistent with the histopathologic demonstration that TTP lesions are rich in VWF and poor in fibrin.47 In contrast, risk factors for venous thrombosis, with lesions characteristically rich in fibrin and poor in platelets, would not usually be thought of as risk factors for TTP. However, among 11 Caucasian patients with TTP and normal ADAMTS13 levels, 4 (36%) were heterozygous for factor V Leiden compared with 6 (3%) of 186 control subjects (P < 0.001).42 The possible involvement of factor V Leiden suggests that some mechanisms of thrombotic microangiopathy may involve fibrin deposition, which is not a prominent feature of TTP.
II. The Role of ADAMTS13 in the Evaluation and Management of Patients with Thrombotic Thrombocytopenic Purpura
Bernhard Lämmle, MD*
Central Hematology Laboratory, University Hospital, Inselspital, CH 3010 Bern, Switzerland
Dedication: I dedicate this paper to my beloved son Gregor, a third-year medical student, who died on July 12, 2002, at the age of 24 years.
Many hypotheses concerning the pathogenesis of TTP have been proposed (for review, see Moake and Chow,1 Ruggenenti and Remuzzi,2 and Furlan and Lämmle3). One of them, originally put forward in 1982 by Moake et al,4 has attracted much interest in the past few years. These researchers observed unusually large multimers of von Willebrand factor (ULVWF) in the plasma of several patients with chronic relapsing TTP and suggested that these extremely adhesive VWF multimers were directly responsible for in vivo platelet clumping in the microcirculation leading to ischemic neurologic and renal dysfunction. ULVWF multimers, present during remission, disappeared from plasma during acute relapses, presumably by consumption during the platelet clumping process. Moake et al4 suggested that their presence in plasma might be due to an excessive release from endothelial cells and/or an impaired degradation by a then-hypothetical VWF “depolymerase.”
In 1996, our group5 and Tsai6 independently isolated and partially characterized a novel protease from human plasma that specifically cleaved VWF at Tyr842-Met843,5 the peptide bond known to be cleaved in vivo.7 Shortly thereafter, 4 patients, including 2 brothers, with chronic relapsing TTP and ULVWF in their plasma were found to completely lack the VWF-cleaving protease activity.8 Infusion of fresh frozen plasma (FFP) into the 2 brothers led to a quantitative recovery of VWF-cleaving protease activity and disappearance of the ULVWF multimers. The half-life of the protease in plasma was estimated to be 2-4 days.9
Another patient with severe sporadic TTP came to our attention whose plasma lacked any VWF-cleaving protease activity due to an autoantibody inhibiting its activity.10 Two large retrospective (multicenter) studies on a large series of patients diagnosed with TTP11,12 or hemolytic uremic syndrome (HUS)11 revealed that most patients with acute TTP had a severely depressed VWF-cleaving protease activity (less than 5% of the activity in normal human plasma [NHP]), most often due to inhibiting IgG autoantibodies,11,12 and that those patients diagnosed with HUS had normal (higher than 50%) or subnormal (26-50%) protease activity.11
The VWF-cleaving protease has been further purified from plasma,13,14 and the N-terminal amino acid sequence allowed its characterization as a novel member of the ADAMTS family of metalloproteases14 and elucidation of its cDNA and gene structure.15–,17 The approach of Levy et al17 using genome-wide positional cloning in patients with hereditary TTP and their family members led to the identification of the ADAMTS13 gene on chromosome 9q34 and of several mutations of this gene as presumably being responsible for severely decreased VWF-cleaving protease activity and disease in doubly heterozygous or homozygous carriers of mutated alleles. This latter observation17 strongly supported a causal linkage of severely deficient ADAMTS13 activity with the hereditary form of TTP.8
In this overview, I will (1) describe the methods to measure ADAMTS13 activity, (2) report on our experience with hereditary ADAMTS13 deficiency in patients with constitutional TTP (the Upshaw-Schulman syndrome), (3) assess the specificity and sensitivity of severe ADAMTS13 deficiency for the diagnosis of TTP, and (4) estimate the value of ADAMTS13 activity measurement for diagnostic purposes and therapeutic decisions.
Assays of ADAMTS13 Activity and of ADAMTS13 Inhibiting Autoantibodies
Several assays have been developed for measuring ADAMTS13 activity (for review, see Furlan and Lämmle18). Our original assay5,8 involves activation of the diluted patient plasma with 10 mM BaCl2, admixture of purified protease-free VWF substrate, digestion of VWF during 16-18 hours on a hydrophilic filter membrane lying on top of a buffer consisting of 1.5 M urea, 5 mM Tris, pH 8.0, followed by sodium dodecyl sulphate (SDS) -1.4% agarose gel electrophoresis and immunoblotting of the degraded multimers. The assay takes 4 working days to be completed, is reproducible, and is very sensitive, allowing one to discriminate 1-3% of protease activity from 0% by comparing the VWF multimeric pattern produced by the sample with that of a standard curve obtained with serial dilutions of pooled normal plasma defined to contain 100% of ADAMTS13 activity (Figure 3 ).
The method reported by Tsai and Lian6,12 measures the generation of disulfide-linked dimers of C-terminal (amino acids 843-2050) and/or N-terminal (amino acids 1-842) proteolytic fragments of the VWF subunit after incubation of guanidinium-treated VWF substrate with diluted plasma in the presence and absence of EDTA, followed by unreduced SDS polyacrylamide gel electrophoresis and immunodetection with 125I-labeled anti-VWF antibodies and autoradiography. The difference of the intensity of the 350 kD band representing the dimer of C-terminal VWF fragments between the reaction mixture with and without EDTA reflects the protease activity in the test sample.
In order to make the measurement of VWF-cleaving protease activity more generally available to routine laboratories, we set out to simplify the assay by measuring the residual collagen binding of the VWF substrate after its degradation by BaCl2 activated protease in patient plasma in the presence of 1.5 M urea and low ionic strength conditions.19 Despite its advantages of simplicity (ELISA technique using microtiter plates) and speed (completion within a few hours), the assay is less sensitive and less precise in the low activity range, and we have sometimes encountered problems with reproducibility.
A very elegant assay was reported by Obert et al,20 in which BaCl2 activated protease in patient plasma digests recombinant VWF substrate. Protease-digested VWF is assayed using a 2-site immune radiometric assay (IRMA) with a capturing monoclonal antibody directed against the C-terminal part of the VWF subunit and a mixture of radiolabeled monoclonal antibodies toward the N-terminal part of VWF for detection. The apparent loss of VWF antigen is directly related to ADAMTS13 activity.
Inhibitor assays in the above tests involve mixing patient plasma with pooled normal plasma, incubating it, and measuring the residual VWF-cleaving protease activity in these mixtures.
It is mandatory that these proposed assays be compared with each other aiming for standardizing and, thus, correctly appreciating the diagnostic value of ADAMTS13 activity values.
Hereditary ADAMTS13 Deficiency and Constitutional TTP (Upshaw-Schulman Syndrome)
In 1978 Upshaw21 reported a young woman having suffered since childhood from recurrent episodes of severe microangiopathic hemolytic anemia and thrombocytopenia who, on one occasion of acute illness, received whole blood instead of the usually transfused red cell concentrates. The better and faster response of the platelet count and hemolysis to whole blood as compared to packed red cells led to the hypothesis that a plasma component might be effective in reversing the thrombocytopenia and hemolysis. This hypothesis was substantiated during an ensuing attack that promptly responded to FFP infusion. Upshaw concluded that his patient was deficient in a plasma factor that protected from hemolytic anemia and thrombocytopenia.21
After establishing a severe, presumably hereditary VWF-cleaving protease deficiency in 2 brothers with chronic relapsing TTP and half normal activity in their parents,8 we have identified a further 26 subjects from 19 families with ADAMTS13 activity less than 3-5% without a circulating inhibitor and slightly lowered protease activity in their parents. According to the (sometimes incomplete) clinical information available to us, there is a striking variability of the clinical phenotype,3 an observation also made by other investigators.22 About half of the patients identified in our laboratory3 had their first TTP attack between the neonatal period and an age of 5 years. Diagnosis was often delayed; in one child constitutional TTP was recognized only after several hospital admissions when severe ischemic brain lesions were detected by magnetic resonance imaging;23 and several siblings of affected patients had died. Very recently, we encountered the case of an 8-year-old boy who died after several bouts of acute illness since childhood; the diagnosis of TTP was made only at autopsy, and VWF-cleaving protease activity in his premortem serum was less than 3%, both parents showing about 50% of activity. Pediatricians should be aware of the existence of constitutional TTP, given that effective treatment is available (see below).
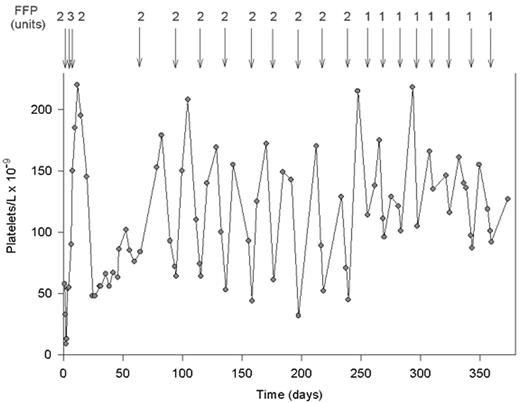
The other half of patients diagnosed by our laboratory became clinically symptomatic only in adulthood, and 2 subjects are still asymptomatic at an age older than 35 years.3 Most patients, both those with early and those with late onset of TTP, showed often multiple relapses once they had suffered their first TTP attack. Response to FFP infusion was rapid, and prophylactic FFP infusions every 2-3 weeks avoided relapses and controlled the platelet count and hemolysis3,23– 25 (Figure 4 ).
It may be concluded that ADAMTS13 activity levels as low as 5-10% are sufficient for prevention of microvascular platelet consumption, and all parents of affected patients, with VWF-cleaving protease activity as low as 6-20%,25 are asymptomatic according to available information.
Levy et al17 identified 12 different mutations in the ADAMTS13 gene accounting for 14 of 15 disease alleles in their families with hereditary TTP (see Section I). More recently, Kokame et al26 found 5 mutations, including 1 silent polymorphism, in 2 Japanese families with constitutional TTP. Expression studies showed that 2 mutant proteins were not secreted. Interestingly, one expressed and secreted mutant with a P475S exchange had only 5% of the wild-type ADAMTS13 activity, and showed an allele frequency of 5.1% in the Japanese population. Whether this 475Pro→Ser substitution or other high-frequency alleles with a similar effect explain the rather wide normal range of about 50-150%, also in healthy Caucasian subjects, remains to be investigated.
Specificity and Sensitivity of ADAMTS13 Deficiency for TTP
The specificity of VWF-cleaving protease deficiency for TTP has been recently challenged.27–,29 Loof et al27 reported decreased VWF-cleaving protease activity (36% ± 24% [mean ± SD] as compared to the activity of normal pooled plasma) in 14 patients with disseminated intravascular coagulation (DIC). Moore et al28 found moderately or severely decreased VWF-cleaving protease activity in several patients with thrombocytopenic disorders different from TTP and even in some healthy controls. Moreover, Mannucci et al29 reported decreased ADAMTS13 activity in newborns; during the second and third trimesters of pregnancy; in liver cirrhosis, uremia, and various acute inflammatory disorders; and in the postoperative period. They concluded that VWF-cleaving protease deficiency was not a specific beacon of TTP. It should be noted, however, that most subjects with low VWF-cleaving protease activity described by these authors29 had activity levels of some 20-50% and none had values less than 10%.
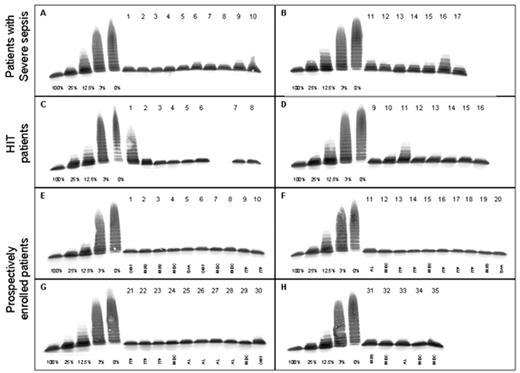
We performed a formal study on 68 patients with thrombocytopenia not due to TTP or HUS, including 17 with severe sepsis or septic shock, 16 with heparin-induced thrombocytopenia, and 35 with thrombocytopenia caused by various hematologic conditions, such as idiopathic (immune) thrombocytopenic purpura, idiopathic osteomyelofibrosis, myelodysplastic syndrome, acute leukemia, severe aplastic anemia, and miscellaneous disorders.30 Twelve of the 68 patients had a VWF-cleaving protease activity ≤ 30% of NHP, but none had a value < 10% (Figure 3). The median ADAMTS13 activity level in the 17 patients with severe sepsis was 40% (range 15-80%), being in agreement with the slightly or moderately decreased values reported in patients with inflammatory conditions.29 The ADAMTS13 values in our series of thrombocytopenic patients30 contrast sharply with our data in TTP patients.11 Our experience is based on the analysis of more than 1500 plasma samples provided by clinicians from more than 100 hospitals in some 25 countries. As of June 2002, we have identified > 130 patients with an ADAMTS13 activity < 5% of NHP. According to available clinical information, all had a clinical picture consistent with TTP with the exception of 3 cases: 2 subjects were brothers of affected siblings from 2 families with adult-onset hereditary TTP, both still asymptomatic at age > 35 years.3 In addition, one child with < 5% ADAMTS13 activity, caused by a transient autoantibody, belonged to a series of 29 children with Escherichia coli 0157:H7-associated D+ HUS.31
Therefore, in contrast to a recent editorial on the debate,32 severe deficiency of VWF-cleaving protease activity (< 5% of the activity in NHP) is a specific feature for a thrombotic microangiopathy commonly diagnosed as TTP.12,30
This statement is supported not only by the above-mentioned study on thrombocytopenic patients,30 but also by data on 74 hospitalized or healthy controls12 and 120 previously investigated healthy subjects,11 all showing ADAMTS13 activity levels of at least 45% of NHP.
Even though a severely deficient VWF-cleaving protease activity is specific for TTP, the sensitivity of this laboratory finding for the diagnosis of TTP remains questionable. In retrospective large-scale studies, Furlan et al11 found 26 of 30 patients (86%) with TTP to lack any measurable protease activity, and Tsai and Lian12 reported a severe VWF-cleaving protease deficiency in 37 of 37 patients (100%) with acute TTP. A recent retrospective study from Japan33 on 27 patients with thrombotic microangiopathy showed a VWF-cleaving protease < 3% due to a circulating inhibitor in 12 of 18 patients (66%) with TTP, the remaining 6 patients having activity levels of 6-28%, whereas the 9 patients diagnosed with HUS had values between 28-70%. These results largely confirmed previous studies from Europe.11,34 In this latter prospective multicenter study on 111 patients with thrombotic microangiopathies, Veyradier et al34 found a severe ADAMTS13 deficiency in 47/66 patients (71%) being diagnosed as TTP, whereas most patients presenting with HUS had normal or subnormal protease activity.
Thus, with sensitivities of 66-100%, it is evident that many but not all patients being diagnosed with acute TTP have severe ADAMTS13 deficiency. This suggests that other pathogenetic factors1– 3 may lead to a clinical condition indistinguishable from that seen with severe acquired or hereditary ADAMTS13 deficiency.
Patients with bone marrow transplantation–associated thrombotic microangiopathy had normal35 and 4 patients with disseminated neoplasia-associated thrombotic microangiopathy had subnormal or normal ADAMTS13 activity.36 In contrast, ticlopidine-induced37 and possibly clopidogrel-induced thrombotic microangiopathy38 were reported to be associated with severe, autoantibody-mediated ADAMTS13 deficiency, as was the thrombotic microangiopathy in one patient with acquired immunodeficiency syndrome.39
Value of Measuring ADAMTS13 Activity and Its Inhibitors for the Diagnosis of and Treatment Decisions in TTP
Diagnosis of acute TTP is often both difficult and urgent.40 It may be difficult because not all patients with TTP will show the complete pentad of diagnostic criteria,40,41 and clinical diagnosis may have to be based on thrombocytopenia and microangiopathic (schistocytic) hemolysis that are not explained by another condition, even in the absence of ischemic organ dysfunction40 (see Section III). Moreover, the diagnosis is urgent because mortality in untreated patients may be as high as 80-90%40,41 and plasma exchange with replacement by FFP has reduced the mortality to about 20%.40,42 Recognition of a large proportion of acute idiopathic TTP patients as displaying an autoantibody-mediated severe VWF-cleaving protease deficiency may explain the empirically established effectiveness of plasma exchange with FFP replacement that removes the inhibiting autoantibody and substitutes the lacking ADAMTS13 activity. Still, I believe that based on the present evidence it would not be justified or appropriate to withhold plasma exchange or FFP infusion in a patient with a bona fide clinical diagnosis of TTP showing normal ADAMTS13 activity.
The retrospective study by Mori et al33 is noteworthy. Ten of the 12 TTP patients with a severe acquired ADAMTS13 deficiency survived the acute episode, whereas 4 of 6 patients with moderate protease deficiency (∼25% activity) died. These results may suggest that treatment with plasma exchange is not the optimal therapy for a thrombotic microangiopathy that is not due to severe acquired VWF-cleaving protease deficiency. However, in the absence of knowledge on the underlying pathophysiology, and hence specific therapy, standard treatment with plasma exchange still seems mandatory until improved therapeutic measures based on the underlying pathogenesis become available.
There is an indication that corticosteroids may have efficacy in TTP.43 Even though no controlled study is available, the demonstration of autoantibodies abolishing VWF-cleaving protease activity in many cases of TTP would certainly support their use. Splenectomy has been performed empirically in desperate cases of plasma refractory or frequently relapsing TTP44,45 and we have provided evidence that its efficacy may be related to the elimination of the autoantibody-producing B cells.10 Establishment of a high-titer autoantibody inhibiting ADAMTS13 activity would certainly facilitate the decision to proceed to splenectomy, which may entail considerable risk in a patient with ongoing active TTP.
The relapse rate in patients surviving an initial TTP attack is rather high.40 The observation of disappearance with remission and reappearance of an autoantibody inhibiting ADAMTS13 heralding a TTP relapse within a few weeks in our initially studied patient with acquired TTP10 may suggest that assaying ADAMTS13 activity and its inhibitor could be useful for estimating the relapse risk in patients having survived an initial bout of TTP.
At present, probably the best clinical indication for measuring VWF-cleaving protease and its inhibitor is to distinguish between acquired and hereditary ADAMTS13 deficiency. A severely deficient VWF-cleaving protease activity in a symptomatic patient, a lack of circulating inhibitor, and mildly decreased activity levels in the parents prove hereditary TTP. Treatment of acute attacks using simple FFP infusion instead of performing plasma exchange is in order for these rare patients,21–,25 and prophylactic FFP infusions are warranted to prevent otherwise frequent relapses3,23– 25 (Figure 4).
Measuring ADAMTS13 activity in other clinical conditions, such as sepsis, heparin-induced thrombocytopenia, various inflammatory disorders, disseminated neoplasia, and other conditions, is not routinely indicated but mayif performed in the setting of appropriate clinical studiesfurther our understanding of this novel member of the ADAMTS family of metalloproteases.
Conclusions
The rather complex assays reported for the measurement of ADAMTS13 activity need comparison and standardization in the frame of multisite studies. Severely diminished activity values (< 3-5%) must be distinguished from moderately (10-25%) or slightly (25-50%) decreased values, the former being a specific beacon of TTP.
Hereditary TTP is often, if not always, caused by severe ADAMTS13 functional deficiency, and various causal mutations of the ADAMTS13 gene have become known. Some childhood-onset cases of Upshaw-Schulman syndrome are probably not diagnosed during the life time because pediatricians are not always familiar with this rare but potentially fatal disease for which clearly effective treatment is available. On the other hand, a sizable proportion of severely ADAMTS13-deficient subjects will not become symptomatic until adulthood. It is unclear at present whether this phenotypic variability is due to different genetic defects or variable residual ADAMTS13 activity levels below the detection threshold of current assays, or whether other constitutional factors or circumstantial triggering events, such as endothelial activation or damage, may be necessary to provoke acute TTP.
Sporadic idiopathic TTP is often caused by autoantibodies inhibiting ADAMTS13 activity, but other pathogenetic mechanisms may lead to a clinically indistinguishable thrombotic microangiopathy.
A diagnosis of acute sporadic TTP without underlying severe acquired VWF-cleaving protease deficiency should notat presentpreclude the patient from being subjected to plasma exchange therapy. However, the distinction between hereditary and acquired ADAMTS13 deficiency is of major importance because patient management will be different.
III. Evaluation and Management of Patients with Clinically Suspected Thrombotic Thrombocytopenic Purpura or Hemolytic-Uremic Syndrome
James N. George, MD*
Hematology-Oncology Section, Department of Medicine, University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73190
The diagnosis of TTP is appropriately suspected in a patient who has acute and severe thrombocytopenia and microangiopathic hemolytic anemia, without another explanation.1–,3 But these are obviously non-specific criteria; there is no “gold standard” for diagnosis. TTP can be associated with a deficiency of ADAMTS13 (see Section I) and a severe deficiency of ADAMTS13 (< 5% activity) may be specific for TTP (see Section II). However, severe ADAMTS13 deficiency does not define TTP and is not a definitive diagnostic test. The sensitivity of severe ADAMTS13 deficiency remains unknown: patients with only moderate ADAMTS13 deficiency or even normal activity can have presenting features and clinical courses, including response to plasma exchange treatment, identical to patients with severe deficiency. The difficulty of diagnosis is emphasized by observations that among patients diagnosed with TTP and who have severe ADAMTS13 deficiency, approximately half have normal renal function and approximately one-fourth have no neurologic abnormalities. These observations are consistent with the current diagnostic criteria of only thrombocytopenia and microangiopathic hemolytic anemia.1– 3
The frequent uncertainty about the diagnosis of TTP makes management decisions difficult. The most important initial decision is whether or not to begin plasma exchange, a treatment with documented efficacy1 but also with a high risk for major complications (discussed fully below and in ref. 4).
This, therefore, is the clinical dilemma: uncertain diagnosis of a disorder that can be rapidly fatal, and availability of an effective treatment which has major risks. Table 1 describes the clinical situations in which patients with suspected TTP are encountered. Each of these clinical categories has distinct diagnostic and management issues; they are the basis for a physician’s evaluation and for this discussion. The distinction of adult syndromes as either TTP or HUS is not important for the initial management decision regarding plasma exchange. Patients with acute renal failure, a defining criterion for HUS, may respond as well to plasma exchange as patients with normal renal function. Therefore all adult syndromes are described in this discussion simply as TTP, even if acute renal failure is present.
Childhood Syndromes
“Typical HUS” is a frequent diagnostic term used for children, usually less than 5 years old, who present with a prodrome of bloody diarrhea caused by an enterohemorrhagic strain of E. coli, usually E. coli O157:H7.5–,7E. coli O157:H7 and related organisms produce Shiga toxin, which causes the diarrhea prodrome and further causes acute HUS in 5-10% of infected children.8 Acute renal failure is the principal abnormality; the associated thrombocytopenia and microangiopathic hemolytic anemia are not critical problems; conventional management is only supportive care for the renal failure; mortality in large case series is 3-5%.5–,7 Because mortality is not great, because plasma infusion has no clearly documented efficacy,9,10 and because plasma exchange catheter insertion may have greater risks in small children, plasma exchange treatment is rarely considered. However if children with HUS have an “atypical” presentation (i.e., without a diarrhea prodrome), mortality may be greater, spontaneous remissions are less certain, and plasma exchange may be considered.11 Rarely, children may present with minimal or no renal insufficiency, comparable to “typical TTP” in adults. These children, like adults, do not spontaneously resolve and plasma exchange treatment is required.
Adult Syndromes
Bone marrow transplantation
Although there are many case series describing TTP and/or HUS following bone marrow transplantation (BMT), whether this is a valid diagnosis is uncertain. Uncertainty is emphasized by the extreme variation of both the reported incidence of TTP following BMT (2-76% following allogeneic BMT, 0-27% following autologous BMT) and its mortality (0-93%).12 Inconsistency of diagnosis and clinical outcomes is inevitable in these patients who have multiple, severe transplant-related complications which share many clinical features with TTP. Diagnosis of TTP following allogeneic BMT is further complicated by dose-dependent renal and neurotoxicities of the GVHD prophylaxis/treatment medications, cyclosporine and FK506 (see Table 1), which can mimic TTP or perhaps may even cause a TTP-like syndrome.
Table 2 describes the experience with patients who had clinically suspected TTP following BMT at the University of Oklahoma.13 These patients had greater risks for transplant-related complications as well as a greater frequency of complications. In the 17 patients who were treated with plasma exchange for suspected TTP, sepsis and/or acute GVHD could have caused the signs suggesting the diagnosis of TTP. Although plasma exchange for possible TTP may seem appropriate for a patient with critical deterioration of uncertain cause, the outcomes are poor. None of these patients responded to plasma exchange and only 1 of 17 patients currently survives. Therefore our practice for patients with clinically suspected TTP following BMT is to intensify efforts to diagnose and treat GVHD and sepsis, and to delay a decision for plasma exchange. This practice is distinct from the urgency for initiating plasma exchange in other patients with clinically suspected TTP.
Pregnancy/postpartum
Clinical features suggesting a diagnosis of TTP in pregnant or postpartum women are described in Table 3 . The distinction of TTP from pregnancy-related complications, pre-eclampsia/eclampsia/HELLP syndrome, may be impossible.14–,16 However the association of TTP with pregnancy is clear. In large published case series of TTP, approximately 70% of patients are women; in 12-25% of all women, TTP is diagnosed during pregnancy or postpartum, with 75% of episodes occurring around the time of delivery.14 The association of pregnancy with TTP is further documented by five reports of familial TTP in which sisters had their initial episode at the end of their first pregnancy.14,17,18
The decision to initiate plasma exchange in a woman with suspected TTP who is pregnant or postpartum is often difficult. The thrombocytopenia and microangiopathic hemolytic anemia associated with pre-eclampsia/HELLP syndrome can be severe, and may only occur following delivery.19–,21 Seizures, defining eclampsia, often first occur after delivery.22 By definition, these syndromes spontaneously resolve within several days following delivery, though case reports describe more prolonged courses. The decision for plasma exchange is based on an estimate of the chance for spontaneous resolution versus the risk of progressive multi-organ failure and death (Table 3). If the woman is not acutely ill, observation for several days after delivery may allow time for spontaneous resolution to begin;23 women with more acute and severe multi-organ failure require prompt plasma exchange.
The risk for recurrent TTP with a subsequent pregnancy is a difficult issue. Subsequent pregnancies have a risk for recurrence of TTP. However even in women with prolonged, severe, and relapsing TTP following an earlier pregnancy, a subsequent pregnancy can be uncomplicated with delivery of a healthy infant (see “TTP, the patient’s perspective: Christy’s story,” http://moon.ouhsc.edu/jgeorge).
Drug association
Drug-associated syndromes appear to be of two types: acute, immune-mediated syndromes caused by drug-dependent antibodies and insidious, dose-related toxic effects.24 In our experience, quinine is by far the most common cause of immune-mediated TTP.25 This is not described in some discussions of drug-induced TTP26 because quinine-associated syndromes are often considered to be HUS, rather than TTP. However the clinical features of quinine-associated syndromes emphasize the inability to distinguish TTP from HUS. Although acute renal failure is a common manifestation, some patients have no renal insufficiency. Even more striking, one reported patient had acute renal failure with her first episode and no renal insufficiency with two subsequent quinine-induced episodes.25 The frequency of neurologic abnormalities and the severity of thrombocytopenia and anemia are not different in patients with quinine-associated TTP than in other patients with TTP; the serum LDH increase is greater, emphasizing the severity of systemic ischemia.25 The severe systemic ischemia may explain the extremely rapid rise of serum creatinine in patients with acute renal failure, far greater than the assumed maximum daily increase of 0.5-1.0 mg/dL per day, derived from observations on anephric patients. The characteristic presenting features of quinine-associated TTP are the sudden onset of nausea, vomiting, diarrhea (occasionally bloody), fever, and chills occurring several hours after quinine ingestion.25 Sepsis is a common initial, incorrect diagnosis.27 Patients may have severe mental status abnormalities, even coma.25 In addition to the signs of TTP, leukopenia, disseminated intravascular coagulation, and liver function abnormalities may occur, all of which can be caused by quinine sensitivity.28–,30 These syndromes are caused by quinine-dependent antibodies to epitopes on multiple cell types.28 Plasma exchange treatment appears to be effective.
Quinine is also one of the most common drugs that can cause isolated thrombocytopenia.31 In patients with isolated thrombocytopenia, the absence of anemia and any other systemic symptoms and signs exclude consideration of TTP. However, one important case report describes a patient with quinine-induced isolated thrombocytopenia who developed TTP following a subsequent exposure to quinine. 32 This observation is consistent with the development of only quinine-dependent antiplatelet antibodies causing the initial episode and the subsequent development of quinine-dependent antibodies to multiple tissues causing the systematic disorder of TTP.32
Ticlopidine24,33 and clopidogrel24,34 have also been reported to be associated with TTP and HUS. In some of these patients, a deficiency of ADAMTS13 and the presence of inhibitors of ADAMTS13 activity have been observed.34,35 However, drug-dependence of the antibodies to ADAMTS13 has not been documented, and therefore how an acute, apparently immune-mediated drug reaction may be associated with these autoantibodies is unknown. In patients with ticlopidine-associated TTP, the ticlopidine had been taken for less than 2 weeks in 15% of patients and less than one month in 80% of patients.33 In the largest report on clopidogrel-associated TTP, the onset of TTP was 3-14 days after beginning clopidogrel in 10 of 11 patients. However since 2 of the 11 reported patients had recurrences of TTP without re-exposure to clopidogrel,34 the initial drug association may have been coincidental.
In contrast to the acute onset of immune-mediated drug-associated TTP, the onset of syndromes, often described as HUS, following mitomycin C, cyclosporine, and related immunosuppressive agents is insidious and may only become manifest after the drug has been discontinued. These toxic syndromes have the same renal pathology as TTP and HUS,24 but hematologic manifestations may be minimal. The efficacy of plasma exchange is uncertain. In some patients with cyclosporine or tacrolimus-associated TTP, simple discontinuation or dose adjustment of the drug is sufficient to reverse the process.
Prodrome of bloody diarrhea
Similar to the typical HUS of young children, enterohemorrhagic strains of E. coli can also cause acute TTP or HUS in adults.36 Although these syndromes may be similar to HUS in children, in some adults no renal insufficiency occurs,36–,38 supporting the use of a comprehensive diagnostic term, TTP-HUS, or simply TTP. Although children with typical HUS are not treated with plasma exchange, mortality is high in adults and plasma exchange may be efficacious.37 Bloody diarrhea may also occur in patients who have severe ADAMTS13 deficiency, without a documented enteric infection.
Association with autoimmune disorders
In some patients with severe multi-organ failure caused by an established diagnosis of systemic lupus erythematosus, antiphospholipid antibody syndrome, scleroderma, or polyarteritis nodosa, the clinical and pathologic features may be indistinguishable from TTP.39– 41 In these critically ill patients, a trial of plasma exchange treatment for presumed TTP may be appropriate, in addition to intensive immunosuppressive treatment.
Alternative disorders that may mimic TTP
Since the diagnostic criteria for TTP are not specific and since there is urgency to begin plasma exchange, an alternative explanation for acute multi-organ failure, such as sepsis,42 disseminated malignancy,43,44 or malignant hypertension,45 may become apparent after plasma exchange is begun. This experience suggests that some patients who die with apparently refractory TTP and who do not have an autopsy could have had a disorder other than TTP. Although it is often stated that HIV-positive patients are at greater risk for developing TTP,46 there are no epidemiologic data to support a causal association of HIV infection and TTP. The abnormalities suggesting a diagnosis of TTP in HIV-positive patients may only be features of opportunistic infections.47 Therefore, similar to the management of patients following BMT described above, it is appropriate to carefully consider alternative diagnoses, specifically infectious etiologies, before initiating plasma exchange treatment.
Idiopathic TTP
When all of the preceding disorders are excluded, patients are considered to have idiopathic TTP, or “typical TTP,” presumably caused by an acquired autoantibody to ADAMTS13. However, even patients within the idiopathic category are heterogeneous. Among the subset of patients within the idiopathic category who have a severe deficiency of ADAMTS13, some may have a rapidly fatal course with multi-organ failure while others may have no neurologic or renal manifestations and require only few plasma exchange treatments to achieve a durable remission.
Management
Once the diagnosis of TTP is made (or seriously considered), urgent plasma exchange is indicated. If plasma exchange is unavailable, as in a distant rural site, initial treatment with plasma infusion and glucocorticoids is appropriate until the patient is moved to a facility with plasma exchange ability. Since plasma exchange is clearly superior to plasma infusion,1 the patient should be moved as soon as possible.
Once the decision for plasma exchange is made, our treatment plan is to exchange one plasma volume once daily.3 In patients with severe disease who do not respond within the first several days, or in patients who improve and then exacerbate while on daily plasma exchange, twice daily plasma exchange may be more effective. Whole FFP and the cryosupernatant fraction of plasma appear to be equally efficacious.48 Glucocorticoids are frequently used, and their potential efficacy is supported by observations that autoantibodies to ADAMTS13 may be the etiology in some patients. However, many patients respond promptly and completely without glucocorticoid treatment,1 even patients who have severe ADAMTS13 deficiency with an associated inhibitor. Therefore, reasonable practice in patients who are not critically ill may be to observe the response to plasma exchange alone, adding glucocorticoids if the response is not prompt or complete, or if an exacerbation occurs when plasma exchange frequency is diminished.3 Rarely are patients truly refractory to plasma exchange. Continued plasma exchange will achieve responses and ultimately remissions in most every patient. The benefit of additional immunosuppressive therapy or of splenectomy is uncertain.3 There are case reports and small series of selected patients describing efficacy of splenectomy, vincristine, cyclophosphamide, rituximab, and other immunosuppressive agents, but the unpredictable clinical course of TTP makes interpretation of these reports uncertain.
Complications of Plasma Exchange
Although plasma exchange is often considered to be a safe procedure, the risks are great in patients treated for TTP, perhaps because of the severity of illness and the requirement for prolonged treatment in many patients (Table 4 ).4 Deaths, or cardiac arrest with near-death, may be caused by complications of central venous catheter insertion, such as hemorrhage, pneumothorax, or pericardial tamponade due to cardiac perforation by the central venous catheter guide wire. Allergic reactions to plasma can cause severe hypotension and hypoxia. Sepsis related to the central venous catheter may be fatal.
An unappreciated complication of plasma exchange is unintentional plateletpheresis, causing persistent thrombocytopenia which may be interpreted as continuing active TTP and may result in inappropriate treatment.49 In our studies, the Fresenius AS 104 apheresis instrument caused greater platelet losses than either COBE Spectra or Haemonetics LN-9000 instruments, but platelet loss occurred with all 3 instruments.49 Inappropriate apheresis instrument settings that accelerate the procedure by removing plasma closer to the centrifuged cell layer can also cause unintentional plateletpheresis.
Long-Term Clinical Outcomes
The risk for relapse is related to the clinical category and presumably related to the presence of autoantibodies causing ADAMTS13 deficiency. Among patients with no renal failure who have severe ADAMTS13 deficiency, the risk for relapse may be greater than 50% over the first 5 years following recovery. Most relapses occur within the first year of the initial episode, although relapses after more than 10 years of complete remission may occur. Patients may have multiple relapses, although the occurrence of only a single relapse may be more common. Mortality is minimal with recurrence of TTP, because the patient responded to plasma exchange previously and because there is no delay in the diagnosis and therefore less delay in the initiation of treatment.
The incidental observation of asymptomatic thrombocytopenia during follow-up of a patient who has recovered from TTP creates a difficult issue. Without knowledge of a patient’s history, TTP would never be suspected, yet asymptomatic thrombocytopenia may be the first sign of an acute relapse. In some patients, intermittent thrombocytopenia has been observed for several months prior to their first episode of TTP. Some descriptions suggest the occurrence of ITP and TTP in the same patient at different times.50 This may be analogous to the report of quinine-induced thrombocytopenia followed on the next occasion of quinine exposure by TTP, as the spectrum of drug-dependent antibodies broadens from only antiplatelet antibodies to antibodies reacting with multiple cell types in multiple organs.32
Many patients describe incomplete recovery of physical and cognitive abilities even though all objective parameters suggest complete remission. These observations suggest that even after complete hematologic and renal function recovery, the effects of profound systemic ischemia may continue for months or years. Fortunately, continued observations suggest improvement in most patients.51
Thrombotic thrombocytopenic purpura (TTP) and hemolytic-uremic syndrome (HUS): clinical presentations and associated conditions.
| Children |
|
| Adults |
|
| Children |
|
| Adults |
|
Clinical features of patients with suspected thrombotic thrombocytopenic purpura following allogeneic bone marrow transplantation.*
| . | Patients with Clinically Suspected TTP (n = 17) . | Patients in Whom TTP Was Not Suspected (n = 245) . | P . |
|---|---|---|---|
| * Adapted from reference 13. These data describe the experience with allogeneic BMT at the University of Oklahoma, 1989-1998. No patients had clinically suspected TTP following autologous stem cell transplants. Clinically suspected TTP was defined by treatment with plasma exchange. TTP is used as a comprehensive diagnostic term, including syndromes with acute renal failure that may be described as HUS. | |||
| Risk factors for BMT-related complications | |||
| Severe primary disease | 29% | 13% | 0.08 |
| >1 transplant | 18% | % | 0.20 |
| Unrelated donor | 71% | 39% | 0.02 |
| HLA mismatch | 35% | 16% | 0.04 |
| Occurrence of BMT-related complications | |||
| Acute GVHD (grade III-IV) | 47% | 13% | <0.01 |
| Bacterial sepsis | 82% | 57% | 0.04 |
| Viral sepsis | 65% | 16% | <0.01 |
| Fungal sepsis | 65% | 28% | <0.01 |
| . | Patients with Clinically Suspected TTP (n = 17) . | Patients in Whom TTP Was Not Suspected (n = 245) . | P . |
|---|---|---|---|
| * Adapted from reference 13. These data describe the experience with allogeneic BMT at the University of Oklahoma, 1989-1998. No patients had clinically suspected TTP following autologous stem cell transplants. Clinically suspected TTP was defined by treatment with plasma exchange. TTP is used as a comprehensive diagnostic term, including syndromes with acute renal failure that may be described as HUS. | |||
| Risk factors for BMT-related complications | |||
| Severe primary disease | 29% | 13% | 0.08 |
| >1 transplant | 18% | % | 0.20 |
| Unrelated donor | 71% | 39% | 0.02 |
| HLA mismatch | 35% | 16% | 0.04 |
| Occurrence of BMT-related complications | |||
| Acute GVHD (grade III-IV) | 47% | 13% | <0.01 |
| Bacterial sepsis | 82% | 57% | 0.04 |
| Viral sepsis | 65% | 16% | <0.01 |
| Fungal sepsis | 65% | 28% | <0.01 |
Clinical features suggesting a diagnosis of thrombotic thrombocytopenic purpura (TTP) and consideration for plasma exchange treatment in pregnant/postpartum women.
| Adapted from reference 14. These abnormalities may all be due to severe preeclampsia/eclampsia/HELLP syndrome, and may all resolve spontaneously following delivery with only continued observation and supportive care. However, the clinical features described here suggest progressive disease and are appropriate indications for plasma exchange treatment, for presumptive TTP. |
| Hematologic abnormalities |
|
| Neurologic parameters |
|
| Renal abnormalities |
|
| Systemic abnormalities |
|
| Adapted from reference 14. These abnormalities may all be due to severe preeclampsia/eclampsia/HELLP syndrome, and may all resolve spontaneously following delivery with only continued observation and supportive care. However, the clinical features described here suggest progressive disease and are appropriate indications for plasma exchange treatment, for presumptive TTP. |
| Hematologic abnormalities |
|
| Neurologic parameters |
|
| Renal abnormalities |
|
| Systemic abnormalities |
|
Complications of plasma exchange treatment for thrombotic thrombocytopenic purpura (TTP).*
| Complications . | Percentage of Patients with Major Complications . |
|---|---|
| * Adapted from references 4 and 49. Major catheter-related complications included pneumothorax or hemothorax caused by the insertion procedure; sepsis requiring systemic antimicrobial treatment; and thrombosis requiring line replacement or anticoagulant treatment. Major plasma-related complications included hypoxemia or hypotension requiring more than oxygen administration or volume replacement and allergic reactions requiring more than Benadryl and hydrocortisone treatment. No transfusion-transmitted infections were observed. Unintentional plateletpheresis may cause persistent thrombocytopenia, that may be mistaken for continued activity of TTP. | |
| Central venous catheter-related | |
| Insertion procedure | 4% |
| Sepsis | 15% |
| Thrombosis | 10% |
| Plasma-related | |
| Allergic | 4% |
| Infection | 0 |
| Instrument-related | |
| Unintentional plateletpheresis | Unknown |
| Complications . | Percentage of Patients with Major Complications . |
|---|---|
| * Adapted from references 4 and 49. Major catheter-related complications included pneumothorax or hemothorax caused by the insertion procedure; sepsis requiring systemic antimicrobial treatment; and thrombosis requiring line replacement or anticoagulant treatment. Major plasma-related complications included hypoxemia or hypotension requiring more than oxygen administration or volume replacement and allergic reactions requiring more than Benadryl and hydrocortisone treatment. No transfusion-transmitted infections were observed. Unintentional plateletpheresis may cause persistent thrombocytopenia, that may be mistaken for continued activity of TTP. | |
| Central venous catheter-related | |
| Insertion procedure | 4% |
| Sepsis | 15% |
| Thrombosis | 10% |
| Plasma-related | |
| Allergic | 4% |
| Infection | 0 |
| Instrument-related | |
| Unintentional plateletpheresis | Unknown |
Structure of ADAMTS13 and mutations in congenital thrombotic thrombocytopenic purpura.
The ADAMTS13 gene (A) contains 29 exons in ∼ 37 kb on chromosome 9q34. Dashed lines show the relationship of exons to the ADAMTS13 protein (B). Structural domains include signal peptide (S), propeptide (P), metalloprotease, disintegrin domain (Dis), thrombospondin 1 repeats (numbered 1-8), cysteine-rich domain (Cys), spacer domain, and CUB domains. Mutations in patients with inherited TTP are shown (C).
Reproduced with permission from Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389-394.
Structure of ADAMTS13 and mutations in congenital thrombotic thrombocytopenic purpura.
The ADAMTS13 gene (A) contains 29 exons in ∼ 37 kb on chromosome 9q34. Dashed lines show the relationship of exons to the ADAMTS13 protein (B). Structural domains include signal peptide (S), propeptide (P), metalloprotease, disintegrin domain (Dis), thrombospondin 1 repeats (numbered 1-8), cysteine-rich domain (Cys), spacer domain, and CUB domains. Mutations in patients with inherited TTP are shown (C).
Reproduced with permission from Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389-394.
Activity of von Willebrand factor (VWF)-cleaving protease (ADAMTS13) in 68 patients with thrombocytopenia.
Multimer analysis of VWF substrate digested by diluted (1:20) plasma samples from patients. On each gel (A-H), a calibration curve using dilutions of pooled plasma from healthy donors (NHP; 1:20 dilution corresponding to 100%) is included. Seventeen patients with severe sepsis (gels A,B), 16 with heparin-induced thrombocytopenia (gels C,D), and 35 with thrombocytopenia due to various causes (gels E-H) were analyzed. Samples of digested VWF were applied on top of the gel.
OMF, osteomyelofibrosis; MDS, myelodysplastic syndrome; ITP, idiopathic thrombocytopenic purpura; AL, acute leukemia; SAA, severe aplastic anemia; Misc, miscellaneous causes.
Reprinted with permission from Bianchi V, et al. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–713.
Activity of von Willebrand factor (VWF)-cleaving protease (ADAMTS13) in 68 patients with thrombocytopenia.
Multimer analysis of VWF substrate digested by diluted (1:20) plasma samples from patients. On each gel (A-H), a calibration curve using dilutions of pooled plasma from healthy donors (NHP; 1:20 dilution corresponding to 100%) is included. Seventeen patients with severe sepsis (gels A,B), 16 with heparin-induced thrombocytopenia (gels C,D), and 35 with thrombocytopenia due to various causes (gels E-H) were analyzed. Samples of digested VWF were applied on top of the gel.
OMF, osteomyelofibrosis; MDS, myelodysplastic syndrome; ITP, idiopathic thrombocytopenic purpura; AL, acute leukemia; SAA, severe aplastic anemia; Misc, miscellaneous causes.
Reprinted with permission from Bianchi V, et al. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–713.
The effect of fresh frozen plasma (FFP) infusions on the platelet count in a patient with severe constitutional deficiency of von Willebrand factor (VWF)-cleaving protease.
This patient had no acute episode of thrombotic thrombocytopenic purpura (TTP) before age 20. The first TTP event was followed by several acute bouts, leading to a diagnosis of chronic relapsing TTP caused by severe VWF-cleaving protease deficiency. After the last relapse at age 36, the patient received 8 months of prophylactic treatment with 2 units of FFP every third week, and subsequently with only 1 unit of FFP every 2 weeks.
Reprinted with permission from Furlan M, Lämmle B. Best Pract Res Clin Haematol. 2001;14:437-454.
The effect of fresh frozen plasma (FFP) infusions on the platelet count in a patient with severe constitutional deficiency of von Willebrand factor (VWF)-cleaving protease.
This patient had no acute episode of thrombotic thrombocytopenic purpura (TTP) before age 20. The first TTP event was followed by several acute bouts, leading to a diagnosis of chronic relapsing TTP caused by severe VWF-cleaving protease deficiency. After the last relapse at age 36, the patient received 8 months of prophylactic treatment with 2 units of FFP every third week, and subsequently with only 1 unit of FFP every 2 weeks.
Reprinted with permission from Furlan M, Lämmle B. Best Pract Res Clin Haematol. 2001;14:437-454.