Abstract
Hematologists are increasingly involved in the diagnosis and management of patients with venous and arterial thromboembolic disorders. There have been major advances in recent years in our understanding of the central role of hypercoagulability in the pathogenesis of thrombosis. This has led to new approaches to the diagnosis of patients at risk for thrombosis and the development of more rational antithrombotic strategies.
In Section I, Dr. Andrew Schafer reviews current concepts of acquired and inherited hypercoagulable states. It is now recognized that most, if not all, patients with venous thromboembolism have a genetic basis for the disorder (“thrombophilia”). The level of lifelong, baseline hypercoagulability in any individual may be determined by the type(s) and number of thrombophilia(s) that are inherited. Clinical episodes of thrombosis are precipitated by acquired thrombogenic triggers, which may be overt (e.g., pregnancy) or subclinical.
In Section II, Dr. Mark Levine discusses the complex problem of thrombosis in patients with cancer. The goals of treating acute venous thromboembolism in cancer patients are to prevent recurrence, minimize the risk of anticoagulant-induced bleeding, and improve quality of life. New developments have improved treatment of venous thromboembolism in these patients, including outpatient therapy and secondary prevention with low-molecular-weight heparin.
In Section III, Dr. Barbara Konkle reviews the diagnosis and management of thrombotic complications associated with pregnancy and hormonal therapy. Patient management is discussed based on data on thrombotic risks associated with hormonal treatment of infertility, pregnancy and the post-partum period in women with and without underlying thrombophilic risk factors.
In Section IV, Dr. Clive Kearon discusses the management of anticoagulation before and after elective surgery. In the past, there has been no consensus on the perioperative management of anticoagulation for patients who require long-term warfarin therapy. This review considers the expected risks and benefits of different approaches to anticoagulation in patients who require warfarin because of atrial fibrillation, a mechanical heart valve, or a history of venous thromboembolism.
I. Inherited and Acquired Causes of Thrombosis
Andrew I. Schafer, MD*
University of Pennsylvania School of Medicine, Dept. of Medicine, 100 Centrex, HUP, 3400 Spruce Street, Philadelphia PA 19104
Hypercoagulability, a state of heightened activation of the coagulation system, plays a major role in the pathogenesis of venous thromboembolism (VTE). As such, the diagnosis and management of VTE is increasingly falling within the realm of the practice of hematology. With about 2 million cases annually in the United States, including an estimated annual mortality of 60,000 from pulmonary embolism (exceeding the number of deaths from breast cancer), VTE represents a major health problem.1 Hypercoagulability also likely plays some less clearly defined role in the pathogenesis of various forms of arterial thrombosis, mostly in the form of increased activation of platelets and loss of the thromboresistant properties of vascular endothelium, but these disorders are largely excluded from the present discussion.
The inherited hypercoagulable states are associated with venous rather than arterial thrombosis, but some considerations should qualify this generalization. First, arterial thromboembolism may originate from deep vein thrombi by paradoxical embolism across a patent foramen ovale (PFO).2 Since PFO is found in 31% to 77% of individuals with cryptogenic stroke,3 this presentation of VTE may be underrecognized. Second, a significant association has been recently reported between spontaneous venous thrombosis and atherosclerotic vascular disease.4 It is postulated that since atherosclerosis is characterized by activation of the coagulation system as well as platelets, the resulting systemic prothrombotic state may promote venous thrombosis. Interestingly, the use of statins reduces not only arterial complications but also the risk of VTE.5
The concept is emerging that most, if not all, cases of VTE represent a convergence of underlying genetic predisposition and acquired precipitating events. The inherited basis of VTE is supported by increasing recognition that these patients have one or more associated genetic abnormalities (“thrombophilia”). Thrombophilia is currently detected with appropriate testing in over 50% of cases following a first clinical episode of VTE. Furthermore, VTE is increasingly viewed as a chronic disease with recurrence rates of 17.5% at 2 years and 30.3% at 8 years of follow-up.6 When recurrence of lower extremity deep vein thrombosis does occur, it arises in the contralateral leg in almost half the cases, highlighting the pathophysiological importance of systemic hypercoagulability as opposed to local, anatomical factors.7 One could argue that the only reason thrombophilia is not currently detected in all cases of VTE is that our recognition of the multiple genetic causes of hypercoagulability is incomplete.
Genetic predisposition is unlikely to be the sole explanation for the occurrence of VTE. Thrombophilia represents a constant, lifelong state of hypercoagulability, yet VTE is an episodic event. In about half of all cases of VTE a clinically recognizable, acquired precipitating cause can be readily identified (e.g., pregnancy, surgery, immobilization). The remaining cases are usually labeled “idiopathic” or “spontaneous.” Again, one could argue that the only reason not all cases of VTE appear to be provoked is that many thrombogenic triggers are subclinical in nature. The notion that all cases of VTE have both genetic and acquired causes is further presented below.
The coagulation system which culminates in the formation of fibrin probably functions in a baseline state of low-level activation under normal circumstances.8 Presumably, it is thus constantly poised to instantaneously respond to injury with a burst of thrombin generation and fibrin production at the site of vascular damage. Coagulation is normally kept in check by several physiological antithrombotic mechanisms that essentially blanket the entire clotting cascade. Antithrombin inhibits thrombin as well as factors Xa and IXa. Activated protein C, along with its cofactor, protein S, exerts its anticoagulant action by inactivating factors Va and VIIIa. Protein Z inhibits factor Xa via protein Z–dependent protease inhibitor (ZPI). Tissue factor pathway inhibitor (TFPI) downregulates the tissue factor-VIIa complex. There are likely to be yet additional antithrombotic pathways that act in concert to physiologically quench fibrin generation. Finally, whatever small amount of fibrin escapes these anticoagulant mechanisms is rapidly degraded by plasmin, the major protease enzyme of the fibrinolytic system. Most (possibly all) of these systems depend on intact vascular endothelial surfaces to exert their physiological antithrombotic actions and thereby promote blood fluidity. Biochemical assays suggest that individuals with thrombophilia, in whom one or more of these anticoagulant systems is genetically deficient, have heightened baseline activation of the coagulation system even when they are clinically asymptomatic.8
Primary hypercoagulable states (thrombophilias) are due to either (a) a qualitative defect or quantitative deficiency of an antithrombotic protein, or (b) increased level of a prothrombotic clotting factor. The best-characterized and most common conditions currently recognized are listed in Table 1 . The risk of thrombosis varies for the different thrombophilias. One cohort family study found the overall incidence of VTE (per 100 patient-years) to be 1.07 for antithrombin deficiency, 0.54 for protein C deficiency, 0.50 for protein S deficiency, and 0.30 for activated protein C resistance.9 Likewise, different acquired conditions are associated with very different degrees of risk for VTE, irrespective of any underlying thrombophilia. The risk is much higher, for example, following hip or knee surgery than during pregnancy; the latter, in turn, poses a much higher risk than prolonged air travel. Furthermore, individuals who have inherited more than one thrombophilia are often at significantly greater risk of thrombosis than those with only a single genetic factor.10,11
The growing list of established thrombophilias reflects our increasing understanding of the genetic basis of thrombosis but, ironically, it is also creating increasing uncertainty about how to use this information for diagnosis and management in clinical practice. To compound the challenge in clinical decision-making, there is increasing recognition that many of these abnormalities are not “all-or-none” mutations in structural genes for the proteins but rather polymorphisms that increase their rates of transcription and translation.12 For example, the prothrombin G20210A polymorphism involves a G-to-A substitation in the 3′ untranslated region of the prothrombin gene at nucleotide 20210, leading to increased concentrations of prothrombin. A genome-wide linkage screen has identified a distinct region on chromosome 1q, genetic variability of which influences the level of free protein S in plasma.13 Polymorphisms can cause various levels of increases in coagulation factors that may be associated with corresponding gradations in risk of thrombosis. Thus, different polymorphisms may cause varyingly elevated plasma levels of factors VII, XI, IX, VIII, and von Willebrand factor, each of which has been associated with increased risk of VTE. For example, another genome-wide linkage screening has found that polymorphisms in the ABO blood group genotype are major genetic determinants of plasma levels of von Willebrand factor, increases in which are associated with thrombotic risk.14 Conversely, specific polymorphisms in the factor VII gene can lead to reduced levels of this clotting factor, with an associated decrease in risk of myocardial infarction.15 Given this increasingly daunting array of individual genetic factors that predispose to VTE, it would be highly desirable to develop simple, reproducible functional assays that measure composite thrombotic risk (i.e. level of hypercoagulability),8,16 but such assays are not presently available in clinical practice.
Individuals with inherited thrombophilias have a higher risk of VTE in the setting of acquired thrombogenic events than those without identifiable genetic defects. One study, for example, showed that the prevalence of the prothrombin-gene mutation was significantly higher in patients with venous thrombosis than in healthy controls (odds ratio of about 10). Likewise, the use of oral contraceptives was more frequent among women with thrombosis than among controls (odds ratio, 22). For women who were taking oral contraceptives and also had the prothrombin-gene mutation, the odds ratio for thrombosis rose dramatically to 150.17 Similarly, while the overall risk of VTE during pregnancy and the puerperium is about 1 in 1500, the risk of thrombosis with pregnancy is increased to 0.2% among carriers of factor V Leiden, 0.5% among carriers of the prothrombin-gene mutation, and 4.6% among carriers of both genetic defects.18
Based on these lines of evidence regarding interactions between genetic and acquired determinants of VTE, the following unifying model can be proposed. Possibly all patients with VTE have a genetic predisposition in the form of one or more thrombophilias. The level of lifelong, baseline hypercoagulability in any individual may be determined by the type(s) and number of thrombophilia(s) that are inherited. Likewise, it is possible that all episodes of VTE are precipitated by acquired thrombogenic triggers. Thus, in an individual with a relatively low level of baseline genetic hypercoagulability (e.g., a single mutation that is associated with a low risk of thrombosis, such as factor V Leiden), a relatively strong acquired thrombogenic event (e.g., pregnancy) would be required to provoke an episode of VTE. Thus, the precipitating event in such individuals is often clinically overt. In most cases, such thrombophilic individuals never suffer VTE throughout their lifetimes, and when they do have an episode it is unlikely to recur. In contrast, an individual with a high level of baseline genetic hypercoagulability (with multiple thrombophilic mutations or polymorphisms) is at such high risk that relatively minor acquired triggers can initiate a thrombotic episode. These triggers are therefore often subclinical, giving the appearance that the patient has had “idiopathic,” “spontaneous,” or “unprovoked” VTE. Furthermore, VTE in these high-risk individuals is more likely to recur. This concept of “thrombosis threshold” based on level of inherited hypercoagulability will have to be further refined as we increase our understanding of risk stratification for thrombosis based on genetic and acquired determinants.
II. Thrombosis in Patients with Cancer
Mark N. Levine, MD*
Departments of Clinical Epidemiology & Biostatistics, and Medicine, McMaster University; Cancer Care Ontario Hamilton Regional Cancer Centre, Room 9, 90 Wing, Main Level, 711 Concession Street, Hamilton, Ontario, L8V 1C3, Canada.
Dr. Levine is the Buffett Taylor Chair in Breast Cancer Research, McMaster University.
In 1865 Professor Armand Trousseau first reported the association between cancer and thrombosis.1 The pathogenic mechanisms of thrombosis in the cancer patient involve a complex interaction between the tumor cell, the patient, and the hemostatic system. Tumors, through expression of tissue factor, can activate coagulation.2 Furthermore, local peritumoral activation of coagulation may have important effects on the biology of cancer.2 In recent years there have been many new developments in understanding basic mechanisms and optimizing clinical care of venous thromboembolism (VTE) in the cancer patient. This article focuses on the treatment of VTE in cancer patients.
Treatment of cancer patients with VTE is difficult because these patients have an increased risk of both recurrent VTE and anticoagulant-induced bleeding compared with noncancer patients.3 In addition, many cancer patients have a compromised quality of life that is further compromised by the occurrence of thrombosis. In some instances of end-stage cancer, there is the difficult decision of whether one should even treat the acute thrombotic event.
Initial Treatment of Venous Thromboembolism
Based on the results of numerous randomized controlled trials, low-molecular-weight heparin (LMWH) has replaced unfractionated heparin (UFH) as the first-line treatment in the majority of patients with acute deep vein thrombosis (DVT). Large meta-analyses of these clinical trials have shown that weight-adjusted subcutaneous LMWH is safer and probably more effective than UFH administered by continuous intravenous (IV) infusion and monitored by the activated partial thromboplastin time (aPTT).4– 7
Despite the observed efficacy and safety of LMWH in these trials, it should be noted that only about 20% of patients in these studies had cancer. Nonetheless, it would seem reasonable to generalize the results of these trials to cancer patients with acute VTE. In terms of optimizing treatment, the use of LMWH avoids IV administration of anticoagulant therapy and the need for laboratory monitoring, thereby improving the quality of life of the patient.
There have been three clinical trials that demonstrated that patients with acute proximal DVT could be treated safely at home with subcutaneous LMWH without hospital admission.8–,10 In these trials, some of the patients were treated entirely at home and some were admitted to the hospital for a short while and then discharged early. Additional cohort studies have shown that about 80% of unselected outpatients with newly diagnosed DVT could be treated entirely at home, and up to 50% of these patients had cancer.11,12 Hence, use of LMWH at home in the cancer patient with acute VTE is recommended because of the substantial positive impact on quality of life. Clearly, some patients with acute VTE will require hospitalization because of symptoms and other complications related to their cancer. If patients are to be treated at home, they must be reliable and compliant and have a good support system.
There are relatively few trials that have compared LMWH with UFH in patients with acute pulmonary embolism (PE). Simonneau et al compared the LMWH tinzaparin with IV UFH in hospitalized patients with PE, and no difference was detected in recurrent VTE and bleeding between treatment groups.13 In the trial performed by the Columbus Investigators, which found no difference in these outcomes between the LMWH reviparin and UFH, the majority of patients were treated at home and 27% of all patients had PE.10 In these two trials, 10% and 23% of patients had cancer, respectively. Finally, in a prospective cohort study, Kovacs et al treated 108 patients with PE as outpatients with the LMWH dalteparin; 22% had cancer.14 The rate of recurrent thrombosis was 5.6%, and major bleeding occurred in 2.9% of the patients. Hence, based on this evidence and the large experience with LMWH in DVT, it seems reasonable to manage acute PE patients who are hemodynamically stable by treating them with outpatient LMWH. However, in patients with acute PE who are hemodynamically unstable, the use of intravenous UFH should be considered because such patients were excluded from the clinical trials that compared LMWH with UFH.
The use of inferior vena caval (IVC) filters reduces the short-term risk of PE but is associated with an increased long-term risk of recurrent DVT, despite concurrent oral anticoagulant therapy. In a large randomized trial conducted in France, in which patients with proximal DVT were treated with anticoagulant therapy and randomized to receive an IVC filter or not, there was a statistically significant reduction in PE during the first 2 weeks of treatment.15 By 1 year, however, there was a statistically significant increase in recurrent DVT in patients with a filter. This was likely a result of thrombosis that developed around and proximal to the filter. Thus, the use of an IVC filter in a cancer patient presenting with acute VTE is not recommended. Filters should be reserved for cancer patients who are actively bleeding and cannot receive anticoagulant therapy and for patients who develop multiple episodes of recurrent thromboembolism despite therapeutic LMWH.
There are recent reports on a new type of IVC filter, the Gunther Tulip Retrievable Vena Caval Filter, that could potentially be useful in a cancer patient who presents with acute VTE and is actively bleeding.16 In such patients, a filter can be inserted and then removed within 7 to 10 days if the bleeding has stopped and is well controlled. This would avoid the long-term potential complications of IVC filters. However, the results of additional studies on cancer patients are required.
Long-Term Anticoagulant Therapy
Long-term anticoagulant therapy using coumarin derivatives is required to prevent recurrent thrombosis. An oral anticoagulant such as warfarin is commenced on the first or second day of heparin treatment, and the aim is to achieve an International Normalized Ratio (INR) of between 2.0 and 3.0. Warfarin therapy is particularly complicated in the cancer patient for a number of reasons. It is often difficult to maintain the INR within the therapeutic range because cancer patients suffer from anorexia and vomiting. They may have chronic disseminated intravascular coagulation or extensive hepatic metastases. In addition, drug interactions (e.g., chemotherapy and antibiotics) can influence the anticoagulant effect of vitamin K–dependent anticoagulants. Often it is necessary to frequently interrupt oral anticoagulant therapy because of thrombocytopenia and procedures such as thoracentesis and abdominal paracentesis. Finally, frequent blood sampling is required for the INR, and accessing veins can often be difficult in the cancer patient.
There are certain features of long-term anticoagulant therapy with LMWH that are attractive in the cancer patient. LMWH does not require laboratory monitoring and can be administered once or twice daily, subcutaneously, based on body weight. There is the clinical experience that LMWH can be effective in patients who develop recurrent thrombosis despite therapeutic warfarin therapy.17 Finally, based on preclinical data and meta-analyses, there is the potential for less bleeding. There have been a number of trials that have compared long-term oral anticoagulant therapy with long-term LMWH.18– 23 These trials were relatively small and had very few cancer patients. No definitive conclusions can be drawn from these trials concerning long-term treatment with LMWH in the cancer patient.
Several recent randomized trials, however, have provided new information concerning the long-term treatment of cancer patients with VTE. In the trial reported by Meyer et al, cancer patients with acute VTE were randomized to 3 months of enoxaparin or warfarin at a targeted INR of 2.0 to 3.0.24 The primary outcome measure was a composite outcome consisting of major bleeding and recurrent VTE. In the 71 patients who received warfarin, the outcome event rate was 21% compared with 10.5% in the 67 patients who received LMWH, P = .09. This observed difference was mainly as a result of the rates of major bleeding in the 2 groups; 16.9% in warfarin patients versus 7.5% in LMWH patients. Recently, Lee et al reported the results of the CLOT trial in which cancer patients with acute VTE and/or PE were randomized to long-term dalteparin versus long-term oral anticoagulant therapy.25 Over the 6-month study period, 27 of 336 patients in the dalteparin group compared with 53 of 336 patients in the oral anticoagulant group experienced recurrent VTE. The probability of VTE at 6 months was reduced from 17.4% in the oral anticoagulant group to 8.8% in the dalteparin group, hazard ratio 0.48, P = .0017. No statistically significant difference was detected in major bleeding between groups, which occurred in 3.6% and 5.6%, respectively. Finally, in a subgroup analysis of a trial that compared long-term tinzaparin LMWH with oral anticoagulant therapy, both administered for 3 months, there was a statistically significant reduction in recurrent VTE in the subgroup of cancer patients.26 Based on the results of these trials, long-term therapy with LMWH is an important advance in the management of cancer patients with acute VTE. It substantially reduces the rate of recurrent VTE without an increase in bleeding, thereby improving the quality of life of the cancer patient.
Duration of Long-Term Treatment
Clinical trials have shown that longer-duration oral anticoagulant therapy is associated with lower rates of recurrent thromboembolism compared with shorter-duration treatment.27–,29 However, it appears that once oral anticoagulant therapy was stopped in the patients treated for more time, the rates of recurrence increased and approached that of the shorter arms.29,30 There is evolving consensus that the duration of antithrombotic treatment should be tailored to the patient’s risk of recurrent thrombosis and bleeding.31 Patients with active malignant disease have an ongoing thrombotic stimulus, and recurrent VTE has a major impact on a patient’s quality of life. Hence, anticoagulant therapy should be continued for as long as the cancer is active. In patients with metastatic disease, anticoagulant therapy should be continued indefinitely or until a contraindication to therapy develops. In patients with nonmetastatic disease, treatment should be for at least 6 months or for as long as the patient is on chemotherapy or hormonal therapy.
Catheter-Related Thrombosis
Long-term indwelling central venous catheters are commonly used in cancer patients. One of the complications related to central venous catheters is catheter-associated venous thrombosis. This can involve the catheter tip (ball-valve clot), the length of the catheter (fibrin sheath), the catheterized vessel in the upper limb, the central vasculature of the neck or mediastinum, or a combination of these sites. The incidence of catheter-related thrombosis varies among studies.32 This variation is due to a number of factors, including the retrospective nature and small size of most of the studies, differences in the populations studied, and variation in the diagnostic tests used to detect the thrombosis.
Four randomized studies have been performed to evaluate the safety and efficacy of prophylactic anticoagulation in patients with central venous catheters.33–,36 Two of the older randomized trials suggested that the rate of thrombosis was relatively high and that prophylaxis reduced the rate of thrombosis.33,34 Bern et al randomized 84 cancer patients to receive 1 mg of warfarin daily or no treatment. Venography was performed at 90 days or sooner if patients had symptoms suggestive of thrombosis.33 The rate of thrombosis was 37% in the control group compared with 10% in the warfarin group. In the study by Monreal et al, cancer patients with central venous catheters were randomized to dalteparin 2500 IU subcutaneously once daily or no prophylaxis.34 Venography was performed at 90 days. One (6%) of the 16 LMWH patients developed thrombosis compared with 8 of 13 (62%) patients in the control group. Two more recent randomized trials reported low rates of thrombosis and no effect of prophylactic anticoagulant therapy.35,36 Couban et al demonstrated no difference between 1 mg of warfarin and placebo in 255 patients.35 The risk of symptomatic catheter-associated thrombosis was approximately 4% in both groups. Reichart et al randomized 425 cancer patients with central venous catheters to 16 weeks of dalteparin or placebo.36 Venography or ultrasound was performed at 16 weeks. The rate of the catheter-associated thrombosis was very low in both groups, approximately 3%. The reasons for the much lower rates of catheter-related thrombosis reported in recent trials are uncertain, but improved catheter material and advances in insertion techniques may contribute. Currently, it is not clear whether cancer patients with central venous catheters should receive antithrombotic prophylaxis. Additional randomized trials are required.
Treatment of central venous catheter–related thrombosis remains a controversial and poorly studied area. Currently, cancer patients with symptomatic central venous catheter–related thrombosis are treated with anticoagulant therapy—that is, initial LMWH (or UFH) followed by long-term oral anticoagulant therapy. Routine removal of the catheter when there is a catheter-associated thrombosis is a controversial subject. However, if the patient has a continued need for central venous access, a functioning catheter does not have to be removed. Often, routine removal of the catheter is not practical because reinsertion of another catheter is difficult and can be associated with considerable morbidity.
Conclusion
There have been many advances in the management of cancer patients with acute VTE. Subcutaneous LMWH has replaced intravenous UFH for the initial treatment of VTE, and in many instances patients can be treated at home. The use of long-term LMWH instead of oral anticoagulants can substantially reduce the risk of recurrent VTE in this high-risk group of patients without increased bleeding. A number of novel agents that target specific coagulation proteases are currently undergoing investigation for both the prevention and the treatment of VTE.37 Such agents could potentially improve thrombosis management in cancer patients.
III. Thrombosis: Diagnosis and Management Issues Before, During, and After Pregnancy
Barbara A. Konkle, MD*
Associate Professor of Medicine; Director, Penn Comprehensive Hemophilia and Thrombosis Program; University of Pennsylvania School of Medicine, Presbyterian Medical Center, MAB 103, 39th and Market Streets, Philadelphia, PA 19104
“Milk leg – a painful swelling of the leg soon after childbirth, due to thrombosis of the large veins, sometimes called phlebitis.” Thrombosis has long been recognized as a risk of pregnancy that results in morbidity and mortality in this generally healthy age group. In women with inherited and/or other acquired thrombophilias there is a complex interplay of factors resulting in the potential outcome of VTE and its sequelae.
Women referred to hematologists for thrombosis-related questions often present complex clinical scenarios. To provide our recommendations to the patient and her referring physicians we must use the data we have available to assess the risk/benefit ratio of thrombosis and treatment in that individual patient. In this section, using a patient case presentation, we will review the currently available data on which to make our recommendation.
Patient Presentation
A 31-year-old female is referred for consultation by her obstetrician. She is early in her third pregnancy. She has a history of polycystic ovarian disease and required hormonal ovarian stimulation for conception of her first 2 children, but not for the current pregnancy. Two years earlier, during her last pregnancy, she developed right arm and neck swelling 7 weeks after insemination and she was diagnosed with a right jugular, innominate and subclavian vein thrombosis. She was treated with heparin during the pregnancy, followed by warfarin post-partum. A thrombophilia evaluation demonstrated heterozygosity for prothrombin G20210A. She now presents for advice on management of this pregnancy.
Assisted Reproductive Treatment and Thrombotic Risk
Thrombosis has been described as a risk with these treatments, particularly in association with ovarian stimulation. Thrombosis is more common in the setting of ovarian hyperstimulation syndrome (OHSS) and following a treatment cycle that results in pregnancy. In a review of 54 cases reported in the literature, 66% were associated with OHSS and 84% with a pregnancy cycle.1 Seventy-five percent of patients had venous thrombosis, and surprisingly 60% of these were in the upper limb, neck, and head veins. Most were not associated with venous catheters. Although there may be some reporting bias in publishing cases of upper extremity thrombosis given its rarity, these reports suggest that upper extremity thrombosis is not an uncommon site of thrombosis in this clinical situation. Twenty-five percent of patients in this report suffered arterial thromboses, most commonly intracerebral.
Why these individuals develop thrombosis is not clear. The hormonal stimulation used typically results in estradiol levels up to 10 times the normal levels.2 OHSS can be manifest by significant hemodilution associated with the development of pleural effusions and ascites and compression of pelvic veins from marked ovarian enlargement. Coagulation studies have been limited but generally show increased coagulation factors, particularly fibrinogen and factor VIII, and decreased fibrinolysis.2 OHSS occurs in 2%–6% of in vitro fertilization treatment cycles.3 Polycystic ovaries are a risk factor for OHSS. When thrombosis occurs, it typically does so between 7 and 10 weeks of gestation,3 findings remarkably similar to our patient’s presentation.
Underlying thrombophilias generally have not been detected in these women, although most patients reported were not tested for the common thrombophilias, including factor FV Leiden and prothrombin G20210A. However, of 54 cases reviewed by Stewart et al,1 6 had a history of prior thrombosis and 2 had a strong family history of thrombosis. Given the risks, it seems prudent to institute prophylaxis in a woman with a documented thrombophilia or a history of prior thrombosis who is to receive assisted reproductive treatment, although the optimal drug and dosing regimen is unknown.
Thrombosis and Pregnancy
Pregnancy and the first 6–8 weeks postpartum carry with them a 5- to 6-fold increased risk of thrombosis, occurring in approximately 1/1500 pregnancies.4,5 Risk factors that further increase a woman’s likelihood of thrombosis have been identified and are listed in Table 2 .6 Most studies have found the risk in the postpartum period to be relatively greater than antepartum. The risk is higher after cesarean section than after a vaginal delivery, including a 10-fold higher risk of fatal pulmonary embolism (PE).7 Deep vein thrombosis (DVT) usually occurs in the left leg (~90%) and involves the iliac system more commonly than in the nonpregnant individual.8 Presenting symptoms of iliac vein thrombosis may include left lower back and flank pain and asymmetric lower extremity swelling, requiring a high index of suspicion in this clinical situation.
Women with a prior history of thrombosis or with thrombophilias, present therapeutic challenges during pregnancy. While there remain many unanswered questions regarding management of the thrombophilic individual during pregnancy, there have been some recent studies to help guide us.
Risk of VTE in women with a prior history of thrombosis
Two recent studies provide data to help address this question. Brill-Edwards and colleagues9 prospectively studied 125 pregnant women with a single previous episode of VTE. Women were not anticoagulated antepartum, but were given 4–6 weeks of warfarin postpartum. With this approach there were no recurrences in the 44 women who had a history of a precipitated DVT and no identifiable thrombophilia, while 3 (5.9%) of the 59 women who had an idiopathic DVT and/or thrombophilia had an antepartum recurrence during the pregnancy under study. The overall risk of recurrent VTE was 2.4%, but with wide confidence intervals (0.2–6.9) because of the small number of women studied. The authors concluded that women with a history of VTE that was associated with a temporary risk factor (including pregnancy) and did not have an identifiable thrombophilia do not need to receive antepartum heparin prophylaxis. In those who suffered their previous episode of VTE without a temporary risk factor and/or those who had an associated thrombophilia, antepartum prophylaxis should be considered. However, they recommended postpartum oral anticoagulation in all women with a previous history of VTE.
In a retrospective study, Pabinger et al10 examined recurrence rates in 109 women who had at least one pregnancy after an episode of VTE. They reported a recurrence rate of 10.9% during versus 2.7% outside of pregnancy per 100 patient-years, a relative risk of 3.5 (95% CI 1.6–7.8). Seven of the 8 women with recurrence during pregnancy versus 2/35 outside of pregnancy had their prior DVT on oral contraceptives, suggesting a strong hormonal influence. Four women with antepartum recurrences were heterozygotes for factor V Leiden. These data support antepartum anticoagulation in women with an identified thrombophilia and in women whose prior DVT was precipitated by hormonal therapy. The strong hormonal effect they observed raises questions concerning withholding antepartum anticoagulation in women with a prior pregnancy-associated VTE, such as suggested by the Brill-Edwards et al study. Clearly, larger studies are needed in this area.
Thrombophilia and VTE
Thrombophilia appears to increase the risk of VTE in pregnancy. Conard et al provide a recent comprehensive review of this topic.11 In 2 case control studies of pregnancy-related VTE, one study evaluating 42 women and the other 119,12,13 the relative risks of VTE in women heterozygous for factor V Leiden were 16.3 (CI 4.8–54.9) and 9.3 (CI 5.1–16.9) and for prothrombin G20210A were 10.2 (CI 4–25.9) and 15.2 (CI 4.2–52.6). Using the data from the Gerhardt et al study,5 carriers of factor V Leiden or prothrombin G20210A have a risk of pregnancy related VTE of 0.2 and 0.5%, respectively. Eleven of the 119 women in their study were heterozygotes for both factor V Leiden and prothrombin G20210A, a condition not found in the control population. Based on an estimated frequency of combined defects of 0.10%, they calculated an odds ratio of pregnancy-related VTE in this group at 107 and the risk of thrombosis in pregnancy of 4.6%.
Several studies have reported a low incidence of DVT in asymptomatic affected relatives with factor V Leiden or prothrombin G20201A.13,14 Martinelli et al15 recently reported a study evaluating inherited thrombophilias (factor V Leiden and prothrombin G20201A) and the risk of first VTE during pregnancy and the puerperium. They studied 119 women who had a first episode of pregnancy-related VTE compared to 232 healthy controls who had at least 1 pregnancy without VTE. In their study, 57% of the thrombotic events were postpartum. The relative risks of VTE were 10.6 (95% CI, 5.6–20.4) and 2.9 (95% CI, 1.0–8.6) for factor V Leiden and prothrombin G20210A, respectively. Assuming a risk of VTE of 1/1000 pregnancies, the recurrence rate during a pregnancy would be 1.1% for factor V Leiden and 0.3% for prothrombin G20210A. These studies support the practice of withholding anticoagulation in individuals without a history of DVT, but with these mild thrombophilic risk factors.
Deficiencies of protein C, protein S and antithrombin (AT) are uncommon and laboratory assays for these used in large population studies problematic, and thus the true incidence of pregnancy-associated VTE in women with inherited deficiencies of these factors is difficult to determine (reviewed in Conard et al11). Studies of propositi and their relatives vary widely in the reported frequency of thrombosis, and not all of the thromboses were objectively confirmed. Familial AT deficiency appears to carry the highest risk of thrombosis (40% in one study),16 and this is generally accepted as an indication for anticoagulation during pregnancy in a woman without a history of prior thrombosis.
Diagnosis of VTE during pregnancy
The diagnosis of VTE in the pregnant woman has recently been addressed in detail by Bates and Ginsberg.17 In pregnant women presenting with lower extremity edema, back pain, and/or chest pain, the prevalence of VTE is less than in the general population because of the frequency of these complaints in the pregnant woman. D-dimer assays, which can be used to exclude VTE in healthy nonpregnant individuals, commonly become positive late in pregnancy, decreasing the utility of this assay in pregnancy.18 Also, radiologic studies used to diagnose VTE in the nonpregnant individual have not been validated in pregnancy, and potential risks to the fetus, particularly in terms of ionizing radiation exposure, need to be considered.19 Compression ultrasonography (CUS) of the proximal veins has been recommended as the initial test for suspected DVT during pregnancy.17 When results are equivocal or an iliac vein thrombosis is suspected, magnetic resonance venography (MRV) can be used. MRV does not carry the radiation risk of contrast venography and is becoming increasingly available in the US. The approach to the diagnosis of PE is similar in the pregnant and nonpregnant individual. V/Q scanning gives relatively low radiation exposure to the fetus, a risk less than that of missing a diagnosis of PE in the mother. With indeterminate V/Q studies in a woman without demonstrated lower extremity thrombosis, angiography with a brachial approach carries less radiation exposure to the fetus than spiral computed tomography (CT).
Anticoagulation During Pregnancy
The optimal anticoagulation regimen has not been established by clinical study. Low molecular weight heparins (LMWH) have generally become the anticoagulant of choice because, like unfractionated heparin (UFH), they do not cross the placenta, but they have better bioavailability and carry less risk of osteoporosis and heparin-induced thrombocytopenia than UFH (reviewed in Bates and Ginsberg17). A recent review of published data on the use of LMWH in pregnancy supports their use as safe alternatives to UFH as anticoagulants during pregnancy.20
A common practice in the United States is to switch patients to UFH near delivery to allow use of the aPTT at presentation in labor to assess anticoagulation. Since the effects of UFH may be prolonged in labor, it is important that the aPTT be determined in women who have received UFH. A small study found an anticoagulant effect of subcutaneous UFH for up to 28 hours in women in labor.21
A difficult situation is anticoagulation in pregnant women with mechanical heart valves. This issue was recently addressed by Ginsberg et al.22 Based on reports of thrombosis in women on enoxaparin, Aventis Pharmaceuticals issued a “warning” that enoxaparin should not be used in patients with prosthetic heart valves and a “precaution” about potential teratogenicity. The incidence of congenital anomalies in infants born to women who receive enoxaparin has not been established to be higher than that in the general population, and, since LMWH does not cross the placenta, teratogenicity seems unlikely. That the risk of thromboembolic complications is less with UFH is also not clear. Warfarin is the drug of choice for nonpregnant women with mechanical heart valves, but carries the risk of warfarin embryopathy for exposures in weeks 6–12, and also crosses the placenta and may be associated with bleeding risks. A recent review of published literature on warfarin embryopathy suggested that this complication is much less frequent than usually cited.23 Thus the chosen anticoagulant in these patients requires patient/physician consultation to determine the optimal choice for each patient.
Optimal dosing of LMWH in pregnancy has not been evaluated by clinical study. Recommendations for using different regimens have been published.11,17 In patients with VTE during pregnancy or with a high risk of recurrent VTE, therapeutic anticoagulation is given. Many practitioners check peak anti-Xa levels during the pregnancy to maintain peak levels of 0.5–1.2 U/mL, and dosing is usually on a twice daily regimen. The use of fondiparinux, which has a longer half-life, has not been reported in pregnancy. A number of regimens have also been proposed for prophylaxis of DVT during pregnancy and the postpartum period.11,17 Heparins and warfarin can be used during breast feeding. Laboratory changes in hemostatic factors that occur during pregnancy will have returned to baseline by 6–8 weeks,24 and prophylaxis for VTE is usually stopped at that time. Patients who suffer a VTE during pregnancy should be anticoagulated at least through 8 weeks postpartum, with the total length of anticoagulation as for nonpregnant individuals, depending on the site of thrombosis and clinical situation.
Because of the relative increased risk of cesarean section deliveries, prophylaxis for this surgical procedure warrants discussion. Risk factors associated with DVT in this setting have been identified and are shown Table 3 .25 A recent review of obstetrical practices at two large institutions documented the low use of prophylaxis, even in women at relatively high risk of VTE.26 Attention to institution of appropriate prophylaxis in this setting would likely reduce the incidence of fatal VTE, which remains the most common cause of death in this population.
The patient presented at the beginning of this section was anticoagulated with LMWH during the pregnancy, switched to UFH near term, then placed on warfarin for 8 weeks postpartum. Because her thrombosis was precipitated by hormonal therapy and her thrombophilia (heterozygosity for prothrombin G20210A mutation) is not an indication for long-term anticoagulation, the warfarin was discontinued after that time.
IV. Management of Anticoagulation Before and After Elective Surgery
Clive Kearon, MB, MRCP(I), FRCP(C), PhD*
McMaster University and Henderson Research Centre, Room 39, 70 Wing, 711 Concession St, Hamilton, ON L8V 1C3, Canada
Long-term anticoagulation presents a problem when the need for surgery arises because anticoagulation is associated with bleeding from the operative site, patients have an increased risk of thromboembolism when therapy is interrupted, and warfarin’s antithrombotic effect takes days to recede after it is stopped and a similar length of time to re-establish after it is restarted. There is uncertainty about the optimal perioperative management of anticoagulation for patients who have been receiving oral anticoagulant therapy. Rational decisions can be made only if one can quantify the risks of thrombosis and bleeding that are associated with different approaches to management (Table 4 ). The risk of thromboembolism and associated morbidity depends largely on (1) the indication for anticoagulation (i.e., prevention of venous or arterial thrombosis) and (2) the likelihood (absolute risk) that an individual will have a thrombotic event. The latter is largely determined by the prevalence of chronic risk factors for thromboembolism and whether or not surgery increases the risk of postoperative thromboembolism.1 The risk of anticoagulant-induced bleeding is generally low preoperatively, but is high during and shortly after major surgery.
Based on an individual assessment of risk factors for arterial or venous thrombosis and the risk of postoperative bleeding, this review will outline an approach to the perioperative management of anticoagulation that is designed to optimize patient safety and efficient health care delivery. The risk of venous and arterial thromboembolism associated with different conditions and the relative risk reduction for thromboembolism achieved by anticoagulation are summarized in Table 5 .1 Derivation of these estimates and an analysis of the risks and benefits of using supplementary intravenous UFH as bridging therapy while oral anticoagulation is subtherapeutic before and after surgery have been detailed elsewhere.1
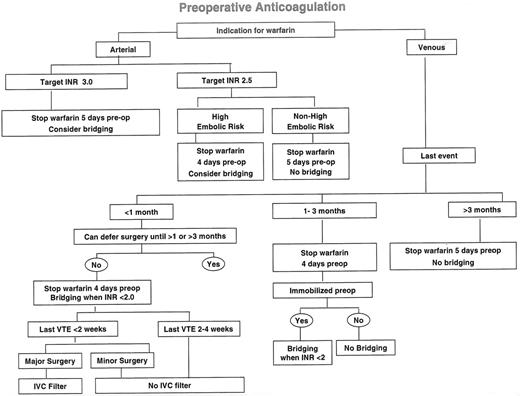
In order to assess the risks associated with temporarily stopping anticoagulants, the consequences, as well as the absolute risk, of thromboembolic events need to be considered. Arterial thromboembolism often results in death (about 40% of events) or major disability (about 20% of events),1 whereas recurrent venous thromboembolism rarely presents as sudden death (about 6% of cases),2 and major permanent disability due to venous thromboembolism is also unusual (estimated at less than 5% of events) in treated patients. It is, therefore, logical to consider patients whose indication for long-term anticoagulation is the prevention of arterial thromboembolism separately from those whose indication is the prevention of venous thrombosis (Figure 1 ). Similarly, as the risk of thromboembolism and bleeding are often influenced by the surgical procedure, it is helpful to consider anticoagulant management separately for the preoperative and postoperative periods.
Preoperative Management of Anticoagulation
Before proposing how patients with different indications for long-term anticoagulant therapy should be managed before surgery, approaches to the delivery of preoperative “bridging therapy” will be described. In this review, the term “bridging therapy” refers to the use of therapeutic-dose UFH or LMWH and does not include lower doses of UFH and LMWH that are used to prevent venous thromboembolism.3
Bridging therapy
Although the practice has not been evaluated by randomized trials, it is generally recommended that patients with the highest risk of arterial or venous thromboembolism who require interruption of oral anticoagulant therapy for surgery should receive therapeutic-dose heparin therapy (UFH or LMWH) during much of the interval when the INR is subtherapeutic.4–,7 As the INR does not start to fall until ~29 hours after a dose of warfarin, and then decreases with a half-life of ~22 hours, if bridging therapy is used preoperatively it is reasonable to start it ~60 hours after the last dose of warfarin (i.e., third morning after last evening dose).8–,10 In the past, this generally necessitated admission to hospital to receive intravenous UFH, which was stopped ~6 hours before surgery.1
A popular alternative to intravenous UFH is to use LMWH, administered subcutaneously as an outpatient, for bridging therapy.4–,7,10–,12 With this approach, doses of LMWH that are recommended for treatment of venous thromboembolism are administered once9,10 or twice11,12 daily, generally for 3 days before surgery. In order to avoid persistence of heparin during surgery, it is suggested that the last dose of LMWH should be given no less than 18 hours preoperatively with a twice-daily regimen (i.e., ~100 U/kg of LMWH); 30 hours preoperatively with a once-daily regimen (i.e., ~150–200 U/kg of LMWH); and that an additional 6-hour interval between the last dose of LMWH and surgery may be appropriate if neuraxial anesthesia is planned.13,14 Use of bridging therapy after surgery will be considered in a subsequent section.
Arterial thromboembolism
Prophylaxis of arterial thromboembolism is most commonly undertaken in patients with atrial fibrillation and/or valvular heart disease (native or prosthetic). Patients with non-valvular atrial fibrillation have an average risk of systemic embolism of about 4.5% per year in the absence of antithrombotic therapy.15 In individual patients, this risk varies from about 1% to 20%, depending on the prevalence of risk factors (e.g., previous embolism; hypertension; age ≥ 75 years; left ventricular dysfunction; diabetes; mitral stenosis).15–,18 The average annual rate of major thromboembolism in non-anticoagulated patients with mechanical heart valves is estimated to be 8%, with the risk in individuals also varying widely according to the prevalence of risk factors (e.g., caged ball or disc valves; mitral position; atrial fibrillation; previous embolism; age ≥ 70 years).19– 22
Previous thromboembolism is the single most important risk factor for stroke in patients with atrial fibrillation15,17,18,23 and it is also an important risk factor in patients with prosthetic heart valves.21,22 Consequently, the period of subtherapeutic oral anticoagulation should be kept to a minimum in patients with previous embolism and in others who are at highest risk for embolism (see below) (Figure 1). In patients whose INR is 2.0–3.0 (i.e., target INR of 2.5), it takes about 4 days for the INR to spontaneously fall to 1.5 or less in most (i.e., 95%) patients,8,9 an intensity of anticoagulation that is not expected to be associated with an increase in intraoperative bleeding.8,24– 28 If the INR is 2.5 to 3.5 (i.e., target INR of 3.0), this is expected to take 5 days. Therefore, in patients with a higher than average risk of embolism, such as those with a previous episode, 4 (target INR of 2.5) or 5 (target INR of 3.0) daily doses of warfarin should be withheld preoperatively, and the INR should be measured the day before surgery to determine if it has decreased adequately.
If the INR is 1.6 or 1.7 the morning before surgery we generally give 1 mg vitamin K orally (if the INR is 1.8 or higher we generally give 2 mg) to accelerate the reversal of anticoagulation and then repeat the INR the day of surgery.29 If necessary, plasma can be given the morning of surgery if the INR is still not acceptable to the surgeon; however, administration of blood products should be avoided for elective surgery. Checking the INR on the morning of the day before surgery may also provide a convenient opportunity to administer a single dose of therapeutic-dose LMWH if the INR is 1.8 or less, without embarking on the more complex task of providing 3 days of outpatient bridging therapy.
Highest risk patients:
Although the efficacy of therapeutic dose UFH and LMWH at preventing cardioembolism is uncertain (see discussion), most authorities recommend use of bridging therapy for patients with the highest risk of embolism.4–,7,30,31 With mechanical heart valves, this would include those for which a higher (i.e., target INR 3.0; range 2.5 to 3.5) intensity of anticoagulation is recommended (e.g., tilting disc and bileaflet mitral valves; bileaflet aortic valves with atrial fibrillation; caged ball or caged disc valves; any valve with previous embolism).22 With atrial fibrillation, this could include those with a history of embolism or multiple risk factors.15,17,18,23 However, as current evidence suggests that neither therapeutic dose UFH nor LMWH is very effective at preventing stroke in patients with atrial fibrillation32,33 (see discussion), I rarely use bridging therapy in such patients.
Patient and physician preference:
After a discussion of the risk and benefits of bridging therapy in patients with mechanical heart valve or atrial fibrillation, some patients express a preference to either receive or not receive such therapy. Their decision may be influenced by previous experience with bridging therapy, aversion to subcutaneous injections (self-administered or by another), fear of stroke, or cost implications. Similarly, referring physicians (e.g., cardiologists, cardiac surgeons) may have a strong preference that their patient receive bridging therapy. As preoperative bridging therapy may reduce embolism and is unlikely to cause bleeding, I do not discourage its use, particularly in patients with prosthetic heart valves where there is less evidence challenging the efficacy of bridging therapy than there is for those with atrial fibrillation.32– 34
Venous thromboembolism
Venous indications for long-term anticoagulation are usually the prevention of recurrent venous thromboembolism, a risk which declines rapidly during the 3 months after an acute episode.35,36 It is estimated that stopping anticoagulation within 1 month of an acute event is associated with a very high risk of recurrent venous thromboembolism (i.e., 40% over a 1-month period) (Table 5 ) and that this risk is intermediate if anticoagulants are stopped during the second and third months of treatment (i.e., 10% over a 2-month period1).
If feasible, surgery should be deferred following an acute episode of venous thromboembolism until patients have received at least 1 month, and preferably 3 months, of anticoagulation (Figure 1 ). If this is not feasible and surgery is performed within 1 month of an acute event, bridging therapy should be used while the INR is less than 2.0. If it is necessary to perform surgery within 2 weeks of an acute episode of venous thromboembolism, the risk of pulmonary embolism is probably acceptable if bridging therapy is withheld for 18 hours or less (e.g., with intravenous UFH, 6 hours preoperatively and 12 hours postoperatively) and the duration of surgery is short (e.g., 1 hour, or less). Consequently, patients who do not have major surgery and do not have a high risk of postoperative bleeding can be managed with bridging therapy. However, patients who have major surgery within 2 weeks of an acute episode of proximal deep vein thrombosis or pulmonary embolism should have a vena caval filter inserted preoperatively or intraoperatively.1
If the most recent episode of venous thromboembolism was between 1 and 3 months previously, warfarin should only be withheld for 4 doses to minimize the period of thrombotic risk; however, unless patients are immobilized (i.e., already hospitalized) neither bridging therapy nor prophylactic doses of UFH or LMWH are necessary preoperatively. As previously described, the INR of outpatients can be checked the day before surgery and, depending on its value, a single dose of oral vitamin K or subcutaneous LMWH can be considered at that time.
Greater than 3, or 6, months of anticoagulation is usually reserved for patients with multiple episodes of venous thromboembolism or a single episode of thrombosis which was not provoked by a temporary risk factor such as recent surgery. The latter group of patients may have had idiopathic venous thromboembolism or may have a chronic risk factor such as active malignancy or an underlying hereditary hypercoagulable state. Interruption of warfarin therapy during this phase of treatment is estimated to be associated with a much lower risk of thromboembolism than if it is stopped during the first 3 months of therapy (i.e., 10–15% per year). Consequently, it is reasonable to withhold 5 doses of warfarin prior to surgery in patients who have already been treated with 3 or more months of anticoagulation. If patients are immobilized in hospital before surgery, they should receive prophylactic doses of UFH or LMWH when the INR decreases to less than 1.8.
Postoperative Management of Anticoagulation
In relationship to the management of anticoagulation, two factors distinguish the postoperative from the preoperative period. First, major surgery is associated with a marked increase in the risk of venous thromboembolism; in the short term, this is estimated to be a 100-fold increase in risk.1 However, unlike venous thromboembolism, there is no convincing clinical evidence that surgery increases the risk of arterial embolism. Second, recent surgery is a major risk factor for anticoagulant-induced bleeding.1 Whereas bleeding is uncommon when warfarin is started after major surgery,3,28,37,38 bleeding is expected to be substantial if therapeutic doses of UFH or LMWH are administered within days of operation.39 For example, when both are started within 24 hours of major orthopaedic surgery, prophylactic doses of LMWH (i.e., less than half the dose used for bridging therapy) are associated with more bleeding than warfarin.3,37,38 Although the consequences of an episode of major bleeding in the postoperative period (case-fatality estimated at 3%)40,41 are generally less severe than those of an episode of thromboembolism, because the absolute risk of thromboembolism prior to re-establishing oral anticoagulation is often extremely low, administration of bridging therapy has the potential to do more harm than good during this interval.1 A number of recent small studies suggest that, with appropriate patient selection, major episodes of bleeding are uncommon (~1%) when LMWH is used for bridging therapy pre- and postoperatively.7,9,11,12 However, a preliminary report of a prospective study of more than 200 patients suggests a higher frequency of perioperative major bleeding and the possibility that such bleeds may predispose to thromboembolism by prolonging interruption of oral anticoagulant therapy.10
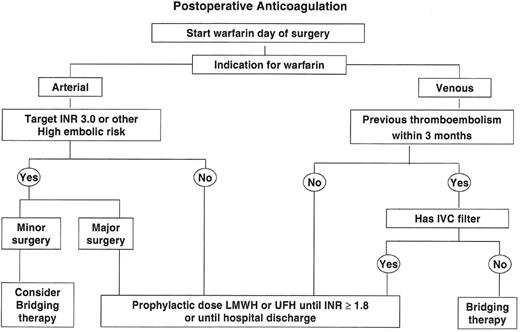
As there is a delay of about 12–24 hours after warfarin administration before the prothrombin time begins to increase, warfarin should be restarted as soon as possible after surgery unless patients have additional invasive procedures planned or are actively bleeding (Figure 2 ).
Arterial thromboembolism
In patients with the highest risk of arterial embolism (see above), it is reasonable to use bridging therapy after surgery provided the risk of bleeding is minimal (e.g., minor surgical or diagnostic procedures) (Figure 2).
If intravenous UFH is being used for postoperative bridging therapy, it should be started without a loading dose, approximately 12 hours after surgery, at a rate of no more than 18 units per kilogram per hour.42 In the absence of a loading dose, the first aPTT measurement should be deferred for 12 hours in order for a stable anticoagulant response to have been attained. Compared with therapeutic-dose subcutaneous LMWH, intravenous UFH has the advantage that it is rapidly eliminated when stopped and can be effectively reversed by protamine sulphate, if bleeding occurs.43
If therapeutic-dose subcutaneous LMWH is being used, it should probably not be started until ~24 hours after surgery and only after hemostasis has been achieved. With hospitalized patients, the LMWH dose can be “stepped up” over 36 hours, starting with a prophylactic dose within 12 hours of surgery. Twice-daily dosing may be preferable to once-daily dosing in the early postoperative period as lower peaks of anticoagulant effect are induced, and the smaller twice-daily dose is expected to be eliminated sooner if bleeding occurs close to the time of injection; however, once daily9,10 and twice daily11,12 regimens have been used postoperatively.
Bridging therapy is not recommended after surgery that is associated with a moderate or higher risk for bleeding, even if patients are considered to have a high risk of arterial embolism.1 Instead, subcutaneous UFH or LMWH, in doses recommended for thromboembolism prophylaxis of high-risk patients, should be given to hospitalized patients until the INR reaches 1.8.3
Venous thromboembolism
As surgery is a major risk factor for venous thromboembolism, the need for antithrombotic prophylaxis is much greater postoperatively than it is preoperatively. Patients who have had an episode of venous thromboembolism within 3 months of surgery have a very high risk of recurrence postoperatively. Consequently, bridging therapy is recommended in this setting until the INR is 2.0 or greater, provided the surgeon does not feel that the patient is at high risk for bleeding.1 Although patients who have a vena caval filter remain at high risk of recurrent venous thrombosis, they are at least partially protected from pulmonary embolism44 and, consequently, bridging therapy can be avoided in these patients in the early postoperative period.
Provided there have been no previous episodes of thromboembolism within 3 months prior to surgery, postoperative bridging therapy is not indicated. Subcutaneous UFH or LMWH is recommended in doses used for venous thromboembolism prophylaxis of high-risk patients while the INR is less than 1.8 and patients are hospitalized. As there is a concern that restarting warfarin may induce a transient hypercoagulable state in patients with protein C or protein S deficiency,45 patients with these conditions should restart warfarin at no more than the expected maintenance dose and should receive at least prophylactic doses of UFH or LMWH until the INR is 2.0 for two days.
Qualifying Remarks
The recommendations outlined above are strongly influenced by a number of assumptions, some of which will be considered in greater detail. It is proposed that, for most patients, warfarin is withheld preoperatively long enough for the INR to spontaneously fall to a value of 1.5 or lower before surgery without the need for bridging therapy. As the INR will be prolonged to some extent for much of this time, it is estimated that this interruption of warfarin will expose patients to a small risk of thromboembolism preoperatively (i.e., equivalent to the thromboembolic risk associated with one day of “no anticoagulation”).46–,48 For the same reason, provided warfarin is restarted the day of surgery, it is estimated that patients are exposed to a similar small risk of thromboembolism postoperatively while oral anticoagulant therapy is being re-established. It has also been assumed that the risk of thromboembolism associated with a day without anticoagulation is one 365th part of the risk associated with a year without therapy. Hence, a 10% per year risk of thromboembolism translates to a daily risk of ~0.03%, or a one in 3650 probability of an event. If stopping oral anticoagulation induces a transient “rebound” hypercoagulable state,1,7,49 or starting anticoagulation induces a transient “paradoxical” hypercoagulable state,45 the daily risk of thromboembolism may be underestimated. However, there is no convincing clinical evidence to support either of these phenomena.49 As patients with inherited protein S and protein C deficiency are believed to have a higher than average risk for warfarin-induced thrombosis, it is recommended that oral anticoagulation be reintroduced slowly, under the cover of concomitant heparin therapy (prophylactic or therapeutic doses), in such patients.
Another approach to management in this setting is to shorten the interval when the INR is subtherapeutic by withholding fewer doses of warfarin preoperatively while giving a small dose of oral vitamin K (i.e., 1 mg) to accelerate reversal of anticoagulation. The safety of this approach is not known, and though it appears reasonable for most patients if surgery needs to be performed before the INR can spontaneously decrease to an acceptable level, unless accompanied by bridging therapy, this practice is discouraged for patients with mechanical heart valves.50,51
There is also uncertainty about the need to reverse anticoagulation before some surgical procedures. It does not appear to be necessary for dental extractions4,52 (a local hemostatic agent may be used with more extensive dental surgery53) or for extracapsular cataract removal under local anesthetic.54 Similarly, the American Society for Gastrointestinal Endoscopy recommends that, because of a low associated risk of bleeding, diagnostic upper endoscopy, flexible colonoscopy with or without biopsy (but not polypectomy), diagnostic endoscopic retrograde cholangio-pancreatography (ERCP) and biliary stent implantation can be performed with an INR of up to 2.5.55 In general, it is more acceptable to perform surgical procedures while on anticoagulant therapy if the site of potential bleeding is accessible (e.g., mouth or skin) rather than remote (e.g., percutaneous biopsy of internal organs).
The assumption that underlies the use of bridging therapy is that it is effective at preventing thromboembolism. While there is good evidence that UFH and LMWH are effective at preventing venous thromboembolism,3,56 there is less certainty for the prevention of cardioembolism, particularly with the use of LMWH. Compared to aspirin, LMWH (dalteparin 100 IU/kg, twice daily) did not reduce the frequency of early recurrent stroke in patients with atrial fibrillation (odds ratio = 1.1 in favor of aspirin, 95% confidence interval 0.6 to 2.2),32 a finding that is consistent with subgroup analyses of other studies that have evaluated LMWH in acute ischemic stroke.33,57,58 However, in the International Stroke Trial, UFH (5000 or 12,500 IU twice daily; analysis by dose not available) was more effective than aspirin at preventing early recurrent stroke (42% risk reduction).59 There are fewer data relating to the efficacy of UFH and LMWH for the prevention of embolism in patients with mechanical heart valves. Indirect comparisons suggest that subcutaneous UFH is substantially less effective than oral anticoagulants at preventing thromboembolic complications in pregnant women with mechanical heart valves; however, less than currently recommended therapeutic doses of UFH were often used in these patients.60,61 As discussed in Section III, a number of cases of fatal mechanical valve thromboses have been documented in pregnant women who were being treated with therapeutic-dose LMWH (~100 IU/kg, twice daily), including 2 of 8 women in a prospective study (Lovenox® [enoxaparin] product monograph).34 At least partly arising from these reports, the product monograph for this LMWH preparation states that it should not be used for thromboprophylaxis in patients with prosthetic heart valves; this does not preclude that LMWH reduces the risk of thromboembolism in patients with mechanical heart valves when oral anticoagulation is interrupted.62 In the absence of treatment with thrombolytic therapy and aspirin (but not with such therapy), therapeutic or near-therapeutic doses of UFH approximately halves the frequency of stroke associated with acute myocardial infarction, supporting that UFH can reduce cardioembolism.63 Taken together, the above data suggest that UFH, and particularly LMWH, are less effective than warfarin at preventing cardioembolism. Doses of UFH and LMWH that are greater than those used to treat venous thromboembolism and for bridging therapy might be more effective at preventing cardioembolism; however, such doses are more likely to cause bleeding, particularly after surgery.
It is concluded that warfarin should be interrupted for as short a time as possible (usually 4 or 5 days) when it is necessary to reverse oral anticoagulant therapy. Most patients can then have invasive procedures performed without the need for bridging therapy and, because of the associated risk of bleeding, bridging therapy should generally be avoided within 2 days of major surgery. However, as this assessment is based on an interpretation of mostly indirect data, and as these data are open to different interpretations, uncertainty as to the optimal management of such patients is acknowledged.
Finally, perioperative management of anticoagulation can cause anxiety for patients, surgeons, anesthetists, and those who manage long-term anticoagulant therapy. Good communication between all of these parties is essential to ensure that an optimal management strategy is identified, that this strategy is then successfully executed, and that the potential for recrimination is minimized in the unlikely event of a serious thrombotic or hemorrhagic complication.
Primary hypercoagulable states (thrombophilias).
| 1. Decreased antithrombotic proteins |
|
| 2. Increased prothrombotic proteins |
|
| 1. Decreased antithrombotic proteins |
|
| 2. Increased prothrombotic proteins |
|
Risk factors for venous thromboembolism (VTE) during pregnancy.
| Cesarean delivery |
| History of prior VTE |
| Family history of VTE |
| Inherited or acquired thrombophilia |
| Obesity |
| Older maternal age |
| Higher parity |
| Prolonged immobilization |
| Cesarean delivery |
| History of prior VTE |
| Family history of VTE |
| Inherited or acquired thrombophilia |
| Obesity |
| Older maternal age |
| Higher parity |
| Prolonged immobilization |
Risk factors for venous thromboembolism (VTE) with cesarean section.
| Age over 35 years |
| Weight over 180 lb |
| Parity > 3 |
| Severe varicose veins |
| Infection |
| Emergency cesarean section |
| Pregnancy-induced hypertension |
| Cesarean section requiring hysterectomy |
| Personal or family history of VTE |
| Age over 35 years |
| Weight over 180 lb |
| Parity > 3 |
| Severe varicose veins |
| Infection |
| Emergency cesarean section |
| Pregnancy-induced hypertension |
| Cesarean section requiring hysterectomy |
| Personal or family history of VTE |
Factors influencing perioperative anticoagulant management.
| Risk of thromboembolism without anticoagulation |
| During the preoperative period |
| During the postoperative period |
| Risk reduction for thromboembolism with: |
| Oral anticoagulation |
| Unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) |
| Incremental risk of bleeding with UFH or LMWH bridging therapy: |
| During the preoperative period |
| During the postoperative period |
| Consequences of thromboembolism (venous or arterial) |
| Consequences of bleeding |
| Patient preference (e.g., fear of thromboembolism or bleeding) |
| Cost of UFH or LMWH bridging therapy |
| Risk of thromboembolism without anticoagulation |
| During the preoperative period |
| During the postoperative period |
| Risk reduction for thromboembolism with: |
| Oral anticoagulation |
| Unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) |
| Incremental risk of bleeding with UFH or LMWH bridging therapy: |
| During the preoperative period |
| During the postoperative period |
| Consequences of thromboembolism (venous or arterial) |
| Consequences of bleeding |
| Patient preference (e.g., fear of thromboembolism or bleeding) |
| Cost of UFH or LMWH bridging therapy |
Rates of thromboembolism associated with different indications for oral anticoagulation and risk reduction with anticoagulation.
| Indication . | Rate of Thromboembolism Without Anticoagulation . | Risk Reduction (%) . |
|---|---|---|
| Reproduced, with minor modifications, from Kearon and Hirsh1 | ||
| * An increase in the risk of venous thromboembolism associated with surgery (estimated to be 100-fold) is not included in these rates. | ||
| ‡ Last episode of venous thromboembolism more than 3 months previously, but require long-term anticoagulation because of high risk of recurrence. | ||
| † Risk reduction with oral anticoagulation; risk reduction with bridging therapy is uncertain but expected to be less (see discussion) | ||
| Abbreviations: NVAF, non-valvular atrial fibrillation | ||
| Venous Thromboembolism | ||
| Acute venous thromboembolism | ||
| 0–1 month | 40%/month* | 80 |
| 1–3 months | 10%/2 months* | 90 |
| Recurrent venous thromboembolism‡ | 15%/year* | 90 |
| Arterial Thromboembolism | ||
| NVAF | 4.5%/year | 75† |
| NVAF and previous embolism | 12%/year | 75† |
| Mechanical heart valve | 8%/year | 75† |
| Acute arterial embolism | ||
| 0–1 month | 15%/month | 75† |
| Indication . | Rate of Thromboembolism Without Anticoagulation . | Risk Reduction (%) . |
|---|---|---|
| Reproduced, with minor modifications, from Kearon and Hirsh1 | ||
| * An increase in the risk of venous thromboembolism associated with surgery (estimated to be 100-fold) is not included in these rates. | ||
| ‡ Last episode of venous thromboembolism more than 3 months previously, but require long-term anticoagulation because of high risk of recurrence. | ||
| † Risk reduction with oral anticoagulation; risk reduction with bridging therapy is uncertain but expected to be less (see discussion) | ||
| Abbreviations: NVAF, non-valvular atrial fibrillation | ||
| Venous Thromboembolism | ||
| Acute venous thromboembolism | ||
| 0–1 month | 40%/month* | 80 |
| 1–3 months | 10%/2 months* | 90 |
| Recurrent venous thromboembolism‡ | 15%/year* | 90 |
| Arterial Thromboembolism | ||
| NVAF | 4.5%/year | 75† |
| NVAF and previous embolism | 12%/year | 75† |
| Mechanical heart valve | 8%/year | 75† |
| Acute arterial embolism | ||
| 0–1 month | 15%/month | 75† |
Algorithm outlining an approach to the management of anticoagulation before elective surgery.
Surgery that is expected to take more than an hour to complete, or that is associated with a high risk of postoperative bleeding that precludes restarting bridging therapy 12 hours after surgery is completed, is considered “Major Surgery” in this context. Classification of planned procedures as “Major surgery” or “Minor surgery” often requires discussion with the patient’s surgeon.
Algorithm outlining an approach to the management of anticoagulation before elective surgery.
Surgery that is expected to take more than an hour to complete, or that is associated with a high risk of postoperative bleeding that precludes restarting bridging therapy 12 hours after surgery is completed, is considered “Major Surgery” in this context. Classification of planned procedures as “Major surgery” or “Minor surgery” often requires discussion with the patient’s surgeon.
Algorithm outlining an approach to the management of anticoagulation after elective surgery.
Surgery that is associated with a minimal risk of postoperative bleeding is considered “Minor Surgery” in this context. Classification of completed procedures as “Major surgery” or “Minor surgery” may require discussion with the patient’s surgeon.
Algorithm outlining an approach to the management of anticoagulation after elective surgery.
Surgery that is associated with a minimal risk of postoperative bleeding is considered “Minor Surgery” in this context. Classification of completed procedures as “Major surgery” or “Minor surgery” may require discussion with the patient’s surgeon.