Abstract
Bacterial contamination of transfusion products, especially platelets, is a longstanding problem that has been partially controlled through modern phlebotomy practices, refrigeration of red cells, freezing of plasma and improved materials for transfusion product collection and storage. Bacterial contamination of platelet products has been acknowledged as the most frequent infectious risk from transfusion occurring in approximately 1 of 2,000–3,000 whole-blood derived, random donor platelets, and apheresis-derived, single donor platelets. In the US, bacterial contamination is considered the second most common cause of death overall from transfusion (after clerical errors) with mortality rates ranging from 1:20,000 to 1:85,000 donor exposures. Estimates of severe morbidity and mortality range from 100 to 150 transfused individuals each year.
Concern over the magnitude and clinical relevance of this issue culminated in an open letter calling for the “blood collection community to immediately initiate a program for detecting the presence of bacteria in units of platelets.” Thereafter, the American Association of Blood Banks (AABB) proposed new standards to help mitigate transfusion of units that were contaminated with bacteria. Adopted with a final implementation date of March 1, 2004, the AABB Standard reads “The blood bank or transfusion service shall have methods to limit and detect bacterial contamination in all platelet components.”
This Joint ASH and AABB Educational Session reviews the risks, testing strategies, and regulatory approaches regarding bacterial contamination of blood components to aid in preparing practitioners of hematology and transfusion medicine in understanding the background and clinical relevance of this clinically important issue and in considering the approaches currently available for its mitigation, as well as their implementation.
In this chapter, Drs. Hillyer and Josephson review the background and significance of bacterial contamination, as well as address the definitions, conceptions and limitations of the terms risk, safe and safety. They then describe current transfusion risks including non-infectious serious hazards of transfusion, and current and emerging viral risks. In the body of the text, Dr. Blajchman reviews the prevalence of bacterial contamination in cellular blood components in detail with current references to a variety of important studies. He then describes the signs and symptoms of transfusion-associated sepsis and the sources of the bacterial contamination for cellular blood products including donor bacteremia, and contamination during whole blood collection and of the collection pack. This is followed by strategies to decrease the transfusion-associated morbidity/mortality risk of contaminated cellular blood products including improving donor skin disinfection, removal of first aliquot of donor blood, pre-transfusion detection of bacteria, reducing recipient exposure, and pathogen reduction/inactivation. In the final sections, Drs. Vostal, Epstein and Goodman describe the regulations and regulatory approaches critical to the appropriate implementation of a bacterial contamination screening and limitation program including their and/or the FDA’s input on prevention of bacterial contamination, bacterial proliferation, and detection of bacteria in transfusion products. This is followed by a discussion of sampling strategy for detection of bacteria in a transfusion product, as well as the current approval process for bacterial detection devices, trials recommended under “actual clinical use” conditions, pathogen reduction technologies, and bacterial detection and the extension of platelet storage.
Bacterial contamination of transfusion products, especially platelets, is a longstanding problem that has been partially controlled through modern phlebotomy practices, refrigeration of red cells, freezing of plasma, and improved materials for transfusion product collection and storage. Indeed, bacterial contamination of platelet products has been acknowledged as the most frequent infectious risk from transfusion occurring in approximately 1 of 2,000–3,000 whole-blood derived, random donor platelets (hereafter RDP), and apheresis-derived, single donor platelets (hereafter SDP)1–,4 (see below). In the US, bacterial contamination is considered the second most common cause of death overall from transfusion (after clerical errors) with mortality rates for platelet-related sepsis ranging from 1:20,000 to 1:85,000 donor exposures.5 Estimates of severe morbidity and mortality range from 100 to 150 transfused individuals each year.6,7
To address this problem, the US Food and Drug Administration (FDA) has sponsored scientific workshops to highlight and clarify relevant issues and to promote the development of technologic interventions.8–,10 Additionally, the Centers for Disease Control and Prevention (CDC) conducted a nationwide surveillance study on bacterial contamination in blood products.11 Many publications (detailed later in this chapter) have added important data attempting to determine and define the true clinical effect of bacterial contamination of blood components, and a similar number of publications have offered data as to the ability of a variety of methods to detect and limit this contamination.
Concern over the magnitude and clinical relevance of this issue culminated in an open letter calling for the “blood collection community to immediately initiate a program for detecting the presence of bacteria in units of platelets” (see http://www.cbbsweb.org/bacterialrisk.pdf). Thereafter, the American Association of Blood Banks (AABB), after publication of a number of Association Bulletins (see AB 96-063 at www.AABB.org), proposed new standards (December 2002) to help mitigate transfusion of units that were contaminated with bacteria. Adopted with a final implementation date of March 1, 2004, AABB Standard 5.1.5.1. now reads:
5.1.5.1The blood bank or transfusion service shall have methods to limit and detect bacterial contamination in all platelet components. Standard 5.6.2 applies. [Arm Prep]
Thus, this Joint ASH and AABB Educational Session was conceived to review the risks, testing strategies, and regulatory approaches regarding bacterial contamination of blood components; to aid in preparing practitioners of hematology and transfusion medicine in understanding the background and clinical relevance of this important issue; and to consider the approaches currently available for its mitigation as well as their implementation.
Background and Significance
Whereas the risk of transfusion related transmission of viral diseases such as human immunodeficiency virus (HIV) and hepatitis has steadily decreased over the last 40 years, the risk of transmission of bacteria has remained about the same. Thus, due to our success with viral pathogens, bacterial contamination now has the dubious distinction of being the most common infectious risk from transfusion and has become a matter of increasing concern and attention.12,13 Recent studies of platelets have suggested a bacterial contamination rate of about 1 per 1–3,000 units, clinical sepsis in about 1 per 20,000 transfusions and related fatality in about 1 per 60,000 transfusions5,14,15 (reviewed in detail below). The fatality risk from bacterially contaminated red blood cells (RBCs) is much lower, about 1 per 500,000.15
Despite this longstanding, persistent infectious risk of transfusion, it has been tolerated by some as an “acceptable risk.” However, with the significant risk reduction of transfusion-transmitted diseases attributable to viruses such as HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV), the magnitude and relative importance of platelet-induced sepsis has moved to the forefront of blood supply safety.16 In order to appreciate the significance of the relative importance of bacterial contamination of platelets, a perspective on the safety of the nation’s blood supply is necessary.
Risk
There are approximately 30 million units of blood and blood products transfused each year in the US, and perhaps an equal number internationally. The American public, the medical profession, and the government hold the transfusion of these units to the highest standards of safety.17 Indeed, the blood supply in the US has been described as “safer than ever relative to known risks,” however, “the usual notions of safety do not necessarily apply where transfusion is concerned.”17 The CDC, the AABB, and the American Red Cross18 have echoed these statements.
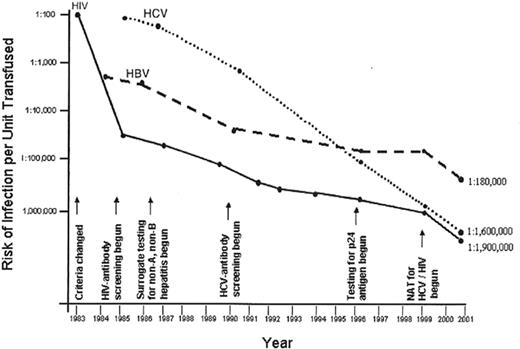
In the early 1980s, the incidence of transfusion transmitted (TT) HIV was greater than 1 case per 1,000 units in some locales, whereas the current incidence of TT-HIV is less than 1 case per 2 million screened and tested units;19 some authorities have posited that this risk may actually be closer to 1 in 5 million screened and tested units especially if there is widespread implementation of “single unit” nucleic acid testing (NAT). Similar improvements have been made for HBV and HCV, two TT-viruses carrying significant morbidity and mortality (Figure 1 ).20 Despite these improvements, many experts argue that the potential US blood recipient demands a “zero-risk” blood supply. Unfortunately, even for HIV in 2002, this goal has not been achieved as there were two reported cases of persons allegedly contracting HIV through transfusion and subsequently suing the blood supplier. In one case, a $100 million dollar negligence lawsuit has been filed. Additionally, a number of “emerging pathogens” still exist, as does the transfusion transmission of pathogens for which testing is not required. Thus, how safe the blood supply is becomes difficult to quantitate and by some criteria the risks to the blood recipient would be considered “very low” to “negligible” (see Table 1 ).21
Safe and safety
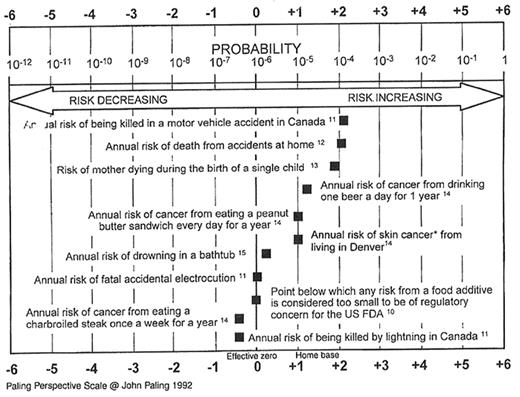
The word “safe” has been defined as free from “harm or risk” or “secure from danger, harm, or evil.” However, safety is a more subjective term emanating from one’s understanding and perspective, as well as the amount of control one has in determining the chance of the adverse outcome occurring. Thus, while the risk of dying in a car accident significantly exceeds the risk of dying from TT-infectious disease, the latter has elements of increased dread and less control and therefore has an increasingly fear-filled association. The Paling Perspective Scale22 provides several examples of risks with which the public is “comfortable” (see Figure 2 ) and allows comparison to the risk of TT-HIV (< 1:2M) and bacterial contamination (see discussion of risk estimates below). Indeed, research has shown that patients, and the majority of the population, have a very poor understanding of risk. It is worthwhile to compare the risk of death from influenza, cigarette smoking, motorcycle riding, or lightning with the risk of dying or acquiring variant Creutzfeldt-Jakob disease (vCJD) though there have been no known TT-cases of vCJD, although animal models support the possibility that vCJD may be transfusion-transmitted.23
Transfusion risks
It is vital to consider the other risks of transfusion such as those termed non-infectious serious hazards of transfusion (often termed NiSHOTs; see Table 2 ) which dramatically outweigh the residual risk of TT-infectious diseases.24 However, the agents that are known to be transfusion transmitted, and those that “might be” are described in Table 3 , for it is only upon this backdrop that a decision can be made on the relative value of new technologies and their ability to abrogate the agents, including bacterial contamination of platelet products.
Typically, four elements are required for an infectious disease–causing agent to be transmitted by transfusion and the criteria are listed in Table 4 .25 However, despite many possible agents that can be transmitted by transfusion, units are regularly tested only for HIV, HCV, HBV, HTLV, and Treponema pallidum. Donor demographics may bring changes in risk level of transfusion-transmitted agents and affect future choices for routine screening. For example, the incidence of malaria and Chagas disease seems to be rising due to increasing travel to endemic areas, climate changes, and immigration.
Clinical relevance
The unique aspect of most bacterial species when compared to viral pathogens is that they can readily proliferate in the nutrient-rich blood product environment during storage. This is particularly true in platelet products that are stored at room temperature, rather than in the cold. It is estimated that the level of contamination at the time of collection is generally relatively low, probably on the order of 1–10 colony forming units/mL or less.26 Once the product is contaminated, the inoculated bacterial seed can proliferate within hours to reach levels of 106/mL or greater. Such quantities of bacteria infused with the transfusion product over a short period of time can cause bacteremia that may progress to sepsis and death. The outcome of a contaminated transfusion is highly dependent on the amount of bacteria transfused, the type of bacteria and its pathogenicity for humans, the rate of transfusion, and the clinical status of the recipient. Immunosuppressed patients and older individuals with poor nutritional status will be the most susceptible population. However, even healthy individuals can have a rapidly fatal outcome when transfused with a large load of endotoxin-producing Gram-negative bacteria.
Thus, there is significant concern about, and demand for, a “safe” or “safer” blood supply in order to provide the best and safest patient care. This, in turn, has the public, government, and blood suppliers’ attention keenly focused on preventing bacterial contamination of platelets and subsequent sepsis due to platelet transfusions. This risk has become “unacceptable” in a climate where the blood supply remains “the safest that it has ever been.”
Prevalence of Bacterial Contamination in Cellular Blood Components
The contamination of RBCs and platelets with bacteria occurs relatively frequently.12,15,27– 31 However, the available prevalence data vary considerably from one study to the next. The data from the available prospective studies evaluating frequency of bacterial contamination of blood components are summarized in Table 5 . The mean prevalence of bacterial contamination in whole blood–derived, RDP platelets is 33.9 per 100,000 units and for apheresis-derived, SDP platelet units is 51.0 per 100,000 units. For RBCs it is 2.6 per 100,000 units. The overall cellular blood product contamination prevalence from all of the data shown in Table 5 is 32.4 per 100,000 units. This translates into an approximate rate of 1 bacterially contaminated cellular blood product unit per 3,000 cellular blood product units released.
In contradistinction to the available data on the bacterial contamination of cellular blood products (RBCs and platelets), the frequency of clinically apparent septic transfusion events or reactions due to such contaminated products is considerably lower. The available data on the prevalence of transfusion-associated septic events is summarized in Table 6 . It should be noted that these summaries report only the more severe or potentially life threatening septic reactions. It is likely, therefore, that these reports represent only one end of the clinical spectrum of transfusion-associated septic transfusion reactions. Moreover, it is very likely that even severe and even fatal septic transfusion reactions go unrecognized as being transfusion-associated. Thus, the actual prevalence of septic transfusion reactions may be considerably higher than reported, due to the observational bias that occurs. For all of these reasons, it has been very difficult to determine the true morbidity and mortality risk of transfusing bacterially contaminated cellular blood products.
The variation in the reported prevalence of clinical events is shown clearly in Table 6 . This table enumerates the risk of septic clinical events per million units transfused from 3 published studies: (1) the BaCon Study from the US, (2) the Hémovigilance system in France, and (3) the Johns Hopkins Hospital, which reports a 10-year follow-up of platelet transfusion episodes detected in that institution.11,41,5 From the data in Table 6 , it is impossible to define a specific prevalence of clinical transfusion-associated septic events, except to say that they probably occur with a frequency of 1 per approximately 25,000 platelet units transfused, and 1 per approximately 250,000 RBC units transfused. This latter estimate excludes the data from the BaCon study (1:5 million), as this study was likely limited by an underestimation bias.11
It is important to note that transfusion-associated septic reactions due to bacterially contaminated cellular blood products often go unrecognized as such, because of the high frequency of febrile non-hemolytic transfusion reactions (FNHTR) that occur following the transfusion of cellular blood products, particularly following platelet transfusions. For platelets, the FNHTR rate may be as high as 15% per unit transfused, whereas for RBCs the FNHTR rate is less than 1% per RBC unit transfused. As the symptoms of septic transfusion reactions often mimic those occurring with FNHTRs, many apparently mild septic transfusion reactions may not be recognized as such. Moreover, most of the organisms isolated from contaminated platelet and RBC units tend to be skin-associated organisms (i.e., Gram-positive cocci), which are not usually implicated in RBC-associated septic transfusion reactions, and are often considered contaminants related to procedure of taking the sample for culture (i.e., a false positive).
Signs and Symptoms of Transfusion-Associated Sepsis
The transfusion of a contaminated cellular blood product unit may be associated with variable signs and symptoms. As indicated above, not all contaminated blood products cause symptoms in the recipient; in fact, it appears that many do not.28 The initial signs and symptoms, when they occur, include fever and chills, which usually begin shortly after (within 2 hours) the start of the transfusion. Subsequent signs may include: hypotension, nausea, vomiting, diarrhea, oliguria, and shock. Other potential presenting symptoms include: respiratory symptoms (dyspnea, wheezing, and/or cough) and bleeding due to the consequence of endotoxin-induced disseminated intravascular coagulation. It is important to note that a majority of septic transfusion reactions associated with contaminated RBCs usually occur with units that have been stored for more than 21 days, whereas septic reactions associated with contaminated platelets usually occur with units that have been stored for 3 days or more.28
The clinical severity of a transfusion-associated septic reaction can vary considerably, depending on: (1) the species of bacteria present in the blood product unit, with Gram-negative organisms tending to cause more severe reactions, due to the presence of endotoxin often elaborated by such organisms; (2) the total number of bacteria infused or present in the cellular blood product unit infused to a recipient; (3) the rate of propagation of the bacteria present; and (4) recipient characteristics, such as underlying disease, leukocyte count, the status of the immune system, and whether the recipient is receiving concomitant antibiotic therapy.
Sources of the Bacterial Contamination for Cellular Blood Products
Possible mechanisms of the bacterial contamination of blood components include: donor bacteremia, contamination during the whole blood collection procedure, contamination of the collection pack, and contamination during the blood processing procedure.
Donor bacteremia
Blood donors with an asymptomatic bacteremia or who are in the recovery phase of a bacterial infection, may have transient bacteremic episodes and still qualify as a blood donor. Approximately 30 cases of Yersinia enterocolitica sepsis associated with RBC transfusion have been reported in the literature. This Gram-negative bacillus may cause enterocolitis, characterized by diarrhea, low-grade fever, and abdominal pain in the donor. Symptoms may be very mild and asymptomatic cases have been documented. In cases of transfusion-associated Yersinia enterocolitica septicemia reported to the CDC, approximately 75% of the implicated blood donors recalled having had diarrhea in the days preceding or following their blood donation, on retrospective questioning.42 Transfusion-associated Yersinia enterocolitica sepsis varies markedly from country to country, with an estimated incidence of 1 per 65,000 transfusions in New Zealand to 1 per 500,000 transfusions in the US.43,44 It is important to note that Yersinia-related sepsis is very episodic. Thus, over longer time intervals, the rates in these two countries might be different.
Bacteremia of short duration may also occur following dental manipulation such as tooth extraction, the use of gum irrigation devices, and even tooth brushing. A case of transfusion-associated Staphylococcus aureus platelet contamination has been documented, likely the result of a bacteremia in a blood donor who had undergone the repair of a tooth 3 hours before the donation.45
Contamination during whole blood collection
Contamination at the time of blood collection is the major cause of bacterial contamination of platelet units. The majority of organisms present in both culture studies and case reports of platelet-associated sepsis is usually normal skin flora as it may be virtually impossible to totally decontaminate human skin. Blood culture results show an incidence of positive cultures, ranging from 2% to 6% after the cleansing of the skin.46 In addition, it has been postulated that a small core of skin may enter the phlebotomy needle at the time of donation and that viable bacteria remain associated with the deep layers of skin, in spite of adequate skin disinfection.47 In the presence of a scarred phlebotomy site, surface cultures may be negative, while blood cultures are positive.48 Organisms not part of the normal skin flora may colonize on the skin and be associated with a septic transfusion reaction. A recent report of a fatal reaction due to Clostridium perfringens was linked to a donor who frequently changed his infant children’s diapers. This organism, as well as other species, normally part of fecal flora, grew in arm swab cultures from the implicated donor.49 Although not linked to episodes of bacterial contamination of blood products, skin disinfection materials including swab sticks containing iodine or alcohol have been reported to be contaminated by various bacterial species.28
Contamination of the collection pack
Leaky seals, damaged tubing, or micro-punctures in collection bags have been linked to episodes of bacterial sepsis.50 Regularly, blood packs used to collect donor blood have been found to have micro-punctures, possibly leading to the bacterial contamination of a cellular blood product.28 Moreover, it has been reported that blood packs in the absence of any defects may be heavily contaminated. A report published in 1999 reported 6 patients who developed septicemia with Serratia marcescens, following transfusions with red cells or platelets.51 This outbreak was traced to the heavy contamination of the exterior of blood packs. Upon investigation, 0.73% of blood bags from the same lot of packs grew the same ribotype of Serratia marcescens. It was postulated that the bacteria probably entered the blood bag at the time of blood donation, either by suction into the needle or due to contamination of the phlebotomist’s hands and subsequently the donor’s skin.51
Strategies to Decrease the Transfusion-Associated Morbidity/Mortality Risk of Contaminated Cellular Blood Products
A variety of strategies have evolved in recent years in an attempt to decrease the morbidity and mortality risk associated with transfusion-associated bacteremic and septic episodes. These measures are aimed at reducing the risk of the transfusion of cellular blood product units that are contaminated with bacteria by: (1) reducing recipient exposure; (2) avoiding contamination (i.e., improved donor screening or better skin disinfection); (3) optimizing blood component processing and storage; and (4) implementing tests and procedures that can be used to detect the presence of bacteria in blood product units.28 These approaches are summarized in Table 7 .
Improving donor skin disinfection
Because most of the bacteria isolated from contaminated blood products are normal skin flora, optimal disinfection of donor venesection sites may significantly reduce the bacterial contamination of blood products.52,53 Recent studies exploring blood culture contamination rates of blood products have demonstrated that both the quality of the antiseptic used and/or the mode of application of the antiseptic can influence the efficacy of skin disinfection. Thus, one possibility of reducing the prevalence of blood product bacterial contamination might be to improve donor skin disinfection.
Removal of first aliquot of donor blood
Even if skin disinfection is optimal, bacteria may be introduced into the blood container by means of a skin core, which is often taken inadvertently when the blood collection needle enters the skin.31 This has been reported to occur in approximately 65% of all venepunctures.47 Recently, studies have suggested that when a needle tip is contaminated, the majority of the contaminating bacteria can be detected in the first few millimeters of blood passing through the venepuncture needle. Several recent studies suggest that the diversion of the initial 15 to 30 mL of whole blood from the main container might be associated with reduced risk of bacterial contamination.54– 56 Several blood bag manufacturers have developed plastic software to accommodate such an option. Several countries, including Canada, have recently introduced this intervention to try to reduce the rate of transfusion-associated sepsis.
Pretransfusion detection of bacteria
The pretransfusion detection of bacteria is an important potential method to reduce the risk of transfusion-associated bacteremia and septic reactions. However, no single currently available laboratory technique is ideal.15 Pretransfusion detection of bacteria is much more complex than traditional transmissible disease testing, because the amount of bacteria present in a particular cellular blood component usually changes over time. Moreover, because the initial inoculum is usually very small (less than 10 organisms per mL) even a very sensitive technique will miss some contaminated units, if the testing is performed very soon after blood collection.15
Automated blood culture methods are being used currently in some European and US centers to test buffy-coat platelets or apheresis platelet concentrates for the presence of bacterial contamination. Because of the larger volume of these products, a 10-mL sample can be used for bacterial culture, substantially improving the sensitivity of detection. Various protocols are in use to culture platelet units: this can be done either shortly after component production, or only for platelet units that have not been transfused by day 3 of storage. A quarantine period of 24–48 hours after inoculation into a culture tube is used in some European centers, prior to issuing such platelet units. In several European countries, the regulatory authorities have accepted the extension of the platelet shelf life to 7 days, for platelet units on which bacterial cultures have been performed. Reduced platelet outdating appears to offset the cost of such bacterial testing.40
Because the number of bacteria present in a contaminated blood unit increases over time of storage, less sensitive methods potentially can be used in the hospital blood bank to perform bacterial screening prior to transfusion. Relatively rapid methods of bacterial detection, such as Gram-staining or the detection of metabolic changes, are being used. However, screening with such low sensitivity methods may be inadequate to prevent some transfusion-associated septic reactions.7
Reducing recipient exposure
One approach that has not been extensively utilized in an attempt to prevent transfusion-associated bacterial contamination is to reduce recipient exposure. In this regard, it would be possible to reduce transfusion-associated septic events by reducing transfusion triggers. Recent studies for both the use of allogeneic RBCs as well as prophylactic platelet transfusions have suggested that reduced transfusion triggers do not endanger recipients. One suggested approach to reducing the risk of transfusion-associated sepsis due to contaminated platelets is to use apheresis platelets in preference to pools of whole blood-derived platelets.5 The universal use of apheresis platelets would significantly reduce recipient exposure to contaminated blood products by a factor of 5. Five is the number of whole blood-derived platelet units usually used per platelet transfusion episode to an adult recipient. Finally, attention should be paid to optimizing transfusion indications. Audits of cellular blood transfusion use have regularly indicated that cellular blood products are often prescribed inappropriately. The reduction of such inappropriate use of cellular blood products should significantly reduce the incidence of transfusion-associated septic reactions.
Pathogen inactivation
Over the past 10 years, a number of photodynamic or photochemical methods have been reported that could lead to the reduction of viruses, bacteria, and protozoa that are present in cellular blood products.15 These pathogen reduction devices include: the use of a combination of a psoralen and ultraviolet A (UVA) light; riboflavin and visible light; ultraviolet B irradiation; and the addition of methylene blue or phthalocyanines with visible light.57–,60 With regard to the bacterial inactivation of contaminated platelet concentrates, Lin et al reported that the novel psoralen amotosalen (S-59), combined with UVA light exposure has effective bacterial inactivation capability.61 Thus, a combination of 150 μmol/L of the psoralen S-59 and 3 joules/cm UVA light result in significant bacterial reduction of contaminated apheresis platelets, as well as whole blood–derived platelets. Such methodologies are undergoing regulatory review in both North America and Western Europe.
Regulation and Regulatory Approaches
Prevention of bacterial contamination
Several approaches have been instituted by the transfusion community and the FDA to help minimize the chances of bacterial contamination of transfusion products at the time of collection. However, the improvement of collection techniques cannot prevent product contamination when the source is occult bacteremia in the donor; this risk may potentially be reduced by more careful and specific questioning of donors to identify recent medical procedures or symptoms that could indicate the onset of an infectious process. For sources of contamination associated with the collection method, steps can be taken that are more likely to decrease the risk.
An obvious first step is more effective sterilization of the phlebotomy site. The FDA has publicly discussed education about proper application of disinfectants and the problems with scar tissue.62 More effective disinfectants have been recommended to sterilize the collection sites and the use of ineffective disinfectants, such as green soap, have been discouraged.62 However, a careful review of the literature by FDA revealed that certain skin disinfection procedures using isopropyl alcohol followed by tincture of iodine that have been thought to be superior to those using povodone iodine actually are comparably effective in studies using hospitalized patients.62 Sterilization of the skin surface also has limits when the phlebotomy needle cores out a skin plug as it passes through the skin. This inherently nonsterile skin plug can be carried into and seed the collected blood.
Recently, two studies have suggested that diversion of the initially collected blood to a separate container (referred to as a diversion pouch) away from the rest of the collected product that is to be used in transfusion could reduce the contamination rate. In a European study of actual transfusion products, diverting the first 15 mL of whole blood appeared to reduce the contamination rate of the final container.63 Similar findings were also shown in an artificial model of blood collection.56 The diversion pouch approach, using two recently FDA approved collection systems, is now being adopted by many blood centers as a way to lower the contamination risk. However, the ability of initial aliquot diversion to prevent clinical cases of transfusion-associated sepsis has not, as yet, been reported. It is important to note that this approach will only reduce the level of skin contaminants in blood products.
Prevention of bacterial proliferation—Refrigeration of platelets?
For red cells that are stored at 4°C, bacterial growth is slow and limited to a few psychrophilic bacteria such as Yersinia enterocolitica, Pseudomonas species and Serratia liquefaciens.64 In comparison, platelets are stored at room temperature and provide a good medium for growth of both Gram-negative and Gram-positive bacteria. For most bacterial species, growth in platelet units can occur, rapidly reaching log phase within 24–48 hours.12,26 An exception is the common skin contaminant, Staphylococcus epidermidis which is a slower growing organism and usually reaches the log phase in 48–72 hours. Since this and similar organisms account for a large proportion of the bacterial species found to contaminate blood units, such slower growth characteristics need to be considered when optimizing sampling strategies for bacterial detection.65
Rapid proliferation of bacteria during storage of transfusion products could be decreased if cold temperatures were used. For red cells, this is generally a standard practice and reduces the growth of most bacterial species and the risk of transfusion related sepsis. On the other hand, platelets do not maintain adequate function when stored at cold temperatures. Even relatively short exposures to cold are associated with irreversible changes in platelets that significantly decrease their ability to remain in circulation.66 Attempts to understand the basis of the temperature sensitivity of platelets have only recently been productive.
A new study has suggested that platelets may respond to cold by clustering of GpIb on their surface which is then recognized by macrophages as a signal for clearance.67 Better understanding of the molecular changes in platelet physiology after cold exposure could lead to alternate storage conditions that would allow cold temperature storage and a reduction of bacterial proliferation while preserving platelet function and viability. Evaluation of novel storage conditions would involve in vitro experiments to demonstrate that the platelets have not been extensively activated or damaged by the conditions. Since in vitro tests have a poor predictive value for clinical performance of platelets, the gold standard for platelet evaluation remains the recovery and survival of radiolabeled transfused platelets.68 Platelets, collected from a single donor by apheresis, are divided into two products and stored under either conventional or novel conditions. At the end of storage a small portion of the cells is labeled with either 51Cr or 111In and the radiolabeled cells are reinfused into the original donor. Serial blood samples are then taken to monitor clearance of the cells from circulation. The double label allows for a direct comparison of the same platelets stored under different conditions in the same recipient.
Detection of bacteria in transfusion products
Ultimately, the reduction of bacterial contamination of transfusion products will require routine testing of the products for the presence of bacteria. Attempts to detect bacteria in transfusion products have spanned the range of simple to highly sophisticated state-of-the-art methodologies. On the low tech side, changes in the appearance of the product, such as platelet swirling or the color of the red cell units, have been utilized but with low specificity and sensitivity.69 Other tests such as determining pH or glucose levels, or direct bacterial staining with Gram’s stain or acridine orange have similarly not been very useful due to relatively low sensitivity when compared with bacterial concentrations that can cause sepsis. Tests with bacteria-specific nucleic acid probes or fluorescent dyes or antibodies are currently being developed.70,71 However, the most useful detection systems currently available appear to be automated, culture-based methods similar to what has been used for clinical detection of bacteremia in blood cultures obtained from patients.65
Sampling strategy for detection of bacteria in a transfusion product
Currently utilized platelet products are either random donor or apheresis units. Both types of units may be stored individually for up to 5 days at room temperature. Approximately 4–6 random donor units are pooled prior to transfusion to achieve a transfusion product with sufficient platelet content. The major factor in sampling either a random donor unit or an apheresis unit is the difference in volume. A typical apheresis unit contains approximately 200–250 mL while the random donor unit contains only 45–55 mL. Thus, the volume of the sample taken for bacterial detection can have a significant impact on the amount of product left for transfusion, particularly for random donor units. On the other hand, a larger sample volume increases sensitivity and thus a balance has to be reached to optimize sensitivity and minimize the impact on the product.
The timing of sampling is highly dependent on the method of detection. For culture-based methods, the product sample is inoculated into a container (bottle or bag) that allows for bacterial growth. Usually this occurs at 37°C and in the presence of sodium polysulfone (SPS), a bacteria growth-potentiating substance, that allows the bacteria to grow faster in the device than in the transfusion product. As the bacteria proliferate in the device, there is a fixed amount of time before a positive signal can be detected. The time to detection, dependent on the initial inoculum and the growth characteristic of the bacteria, is usually 24–48 hours when employing currently available detection methods. Because of this time required, the ideal sampling time for culture-based devices is as early as possible in the storage period so that a test-based decision about the clinical usefulness of the product can be made prior to its use. However, since the level of contamination at the time of collection is low and bacteria could be missed by a sampling error (especially with a low sample volume) at the beginning of storage, a certain amount of presampling storage time is usually allowed for the bacteria to begin to proliferate in the product. For platelets this is usually 24–48 hours.
The length of this presampling incubation period also depends on the sensitivity of the detection device. A more sensitive device can utilize a sample collected earlier in the product storage period while a less sensitive device would require a sample that contains more bacteria as occurs later in storage. The sampling strategy can be altered for a detection method that detects bacteria in real time. These approaches utilize probes, antibodies or nucleic acid–based detection system and can produce an answer within hours of sampling. The sensitivity of culture-based assays should be in the range of 10–100 CFU/mL and the sensitivity of methods that could be used at the point of transfusion would most likely be in the range of 100–1000 CFU/mL.26
Current approval process for bacterial detection devices
The FDA has encouraged manufacturers to submit applications for devices that will detect bacteria in transfusion products. The most urgent need is for bacterial screening of platelet products. Two devices have recently been cleared by the FDA for use in quality control monitoring of the platelet collection process that, in the past, was performed by manual culture (BioMeriuex, Inc. BK 000042; Pall Corp. BK 020014). The current devices allow sampling of the platelet product and automation of the quality control culture process. One device detects bacteria based on elaboration of CO2 in the sample culture, while the other detects a decrease in pO2. However, these devices have not yet been specifically approved for use in release of platelets for transfusion because the effectiveness of the devices to detect bacterial contamination in the clinical setting has not been established.
For the quality control indication, the validation was based on in vitro “spiking” experiments. A unit of platelets, either whole blood–derived, random donor, or apheresis-derived, single donor, was deliberately contaminated with a known amount of a single bacterial species. The unit was then repeatedly sampled 10 times and each time the sample was inoculated into the device. Thus, 10 separate readings were performed by the device for each bacterial species. The manufacturers were encouraged to validate their devices for as many bacterial species as possible and a list of the most common relevant bacteria was agreed on based on literature search, FDA advisory committee input and public workshops input.10,65
Trials recommended under “actual clinical use” conditions
The FDA has proposed that validation of bacterial detection devices for release of platelets for transfusion would require a field trial of the device under actual clinical use conditions.62 The design of such a trial would be to obtain a sample for bacterial detection by a culture-based device early in the storage period. A second sample would then be taken at the end of the storage period or at the time of release of platelets. The results of both cultures would be compared to determine if the first culture was predictive of the result of the second culture. If the unit is contaminated at collection, then the bacteria will have time to grow between the first and second sampling. The bacterial load should be higher and detection of bacteria should be easier at the second sampling.
The needed studies should demonstrate that a sample taken early in the storage period and screened by the automatic culture device is predictive of the culture results up to the time of outdate of the product. Although some aspects of the study could be modeled in vitro it is not clear what is the expected level of bacterial contamination under actual clinical conditions, whether the devices will have sufficient sensitivity or whether sampling errors may occur when low levels of bacteria are present early in the storage period. In addition, such studies will also demonstrate the false positive rate and rate of contamination, if any, from bacteria introduced by sampling and inoculation procedures.72 An excessive false positive rate would lead to an unnecessarily high discard rate for transfusion products.
Such issues raise the level of safety and efficacy concerns for devices intended for release of blood products for transfusion and are the basis for the current FDA practice of requesting clinical or field study data prior to approval of the devices. The field trials of the bacterial detection devices could be combined, under an IND or IDE application, with an extension of storage to day 7 or with pre storage pooling, allowing some costs to potentially be offset by savings from decreasing platelet product loss due to expiration and/or from pre storage leukoreduction of a pool instead of individual units.
Pathogen reduction technologies
As an additional measure of safety, transfusion products could potentially be treated with a pathogen reduction process to decrease or eliminate pathogens, including bacteria, which were not detected by testing. Methods are being developed that allow selective targeting of pathogens in blood products. The selectivity is based on absence of nucleic acids in platelets and red cells. A chemical compound is added to the transfusion product that interacts with the nucleic acids of pathogens and, upon spontaneous or light energy– induced activation, cross links nucleic acids and prevents their transcription and replication. Efficacy of these methods is usually established by in vitro studies where various pathogens are “spiked” into a platelet or red cell unit and then treated using the pathogen reduction methodology. The efficacy of these treatments should be in the range of 6–10 log reduction of common bacterial pathogens.10
However, from an FDA perspective, evaluation of the safety aspects of these methods is more complex and challenging. It includes assessment of possible damage caused by the treatment to the transfusion product (including functionality and survival of transfused cells) and evaluation of the potential toxicities of any residual chemicals to the recipients of the product. In fact, concerns about the safety of these products may arise because of mutagenicity of some of the chemical agents employed and thus their potential to be carcinogenic and genotoxic. In addition, the reactions of the chemical agent(s) with plasma and cellular proteins and lipids can create novel chemical entities that may have unexpected toxicities and increased immunogenicity when administered to certain patient populations. Because of the uncertainties that surround the treated products, even after extensive preclinical and clinical evaluation, it is likely that postmarketing studies will be needed to identify any low frequency adverse events associated with their use in a broader patient population. Another issue is whether the therapeutic efficacy of the treated transfusion products has been fully preserved after treatment with the pathogen reduction process. This is again being evaluated both in vitro and in Phase 2 and 3 clinical trials designed to follow efficacy endpoints, such as hemostasis in the case of platelet transfusions.
The final analysis of the documented and theoretical risks of the pathogen reduction process to the recipient of treated transfusion products will need to be weighed against the risk of the transfusion-transmitted diseases they could prevent, a risk which currently is relatively low. Thus, the clinical use of these products will ultimately depend on the appropriate risk benefit analysis for proposed uses and upon the data submitted for evaluation.
Transfusion product substitutes
The risk of transmitting pathogens by transfusion has been a major driving force for the development of manufactured substitutes for transfusion products. Such products would ideally be free of pathogens, have a long shelf life and may also not present the requirement of having to be serologically typed for each recipient. However, initial results of efforts to engineer red cells or platelets have so far fallen short of matching the natural cells. For example, initial efforts using the hemoglobin molecule to carry oxygen has been associated with a variety of toxicity issues.73 Additional research and clinical testing will need to be done to fully evaluate and, hopefully, realize the potential of these types of products.
Bacterial detection and the extension of platelet storage
Increased risk of bacterial contamination has been the major concern regarding prestorage pooling of platelets and extension of platelet storage beyond the currently allowed 5 days. For pooled platelets it has been demonstrated that a platelet pool created by combining 1 contaminated unit with 3–4 other units permits greater levels of bacteria at stationary phase.74 Until 1986, platelets could be stored for up to 7 days at room temperature. However, due to concerns over a rise in clinically significant transfusion-associated infections from an increased bacterial load at the end of an extended storage period, this was shortened to 5 days on the advice of an FDA scientific advisory committee.
Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately validated effective bacterial detection system. Such validation would involve in vitro testing with pooled products and a field trial in a clinical setting. In addition to bacterial detection the storage conditions for pooled platelets and platelets stored beyond 5 days will need to be validated to demonstrate that platelet quality is preserved under these conditions.75 Use of FDA-approved sterile connection devices otherwise allows pooling and sampling of platelets without introducing bacterial contamination through the pooling process itself.62
Regulatory summary
Bacterial contamination of transfusion products, especially platelets, is a significant clinical problem with multiple causes. Based on recognition of this issue, the FDA and the blood industry have taken steps to improve control of bacterial contamination at multiple steps during blood collection and processing. Dealing effectively with this problem will involve continued and enhanced education of phlebotomy, technical, nursing, and medical staff with regard to sources of contamination and the importance of the problem. Using approved products and procedures, blood collection centers can implement practices that may decrease bacterial contamination, including better skin disinfection, collection of blood with a diversion pouch set, testing for bacteria, and, potentially, the use of safe and effective pathogen reduction technologies if and when available. Promising technologies for bacterial detection appear to be close at hand and offer significant potential to screen out contaminated units. The FDA and the blood community share a common goal that increased efforts focused on the problem of bacterial contamination will be translated into successes comparable to those we have already achieved in decreasing risks from most transfusion transmitted viral diseases.
Concluding Comments
Septic transfusion reactions associated with the use of contaminated blood products remain a very significant adverse event. Nonetheless, clear-cut approaches to reduce the frequency of such events are appearing. A wide variety of measures have been proposed in order to reduce the frequency of transfusion-associated septic reactions and many of these measures are being actively investigated, and in some countries being instituted.15,76 Thus, the potential reduction in the risk of transfusion-associated septic reactions is a realistic possibility in the foreseeable future.
Risk of an individual dying (D) in 1 year or developing an adverse response (A).21
| Term Used . | Risk Range . | Example . | Risk Estimate . |
|---|---|---|---|
| High | > 1:100 | (A) Transmission to susceptible household contacts of measles and chickenpox | 1:1–1:2 |
| (A) Transmission of HIV from mother to child (Europe) | 1:6 | ||
| Moderate | 1:100–1:1,000 | (D) Smoking 10 cigarettes per day | 1:200 |
| (D) All natural causes, age 40 | 1:850 | ||
| Low | 1:1,000–1:10,000 | (D) All kinds of violence | 1:3,300 |
| (D) Influenza | 1:5,000 | ||
| (D) Accident on road | 1:8,000 | ||
| Very low | 1:10,000–1:100,000 | (D) Leukemia | 1:12,000 |
| (D) Playing soccer | 1:25,000 | ||
| (D) Accident at work | 1:43,000 | ||
| Minimal | 1:100,000–1:1,000,000 | (D) Accident on railway | 1:500,000 |
| Negligible | < 1:1,000,000 | (D) Hit by lightning | 1:1,000,000 |
| (D) Release of radiation by nuclear power station | 1:10,000,000 |
| Term Used . | Risk Range . | Example . | Risk Estimate . |
|---|---|---|---|
| High | > 1:100 | (A) Transmission to susceptible household contacts of measles and chickenpox | 1:1–1:2 |
| (A) Transmission of HIV from mother to child (Europe) | 1:6 | ||
| Moderate | 1:100–1:1,000 | (D) Smoking 10 cigarettes per day | 1:200 |
| (D) All natural causes, age 40 | 1:850 | ||
| Low | 1:1,000–1:10,000 | (D) All kinds of violence | 1:3,300 |
| (D) Influenza | 1:5,000 | ||
| (D) Accident on road | 1:8,000 | ||
| Very low | 1:10,000–1:100,000 | (D) Leukemia | 1:12,000 |
| (D) Playing soccer | 1:25,000 | ||
| (D) Accident at work | 1:43,000 | ||
| Minimal | 1:100,000–1:1,000,000 | (D) Accident on railway | 1:500,000 |
| Negligible | < 1:1,000,000 | (D) Hit by lightning | 1:1,000,000 |
| (D) Release of radiation by nuclear power station | 1:10,000,000 |
Noninfectious serious hazards of transfusion (NiSHOTs).24
|
|
Agents and their likelihood of transfusion transmission in qualitative terms.
| Agent . | TransfusionTransmission . |
|---|---|
| * Animal models support the possibility that this agent may be able to be transmitted by transfusion | |
| Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; HBV, hepatitis B virus; HTLV, human T-cell lymphotrophic virus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HGV, hepatitis G virus; TTV, transfusiontransmitted virus; HHV-8, human herpes virus–8; WNV, West Nile virus; CJD, Creutzfeld-Jakob disease; vCJD, variant CJD. | |
| Parasites | |
| Malaria | Rare cases |
| Babesia | Occasional cases, endemic |
| T. cruzii | Occasional cases, US |
| T. gondii | Rare cases |
| Leishmania | Rare cases (< 5 worldwide) |
| Bacteria | |
| Contaminants | Approximately 1:3,000 platelet units |
| Specific Agents | |
| T. pallidum | Rare cases |
| B. burgdorferi | No known cases |
| E. chaffeensis | No known cases |
| E. cytophagophilia | One possible case |
| R. rickettsii | One reported case |
| Viruses | |
| HIV | Rare cases |
| HCV | Rare cases |
| HBV | Occasional cases |
| HTLV | Rare cases |
| CMV | Occurs, threat to specific pts |
| EBV | Occurs, threat to specific pts |
| B19 | Occurs, threat to specific pts |
| HAV | Rare cases |
| HGV | Occurs, not a threat to pts |
| TTV | Occurs, not a threat to pts |
| Sen-V | Occurs, not a threat to pts |
| HHV-8 | No known cases |
| WNV | Several cases reported |
| Enterovirus | Known donor viremia |
| Prions | |
| CJD | No known cases |
| vCJD | No known cases* |
| Agent . | TransfusionTransmission . |
|---|---|
| * Animal models support the possibility that this agent may be able to be transmitted by transfusion | |
| Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; HBV, hepatitis B virus; HTLV, human T-cell lymphotrophic virus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HGV, hepatitis G virus; TTV, transfusiontransmitted virus; HHV-8, human herpes virus–8; WNV, West Nile virus; CJD, Creutzfeld-Jakob disease; vCJD, variant CJD. | |
| Parasites | |
| Malaria | Rare cases |
| Babesia | Occasional cases, endemic |
| T. cruzii | Occasional cases, US |
| T. gondii | Rare cases |
| Leishmania | Rare cases (< 5 worldwide) |
| Bacteria | |
| Contaminants | Approximately 1:3,000 platelet units |
| Specific Agents | |
| T. pallidum | Rare cases |
| B. burgdorferi | No known cases |
| E. chaffeensis | No known cases |
| E. cytophagophilia | One possible case |
| R. rickettsii | One reported case |
| Viruses | |
| HIV | Rare cases |
| HCV | Rare cases |
| HBV | Occasional cases |
| HTLV | Rare cases |
| CMV | Occurs, threat to specific pts |
| EBV | Occurs, threat to specific pts |
| B19 | Occurs, threat to specific pts |
| HAV | Rare cases |
| HGV | Occurs, not a threat to pts |
| TTV | Occurs, not a threat to pts |
| Sen-V | Occurs, not a threat to pts |
| HHV-8 | No known cases |
| WNV | Several cases reported |
| Enterovirus | Known donor viremia |
| Prions | |
| CJD | No known cases |
| vCJD | No known cases* |
Criteria for transfusion transmission of viruses and other infectious agents.25
|
|
Summary of the available prospective studies evaluating the prevalence of bacterial contamination in whole blood–derived platelets, apheresis platelets, and red blood cell units.
| Blood Produce Type . | Reference . | Positives per Number of Units Tested . | Contamination Prevalence per 100,000 Units . |
|---|---|---|---|
| * 95% confidence intervals 25.6 to 39.1 | |||
| Abbreviations: A-plts, apheresis platelets; WB-plts, whole blood–derived platelets; RBCs, red blood cells; n, number of studies. | |||
| WB-plts | Morrow et al32 | 6 per 74,598 | 8 |
| WB-plts | Barrett et al33 | 1 per 4,272 | 23 |
| WB-plts | Yomtovian et al34 | 6 per 15,705 | 38 |
| WB-plts | Chiu et al35 | 10 per 21,503 | 46 |
| WB-plts | Blajchman et al36 | 16 per 31,610 | 51 |
| WB-plts | Leiby et al37 | 4 per 4,995 | 80 |
| WB-plts | Blajchman et al38 | 7 per 10,065 | 70 |
| A-plts | Morrow et al32 | 1 per 9,519 | 5 |
| A-plts | Blajchman et al31 | 14 per 6,055 | 230 |
| A-plts | Barrett et al33 | 5 per 17,928 | 28 |
| A-plts | Yomtovian et al34 | 0 per 2,476 | 0 |
| A-plts | Dzieczkowski et al39 | 1 per 5,197 | 19 |
| A-plts | Aubuchon et al40 | 1 per 2,678 | 37 |
| RBCs | Barrett et al33 | 1 per 31,385 | 3 |
| RBCs | Dzieczkowski et al39 | 1 per 7,080 | 0 |
| Overall | n = 15 | 89 per 274,379 | 32.4* |
| Blood Produce Type . | Reference . | Positives per Number of Units Tested . | Contamination Prevalence per 100,000 Units . |
|---|---|---|---|
| * 95% confidence intervals 25.6 to 39.1 | |||
| Abbreviations: A-plts, apheresis platelets; WB-plts, whole blood–derived platelets; RBCs, red blood cells; n, number of studies. | |||
| WB-plts | Morrow et al32 | 6 per 74,598 | 8 |
| WB-plts | Barrett et al33 | 1 per 4,272 | 23 |
| WB-plts | Yomtovian et al34 | 6 per 15,705 | 38 |
| WB-plts | Chiu et al35 | 10 per 21,503 | 46 |
| WB-plts | Blajchman et al36 | 16 per 31,610 | 51 |
| WB-plts | Leiby et al37 | 4 per 4,995 | 80 |
| WB-plts | Blajchman et al38 | 7 per 10,065 | 70 |
| A-plts | Morrow et al32 | 1 per 9,519 | 5 |
| A-plts | Blajchman et al31 | 14 per 6,055 | 230 |
| A-plts | Barrett et al33 | 5 per 17,928 | 28 |
| A-plts | Yomtovian et al34 | 0 per 2,476 | 0 |
| A-plts | Dzieczkowski et al39 | 1 per 5,197 | 19 |
| A-plts | Aubuchon et al40 | 1 per 2,678 | 37 |
| RBCs | Barrett et al33 | 1 per 31,385 | 3 |
| RBCs | Dzieczkowski et al39 | 1 per 7,080 | 0 |
| Overall | n = 15 | 89 per 274,379 | 32.4* |
| Study . | Country . | A-Plts . | WB-Plts . | Case Fatality Rate - Plts . | RBCs . | Case Fatality Rate - RBCs . |
|---|---|---|---|---|---|---|
| Abbreviations: A-plts; apheresis platelets; WB-plts, whole blood–derived platelets; RBCs, red blood cells; ND, not determined. | ||||||
| Perez et al41 | France | 31.8 | 71.8 | 31.3% | 5.8 | 24.0% |
| Kuehnert et al11 | US | 9.9 | 10.6 | 20.7% | 0.2 | 60.0% |
| Ness et al5 | US | 74.5 | 67.0 | 17.4% | ND | ND |
| Study . | Country . | A-Plts . | WB-Plts . | Case Fatality Rate - Plts . | RBCs . | Case Fatality Rate - RBCs . |
|---|---|---|---|---|---|---|
| Abbreviations: A-plts; apheresis platelets; WB-plts, whole blood–derived platelets; RBCs, red blood cells; ND, not determined. | ||||||
| Perez et al41 | France | 31.8 | 71.8 | 31.3% | 5.8 | 24.0% |
| Kuehnert et al11 | US | 9.9 | 10.6 | 20.7% | 0.2 | 60.0% |
| Ness et al5 | US | 74.5 | 67.0 | 17.4% | ND | ND |
Proposed strategies to reduce transfusion-associated septic risk.
| Abbreviations: NAT, nucleic acid test; RBCs, red blood cell units. |
|
| Abbreviations: NAT, nucleic acid test; RBCs, red blood cell units. |
|
New test implementation and declining risk of viral infections from transfusion.20
Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; NAT, nucleic acid testing. Modified by M.P. Busch from table in AuBuchon JP, Birkmeyer JD, Busch MP. Safety of the Blood Supply in the United States: Opportunities and Controversies.
New test implementation and declining risk of viral infections from transfusion.20
Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; NAT, nucleic acid testing. Modified by M.P. Busch from table in AuBuchon JP, Birkmeyer JD, Busch MP. Safety of the Blood Supply in the United States: Opportunities and Controversies.
Risks with which the public is comfortable.
The numbers denoting references in this figure are those from the primary article and are not notations of the references found at the conclusion of this chapter.
Abbreviations: FDA, Food and Drug Administration.22
Reprinted with permission from
Risks with which the public is comfortable.
The numbers denoting references in this figure are those from the primary article and are not notations of the references found at the conclusion of this chapter.
Abbreviations: FDA, Food and Drug Administration.22
Reprinted with permission from
Author notes
Christopher D. Hillyer, MD, Emory University School of Medicine, Atlanta, GA
Cassandra D. Josephson, MD, Emory University School of Medicine, Atlanta, GA
Morris A. Blajchman, MD, FRCP(C), McMaster University and Canadian Blood Services, Hamilton, Ontario, Canada
Jaroslav G. Vostal, MD, PhD, Center for Biologics Evaluation and Research, FDA
Jay S. Epstein, MD, Center for Biologics Evaluation and Research, FDA
Jesse L. Goodman, MD, MPH, Center for Biologics Evaluation and Research, FDA