Abstract
High-dose therapy with stem cell transplantation (SCT) and novel targeted therapies (thalidomide, its more potent analogues, and bortezomib) represent two approaches for overcoming resistance of multiple myeloma (MM) cells to conventional therapies. While it is now clear that dose-intensification improves the outcome in younger patients, long-term remissions are obtained in a minority of patients. Therefore, the impact of novel agents as part of front-line therapy is the objective of ongoing trials. Gene expression profiling (GEP) will help to improve the management of MM not only by identifying prognostic subgroups but also by defining molecular pathways that are associated with these subgroups and that are possible targets for future therapies.
In Section I, Dr. John Shaughnessy describes recent data obtained with GEP of CD138-purified plasma cells from patients with MM. His group has already shown that overexpression of the Wnt signaling inhibitor DKK1 by MM plasma cells blocks osteoblast differentiation and contributes to the development of osteolytic bone lesions. Recent data allow identification of four subgroups of MM in which GEP is highly correlated not only with different clinical characteristics and outcome but also with different cytogenetic abnormalities. In addition, abnormal expression of only three genes (RAN, ZHX-2, CHC1L) is associated with rapid relapses. In the context of intensive therapy with tandem autotransplantations, this model appears to be more powerful than current prognostic models based on standard biologic variables and cytogenetics. Understanding why the dysregulation of these three genes is associated with a more aggressive behavior of the disease will help to define new therapeutic strategies.
In Section II, Dr. Jean-Luc Harousseau presents recent results achieved with tandem autologous SCT (ASCT) and with reduced intensity conditioning (RIC) allogeneic SCT. ASCT is now considered as the standard of care in patients up to 65 years of age. The IFM (Intergroupe Francophone du Myelome) has recently shown that double ASCT is superior to single ASCT. Current results of three other randomized trials confirm that double ASCT is superior, at least in terms of event-free survival. However, patients with poor prognostic features do poorly even after tandem ASCT. Strategies to further improve the outcome of ASCT include more intensive therapies and the use of novel agents such as thalidomide and immunomodulatory analogs (IMiDs) or bortezomib. Results of allogeneic SCT remain disappointing in MM even with T cell–depleted grafts. Preliminary results of a strategy combining ASCT to reduce tumor burden and RIC allogeneic SCT are encouraging, although the follow-up is still short. However, again, patients with chromosome 13 deletions have poor results with RIC. Longer follow-up of ongoing multicentric studies will help to clarify the indications of RIC.
In Section III, Dr. Paul Richardson summarizes current knowledge of novel targeted therapies in MM. A better understanding of interactions between MM cells and bone marrow stromal cells and of the signaling cascades whereby cytokines mediate proliferation, survival, drug resistance and migration of MM cells provide the rationale for testing novel agents in relapsed/refractory MM.
Increased angiogenesis coupled with the known anti-angiogenesis activity of thalidomide justified its use in refractory MM. The remarkable responses initially achieved prompted a number of clinical studies in different indications and the development of more potent IMIDs. Among them CC-5013 (Revlimid®) has been tested in Phase I/II studies and a randomized Phase III study has just been completed.
Blockade of NF-κB using the proteasome inhibitor bortezomib (Velcade®) may mediate anti-MM activity by inhibiting interleukin (IL)-6 production in stromal cells and other mechanisms of action have been shown in preclinical studies. Based on the promising results of the Phase II trial, a large randomized trial of bortezomib versus dexamethasone has been completed. Studies of bortezomib combined with other drugs are ongoing.
Arsenic trioxide has a number of properties showing that it targets MM cells interacting with the microenvironment. Clinical studies are ongoing as well. Other agents in MM have already been or will probably be translated soon from the bench to the bedside.
I. Toward the Use of Gene Expression Profiling in the Clinical Management of Multiple Myeloma
John Shaughnessy, Jr., PhD*
University of Arkansas for Medical Sciences, Myeloma Institute/Lambert Laboratory of Myeloma, 4301 West Markham St., Little Rock AR 72205 Acknowledgments: I would like to acknowledge Bart Barlogie (1,2), Fenghuang Zhan (1,2), Yongsheng Huang (1,2), Hongwei Xu (1,2), Erming Tian (1,2), Ichiro Hanamura (1,2), Madhumita Santra (1,2), Sushil Gupta (1,2), Anthanasios Fassas (1), Klaus Hollmig (1), Choon Kee-Lee (1), Giampaolo Talamo (1), Raymond Thertulien (1), Frits Van Rhee (1), Maurizio Zangari (1), Ellias Anaissie (1), Guido Tricot (1), Jeffrey Sawyer (3), Yupo Ma (3), Ronald Walker (4), Erik Rasmussen (5), William Barlow (5), and John Crowley (5). I would also like to thank the research and clinical staff of both the Lambert Laboratory and MIRT for their invaluable contributions to this study. Donna D. and Donald M. Lambert Laboratory of Myeloma Genetics (1), Myeloma Institute for Research and Therapy (MIRT) (2), Department of Pathology (3), Department of Radiology (4), University of Arkansas for Medical Sciences, Little Rock, AR; and Cancer Research and Biostatistics, Seattle WA (5).
Multiple Myeloma: The Disease, Its Therapy and Prognosis
Multiple myeloma (MM) is associated with a constellation of disease manifestations, including osteolytic lesions due to uncoupled bone metabolism, anemia and immunosuppression due to loss of normal hematopoietic stem cell function, and end-organ damage due to monoclonal immunoglobulin secretion.1 The presence of somatic hypermutations of the immunoglobulin variable region genes in myeloma plasma cells suggests that malignant transformation occurs in a B cell that has traversed the germinal centers of lymph nodes. However, the hypoproliferative nature of myeloma has led to the hypothesis that the bulk of the tumor arises from a transformed B cell with the capacity for both self-renewal and production of terminally differentiated progeny.2– 4
The clinical course of patients requiring therapy for myeloma varies markedly. Even with tandem autotransplants yielding complete remission (CR) rates in excess of 60%, survival ranges from a few months to greater than 15 years. The extended time (almost 2 years) for those patients to achieve CR, and the even longer time to achieve magnetic resonance imaging (MRI)–CR, strongly suggests enormous tumor cell population heterogeneity in terms of drug responsiveness/resistance.
Traditional prognostic factors, such as β2-microglobulin (β2M), albumin, and C-reactive protein (CRP), account for only 15%–20% of outcome heterogeneity. Abnormal metaphase karyotypes, present in one-third of newly diagnosed patients and reflecting stroma independence, have been consistently associated with a rapidly fatal outcome, and fewer than 10% of patients with these abnormalities survive > 5 years.
Recent advances in molecular cytogenetics have identified primary translocations involving the immunoglobulin heavy chain locus at 14q32 in 40% of patients.5 According to a consensus report of a recent Paris workshop on myeloma genetics, hyperdiploid and t(11;14)(q13,q32)-positive myeloma are associated with a good prognosis, whereas non-hyperdiploidy, often associated with translocations other than t(11;14) and chromosome 13 deletion, imparts a strikingly dismal prognosis.6
Gene Expression Profiling of Myeloma
Gene expression profiling (GEP) has become a routine method for the detailed analysis of gene activity in normal cell development and the disease process. We have used comparative GEP with a limited number of genes (~6800) from CD138-enriched plasma cell RNA, obtained from patients with newly diagnosed myeloma, patients with monoclonal gammopathy of undetermined significance (MGUS), and normal healthy subjects, to identify genes that might play a role in the initiation and progression of myeloma.7,8 These studies revealed that newly diagnosed myeloma could be classified into four subgroups, with the two extremes being similar to either MGUS or myeloma cell lines. The myelomas with a myeloma cell line–like GEP signature also tended to have poor-risk features at presentation, such as elevated serum β2M (> 4 mg/mL) and cytogenetic abnormalities. These studies also revealed that, whereas MGUS and myeloma could be distinguished from normal plasma cells, these two conditions were difficult, if not impossible, to differentiate from each other. It is curious that MGUS appears to harbor all the hallmarks of overt malignancy, yet this condition rarely converts to overt myeloma requiring systemic therapy.9 These data suggest that changes in the bone marrow microenvironment or failure of immune surveillance, rather than a genetic change in the tumor cell, may account for the malignant conversion of this benign plasma cell dyscrasia.
Although myeloma manifests itself at the terminal differentiated stage of the B cell lineage, there is much speculation about the actual origins of the disease. Thus, to place myeloma in the context of plasma cell differentiation, we determined the molecular signatures of cells representing the late stages of human B cell development and showed that the GEP-defined high-risk form of myeloma (described above) has a tonsil B cell–like signature, whereas cells with low-risk features tend to be either bone marrow or tonsil plasma cell–like.10 GEP profiling of CD138-selected plasma cells and CD19-selected B cells from the bone marrow of patients with Waldenström’s macroglobulinemia revealed that both compartments exhibit signs of malignancy, with the plasma cells having either a tonsil plasma cell-like or a myeloma-like GEP signature.11 Taken together, these data support the concept that Waldenström’s macroglobulinemia, and possibly myeloma, may be derived from either terminally differentiated cells with various degrees of plasticity or from cells transformed at distinct stages of late-stage B-cell differentiation, with the ability to self-renew and produce progeny with partially differentiated phenotypes and transcriptome profiles.
Nearly 40% of myelomas harbor one of five recurrent chromosomal translocations involving the immunoglobulin heavy (IGH) chain locus.5 Given the transcription-activating nature of IGH translocations, GEP represents a valuable tool for identifying all myeloma-specific IGH translocations.7,12,13 Furthermore, genes that exhibit highly correlated expression patterns with these activated oncogenes may point to downstream targets of the deregulated genetic pathways resulting from their hyperactivation. Approximately 50% of myelomas harbor deletion of chromosome 13, and this abnormality is linked to poor survival.6 We have mapped a putative myeloma tumor suppressor gene to a minimal region of deletion at 13q14, and we have recently used a combination of triple-color-interphase fluorescence in situ hybridization (FISH) and GEP to predict, with a high degree of accuracy, chromosome 13 deletion by GEP alone.14,15 Finally, we have used GEP to investigate the mechanisms by which myeloma plasma cells contribute to the development of osteolytic bone lesions. Comparative GEP between myeloma patients with and without bone lesions demonstrated that elevated expression of the Wnt signaling inhibitor DKK1 by myeloma plasma cells is highly correlated with bone disease and that treatment of osteoblast precursors with serum from myeloma patients with high serum DKK1 could block BMP2/Wnt-induced differentiation of these cells into osteoblasts, suggesting a mechanism by which myeloma cells directly contribute to the development of osteolytic bone lesions in this disease.16
Gene Expression Profiling to Model the Effectiveness of Tandem Autotransplantation for the Treatment of Myeloma
GEP has been used to develop risk-adapted prognostic models for leukemias and lymphomas.17– 23 The highly variable outcome in patients with MM, with very little of this variability being accounted for by current laboratory tests, prompted us to investigate if GEP could better model event-free (EFS) and overall (OS) survival in this disease as well. The GEP studies to be described are on CD138-enriched (> 90% CD38+/CD45−) plasma cells from bone marrow aspirates taken from newly diagnosed myeloma patients entering the National Cancer Institute–sponsored Phase III clinical trial (Total Therapy II), which enrolled 660 patients from 1998 to 2003. In addition to tandem transplants with melphalan (200 mg/m2), Total Therapy II also randomized patients upfront to thalidomide or no thalidomide and provided one year of intensive consolidation therapy and an additional year of dexamethasone maintenance. Since 2000, we have performed GEP on pretreatment plasma cells from 351 consecutive patients entering the Total Therapy II trial with a median follow up of 25 months.
The following is a brief synopsis of studies aimed at developing clinically relevant risk-adapted prognostic models for myeloma using gene expression signatures.
Unsupervised Hierarchical Clustering of Global Gene Expression Patterns of Pretreatment Myeloma Plasma Cells Defines Biological and Clinical Subgroups
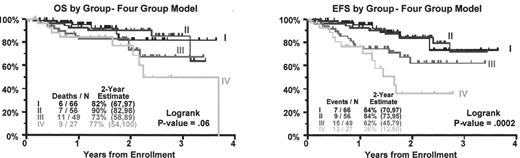
Unsupervised hierarchical cluster analysis, based on a set of 4580 highly variable genes from ~10,000 tested (SD = 0.6) genes, segregated 221 myelomas into four discrete subgroups (Figure 1; see Color Figures, page 510). These groups were characterized by significant differences in genetic features, such as chromosome ploidy (P < 0.01), trisomy 11 (P < 0.001), and 14q32 IGH translocations (P < 0.01), as well as by clinical parameters, such as IgA isotype, albumin (< 3.5 g/dL), β2M (> 4 mg/L), creatinine (> 2 mg/d), MRI lesions (P < 0.05), and EFS (P = 0.002) and OS (P = 0.06).
In the current unsupervised hierarchical cluster analysis, we noted a nonrandom distribution of spiked expression of recurrent translocation partners within the dendrogram-defined subgroups (Figure 2; see Color Figures, page 511). The data revealed that 26 of 30 CCND1 spikes were clustered in one subgroup whereas 28 of 32 MMSET/FGFR3, 4 of 5 MAF, and 3 of 4 MAFB spikes were all located in the same cluster branch. CCND1 expression, either low-level expression associated with chromosome 11 trisomy and hyperdiploid karyotypes or spiked expression linked to the t(11;14)(p13;q32) translocation and presence of normal karyotypes, defined two distinct subgroups with relatively low risk for relapse (Figure 3 ). Elevated expression of proliferation-associated genes, whether in a hyperdiploid or hypodiploid karyotype or MMSET/MAF/MAFB, defined two poor-prognosis subgroups. The MMSET/MAF/MAFB groups was more frequently associated with hypodiploid karyotypes, an IgA isotype, increased incidence of 14q32 translocations by FISH, and reduced incidence of MRI lesions, whereas the CCND1 spike group was significantly associated with normal cytogenetics. Taken together, our results highlight the relationship between the transcriptome, cytogenetics, and the biological and clinical features of myeloma. These findings extend and refine our previous gene expression classification system and provide further evidence that distinct molecular entities of myeloma exist.
Cox Regression Modeling of Gene Expression Patterns in Pretreatment Plasma Cells Identifies Three Genes Associated with Rapid Relapse
Because microarrays are not likely to become routine clinical tests, comprehensive global GEP studies will probably be used to identify a small subset of genes whose expression can be applied in the development of gene-based risk-adapted patient stratification, as has been recently done in lymphoma.21 We used Cox regression modeling of gene expression on EFS in 212 newly diagnosed myeloma patients using data from U95Av2 microarray (~10,000 genes). The median follow-up at the time of this analysis was 20 months. There were 34 events representing either disease-specific death or progression/relapse. EFS was modeled by using standard prognostic values as well as by GEP using the Affymetrix signal. A generalized estimate of R2 was obtained by using the approach suggested by Cox and Snell.24 Gene expression values considered were based on significance of univariate association with EFS. The median signal call was used as a cut point prior to modeling inclusion. The 100 genes most significantly associated with EFS based on the score test were potential variables in multivariate modeling. Using standard prognostic variables only, the model that best fit disease-specific EFS was FISH13 (chromosome 13 deletion detected by FISH) and the presence of chromosomal abnormalities (CA).
Adjusting for CA and FISH13, three genes—RAN, ZHX-2, and CHC1L—were simultaneously significant, each at the P < 0.005 level. After adjustment for other model variables, patients with high RAN expression had increased risk of event while patients with high ZHX-2 or high CHC1L had decreased risk of event. After adjusting for these three genes and each other, FISH13 patients had an increased risk of event but those with CA did not. R2 for the FISH13 and CA model was 30%. The R2 value for the RAN, ZHX-2, and CHC1L model was 66%. It is important to note that each of these genes has independent prognostic influence, so the combination of RAN overexpression and loss of expression of ZXH-2 and CHC1L represents a much more powerful model than any other combination (Figure 4 ).
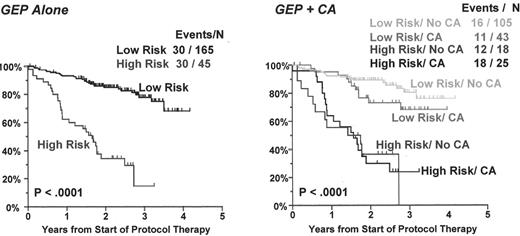
We next compared the EFS between a high-risk entity (patients whose plasma cells express RAN above the median and ZHX-2 and CHC1L below the median; n = 45) and a low-risk entity (all remaining cases; n = 165) (Figure 5, left panel). In this population, there were 30 events in the 45 high-risk patients (66%) and 30 events in the 165 low-risk patients (18%) (P < 0.0001). We next calculated the EFS based on the combination of GEP risk groups and CA (Figure 5, right panel). EFS rates were not significantly different between high-risk and low-risk disease with and without CA. However, high-risk and low-risk disease groups were significantly different irrespective of the presence of CA (P < 0.0001).
RAN, ZHX-2, and CHC1L Function Provides Insight into How These Genes Might Impart a High-Risk Phenotype
RAN, which maps to 6p21, is a member of the Ras family of GTPase proteins that has a role in many aspects of cell biology, including shuttling protein and RNA in and out of the nucleus as well as regulating chromosome condensation, spindle formation, nuclear assembly, and cell-cycle progression.25,26 During interphase, RAN promotes nuclear localization signal-mediated protein import through the association and dissociation of transport complexes. A complex containing aster-promoting activities, importin α/importin β, forms in the cytosol and translocates across the nuclear pore. In the nucleus, Ran-GTP binds to importin β and dissociates the transport complex. Ran-GTP and importin β shuttle back to the cytosol, where Ran-GTP is hydrolyzed by cytosolic RanGAP1 and RanBP1, to form Ran-GDP. The polarized distribution of Ran-GTP across the nuclear envelope is maintained by the compartmentalization of RCC1 (RanGEF), RanGAP1, and RanBP1. The localization of RCC1 on chromatin promotes microtubule assembly in mitosis. During mitosis, importin α and importin β sequester APA in an inactive form in regions where Ran-GTP is low. Increased levels of Ran-GTP near the chromosome promote local disassembly of APA components from complexes containing importin α and importin β. In high Ran-GTP regions, importin β associates with Ran-GTP, maintaining it in an inactive state until RanGAP1 and RanBP1 catalyze Ran-GTP hydrolysis. After dissociation from importin complexes, APA becomes active in promoting microtubule assembly.27
The CHC1L gene (CHromosome Condensation 1-Like, also called E4.5)28 maps to 13q14.3 in a region suspected to harbor a tumor suppressor gene for myeloma.15,29,30 Although we have evidence that the chromosome 13 tumor suppressor gene may be RB1,15 this has not been proven. Reduced CHC1L expression has emerged as an integral component of a three-gene model for high-risk disease, and, consequently, it must be considered as a potential candidate tumor suppressor gene. Although the function of the product of CHC1L has not been completely described, it is a homologue of RCC1, which, as pointed out above, is the GTP exchange factor (GEF) for RAN.31CHC1L has been proposed to be a candidate tumor suppressor gene in both B-CLL30 and prostate cancer.32 Thus, a loss of function of CHC1L may somehow influence the function of RAN and, together with the increased expression of RAN and loss of ZHX-2, may impart the aggressive phenotype on myeloma with this particular gene expression pattern. The poor MSK phenotype is conferred only by genetic interaction, in that gain of RAN or loss of CHC1L alone does not confer the aggressive phenotype. We are currently investigating whether CHC1L may have a RanGEF function in myeloma, if myeloma with activated RAN and loss of CHC1L has an altered ratio of nuclear and cytoplasmic RAN, and whether levels of Ran-GTP in the nucleus are altered in cases or/altered CHCTL.
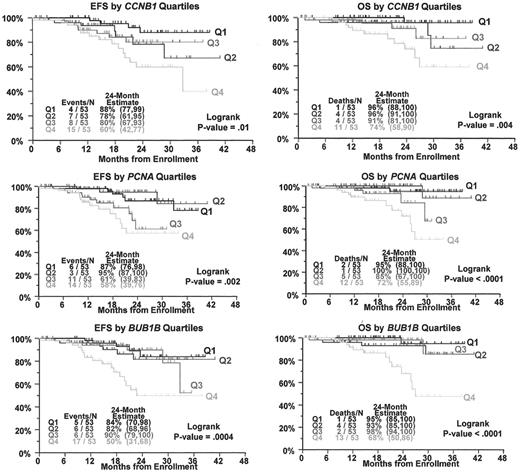
ZHX-2, the third gene in the model, has recently been cloned and has been shown to be a negative regulator of the NF-Y transcription factor.33 This has important implications as the NF-Y complex is a master transcriptional regulator of many genes involved in cell-cycle control and proliferation, including CCNB1, CCNB2, CCND2, CDC2, CDC25, TOP2A, TK1, and others. We have shown that there is a negative correlation between ZHX-2 expression and the expression of a panel of 30 proliferation-associated genes, including genes known to be positively regulated by NF-Y (Figure 6; see Color Figures, page 512). The gene ZHX-2 maps to 8q24.3, very near the MYC oncogene. We have previously shown that the loss of chromosome 8 by cytogenetic analysis imparts a very high risk of relapse, but not to death when patients are treated with high-dose therapy and peripheral blood stem cell transplants.15 Reduced expression of ZHX-2 was linked to poor survival in Total Therapy II, which, based on its chromosomal locus, may provide a mechanistic explanation for why loss of chromosome 8 by conventional cytogenetics imparted such a high risk in Total Therapy I.15
As would be expected from the results of the ZHX-2 correlation studies, overexpression of proliferation-associated genes is also linked to poor survival (Figure 7 ). Given the link between ZHX-2 loss and rapid development of resistance in myeloma to combination chemotherapy, it is noteworthy that NF-Y–mediated transactivation of the human ribonucleotide reductase subunit M2 is related to Gemcitabine resistance.34 The transcription factor homeobox B4 (HOXB4) gene is preferentially expressed in immature hematopoietic cells and implicated in the transition from primitive hematopoiesis to definitive hematopoiesis as well as in immature hematopoietic cell proliferation and differentiation. Recent studies have shown that NF-Y cooperates with USF1/2 to induce the hematopoietic expression of HOXB4.35 Thus, it is possible that myeloma cells that lose ZXH-2 upregulate HOXB4, and these cells acquire a stem cell-like phenotype and resistance to chemotherapy. The mechanism by which ZXH-2 expression is reduced and its potential function in multiple myeloma are currently under investigation.
Conclusion
Unsupervised hierarchical cluster analysis of gene expression patterns in CD138-enriched plasma cells from untreated myeloma patients allowed the identification of four subgroups of patients whose GEP signatures are highly correlated with distinct cytogenetic abnormalities, clinical parameters, and survival after high-dose therapy with peripheral blood stem cell transplant. These data lend credence to the concept that multiple myeloma consists of multiple disease entities with distinct mechanisms of transformation, as evidenced by the highly coordinated gene expression changes seen in each subtype. It is currently not clear whether this coordinated subgroup-specific expression occurs through the deregulation of distinct molecular pathways. However, if confirmed, these data could provide a framework for the development of novel therapeutic interventions based on the molecular biology of disease subtypes. Our experience over the last five years with the prospective application of genomic analysis of tumor cells from a uniformly treated population at diagnosis and follow-up suggests that myeloma is amenable to genomic classification. We firmly believe that a molecular classification of myeloma will prove useful in promoting the more effective application of current therapies and provide the framework for the development of novel therapeutic strategies based on common molecular features of each distinct subtype.
Please note that data described here are preliminary and based on the use of microarrays containing ~12,000 genes in a population of 221 patients. These models represent the most current iterations in our attempts to create a genomic classification of myeloma and have not been validated.
Note in proof: Since writing this chapter, we have developed and validated a molecular classification schema of MM using 351 newly diagnosed patients profiled with the U133Plus2.0 microarray. This new analysis benefited from a near doubling of the number of patient samples and a tripling of the number of genes analyzed (~33,000 vs ~12,000) compared to past studies. These results will be presented in the near future.
II. Stem Cell Transplantation in Multiple Myeloma
Jean-Luc Harousseau, MD,* Michel Attal, MD, Thierry Facon, MD, Hervé Avet-Loiseau, MD, and Philippe Moreau, MD
Hotel Dieu, Place Alexis Ricordeau - BP 1005; CEDEX 01, Nantes 44093, France
Multiple myeloma is one of the diseases in which the impact of dose intensity has been demonstrated. Consequently, in 2002 MM was the first disease for which autologous stem cell transplantation (ASCT) was deemed indicated in Europe. On the other hand, the results of allogeneic SCT remain disappointing due to high transplant-related mortality, increasing the need to explore strategies such as reduced-intensity conditioning. The introduction of novel agents such as thalidomide, bortezomib (Velcade®) or revlimid (Revlimid®) will probably change the field in the next few years.
Autologous Stem Cell Transplantation
Autologous stem cell transplantation versus conventional chemotherapy
The Intergroupe Francophone du Myelome (IFM) was the first, 8 years ago, to demonstrate the superiority of high-dose therapy (HDT) supported by autologous bone marrow transplantation (BMT) compared to conventional chemotherapy (CC) for MM.1 In this randomized IFM90 trial HDT significantly increased the complete remission (CR) rate, the EFS and the OS in patients with newly diagnosed MM up to the age of 65. Following this publication, the number of ASCT performed worldwide as part of front-line therapy in younger patients increased dramatically. However some concerns remained until last year when the IFM90 results were fully confirmed by a larger trial published by the British Medical Research Council (MRC).2 Other randomized trials have been presented in meetings but not yet published.3– 6 Although the details of CC and of HDT varied, the general outline of these studies were the same, except for the Spanish group trial where only patients responding to their initial CC were randomized. The results of all these trials are summarized in Table 1 .
CR defined either by a negative immunofixation (true CR) or by a negative electrophoresis (near CR) was significantly more frequent after HDT, except in the US Intergroup Study where the CR rate obtained with CC was higher than expected (15%). Median EFS was always longer with HDT compared to CC (4–12 months). The difference was significant in 5/6 trials (except in the Spanish trial, where patients refractory to initial CC were excluded). The median EFS is remarkably constant (25–31 months). It is more difficult to analyze OS results since OS partly depends on salvage therapy. However, median OS was significantly longer in the three studies where differences in EFS were more marked. In all studies procedure-related death rate was < 5% and not greater than that observed with CC. From this large experience one can now conclude that HDT supported by ASCT is the standard of care of patients with newly diagnosed MM, at least up to the age of 65.
Tandem autologous transplantation
In the IFM90 trial, achievement of a least a very good partial remission (> 90% reduction of the M-component) was significantly correlated with longer survival. The impact of achieving CR on the duration of disease control and survival was confirmed by several investigators.7 Thus, in MM as in other hematological malignancies, the primary objective of therapy should be to achieve CR. One way to increase the CR rate is to repeat intensive treatments. To this end, Barlogie and colleagues in Arkansas used a strategy with tandem ASCT8 that appeared to increase the CR rate to approximately 40%. In newly diagnosed patients this better tumor cell reduction was translated into encouraging EFS and OS of 43 months and 68 months, respectively.9 However, comparison with less aggressive strategies is needed to evaluate the actual impact of tandem ASCT on EFS and OS.
The IFM was again the first to conduct a randomized trial (IFM94) comparing single and double ASCT.10 In this study, 399 previously untreated patients under the age of 60 years were randomly assigned to receive either a single ASCT prepared by melphalan 140 mg/m2 (Mel 140) plus total body irradiation (TBI) or a double ASCT, the first being prepared by Mel 140 and the second by Mel 140 plus TBI. On an intention-to- treat basis, CR or very good partial remission was achieved by 42% of patients in the single ASCT group versus 50% in the double ASCT group (P = 0.10). The probability of 7-year EFS was 10% versus 20% (P = 0.03) and the probability of 7-year OS was 21% versus 42%, respectively. These results are confirmed by a preliminary analysis of a comparable study conducted by the Bologna Group.11
The preliminary analysis of a Dutch trial reported by Segeren and colleagues yielded negative results, in that there was no improvement in outcome with more intensive therapy.12 However, the updated results showed a significant advantage in term of EFS in the more intensive treatment group.13 Two other studies have been presented in part in meetings.14,15 Current results of these five trials are summarized in Table 2 . Although the CR rate is significantly higher in only 1 trial, the median EFS is significantly longer in 4/5 trials. A significant difference in OS was found only in the IFM94 trial but, since divergence of the OS curves occurred only after 4 years, longer follow-up is probably needed before drawing definite conclusions from other trials. The available data are in favor of tandem autotransplants.
Prognostic factors in the context of autologous stem cell transplantation
As with CC, a number of parameters have been shown to significantly influence the outcome after ASCT in newly diagnosed patients.16 The prognostic value of standard biological markers that are important predictors of outcome after CC has also been demonstrated for ASCT. High levels of β2M, C-reactive protein or LDH, and low levels of albumin have all been associated with poorer outcome.8,10
Genetic abnormalities as evaluated by conventional cytogenetics or by molecular biology techniques appear to be powerful prognostic markers as well. Based on a large experience with tandem ASCT, statistical analysis performed by the Arkansas group has shown that hypodiploidy and cytogenetic abnormalities such as chromosome 13 deletions17,18 or myelodysplastic-like abnormalities19 are associated with a poor prognosis. The presence of chromosome 13 deletions either by conventional cytogenetics17,18 or by FISH20 is an adverse prognostic factor. While metaphase-defined chromosome 13 deletion is found in only 15% of karyotypes, FISH detects this abnormality in approximately 45% of patients. However, when the deletion is found by conventional cytogenetics, the prognosis appears to be even poorer.21
The impact of translocations involving 14q32 has also been studied retrospectively. By FISH analysis, t(4;14) is present in approximately 13% of patients and is associated with a short EFS and OS.22,23 On the other hand, patients with t(11;14) identified by elevated cyclin D1 levels as evaluated by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) appear to enjoy longer remissions and to have longer survival.22,24
The combination of multiple prognostic factors can define prognostic subgroups. For instance, Barlogie and colleagues in Arkansas have shown that patients with low β2M (or low LDH) and no cytogenetic abnormalities have a good prognosis when treated with tandem ASCT.17,25 Patients with high β2M and cytogenetic abnormalities (especially hypodiploidy and/or chromosome 13 deletion) have a poor prognosis even with tandem ASCT, and for these patients new treatments are clearly needed.8,17 The IFM group has shown similar prognostic implications for the combination of β2M and chromosome 13 deletions detected by FISH analysis.20 Patients with low β2M and no chromosome 13 abnormality by FISH had a median OS of 111 months, while patients with high β2M levels and chromosome 13 deletions had a median OS of only 25 months. These findings led to the risk-adapted IFM99, with two different strategies for standard-risk patients (0 or 1 adverse prognostic factor) and for high-risk patients (2 adverse prognostic factors).
The use of initial prognostic factors could also help in determining which patients benefit more from HDT. Unfortunately, in most randomized trials, the number of patients is too small to evaluate the impact of HDT in each prognostic subgroup. In the MRC7 trial comparing CC and HDT, there was a trend toward a greater survival benefit with HDT for the group of patients with a poor prognosis defined by a high β2M level (> 8 mg/L).2 In the IFM94 trial, double ASCT was superior to single ASCT in prognostic subgroups defined by β2M or LDH but, due to a small number of patients in each group, the difference was not statistically significant. The superiority of double ASCT was only significant in patients failing to achieve at least 90% reduction of the M-component within 3 months after one ASCT.10
Therefore, according to available data HDT could be proposed to all patients and could be even more beneficial for patients with adverse prognostic factors. Blade and colleagues reported that there was no significant difference between CC and HDT in patients responding to initial CC in the Spanish Cooperative Group PETHEMA randomized trial,4 suggesting that patients who fail to respond to their first treatment should undergo ASCT as well.
How to further improve the outcome of ASCT
In the IFM94 trial, the 7-year EFS following ASCT is only 20%. Strategies to further improve these results are clearly warranted. One possibility would be to improve upon pretransplant chemotherapy regimens. In current protocols, melphalan 200 mg/m2 (Mel 200) is considered as the standard preparation regimen prior to ASCT.16 In the absence of randomized trials, the benefit of other regimens using higher doses of melphalan, combinations of drugs, or melphalan coupled with bone-seeking radioisotopes remains unknown. It is clear, however, that in the context of tandem ASCT, the dose intensity of the preparative regimen is crucial. In the IFM94 trial10 the CR rate was not significantly higher in the double ASCT arm, but the preparative regimen prior to the first ASCT was only Mel 140, while in the Arkansas experience the CR rate appears higher with two ASCT prepared by Mel 200.6
Barlogie and colleagues have reported impressive results with a more dose-intensive regimen termed “Total Therapy II.”26 This is an aggressive protocol with four consecutive phases: increased intensity induction treatment (including combination chemotherapy with dexamethasone, cyclophosphamide, etoposide, platinum), tandem ASCT, consolidation chemotherapy and maintenance therapy with interferon and dexamethasone. This trial also addressed the possible contribution of thalidomide in a randomized trial design from initiation of treatment, but the thalidomide arm is still blinded. Current analysis of 462 patients included into Total Therapy II program compared to 231 patients treated with the previous “Total Therapy I” protocol shows a higher CR rate (66% versus 43%) and an increased 4-year EFS rate. This improvement was more marked for patients without cytogenetic abnormalities (4-year EFS 70% versus 37%).
The introduction of new agents in frontline therapy is another attractive option. In the last few years, thalidomide has been tested by different groups in different ways. The combination of thalidomide plus dexamethasone is frequently used as induction treatment in the US and is currently compared to dexamethasone alone in an Eastern Cooperative Oncology Group (ECOG) trial.27 As described above, the impact of thalidomide given in addition to chemotherapy throughout the whole program is currently being tested in the Arkansas Total Therapy II. Several trials including IFM99-02 have evaluated the impact of thalidomide given as maintenance therapy. Preliminary results of the IFM99-02 trial show that, in patients with standard prognosis, maintenance treatment with thalidomide significantly prolongs EFS. Bortezomib was recently approved in the US and in Europe for the treatment of relapsed MM. This drug is currently being evaluated as part of induction treatment prior to ASCT, alone or in combination with dexamethasone or with chemotherapy. It is probably more attractive to test the impact of these new agents given in addition to HDT rather than to again compare HDT with standard dose chemotherapy including these drugs.
Allogeneic Stem Cell Transplantation
Standard conditioning regimen
Allogeneic SCT with a conventional myeloablative preparative regimen still has a limited role in MM. Because of its toxicity, it can be proposed only to a small number of patients with MM (patients aged less than 55 years with an HLA-identical sibling). Even in younger patients transplant-related mortality is very high with standard regimens, mainly as a consequence of infection and graft-versus-host disease (GVHD). However, there are several arguments in favor of pursuing allogeneic SCT in MM:
Analysis of the European Blood and Marrow Transplantation Registry showed improved results in the late 1990s, mostly due to a higher frequency of early transplantation.28
Comparison of autologous and allogeneic transplantations show that short-term survival is superior with autologous SCT, mainly due to a higher transplant-related mortality in the first year with allogeneic SCT. However, while patients treated with autologous SCT can experience late relapses, there is a plateau of the EFS and OS curves after 5 years for patients receiving allogeneic transplantation. Long-term survival could be superior with allogeneic transplantation.7
The so-called graft-versus-myeloma effect has been demonstrated by the efficacy of donor lymphocyte infusions in patients relapsing after allogeneic BMT.29
Therefore, recent clinical studies on allogeneic SCT in MM have focused on frontline therapy with the goal of reducing transplant-related mortality while harnessing the graft-versus-myeloma effect.
One way to influence transplant-related mortality is to reduce the incidence and severity of GVHD by T cell depletion. While attempts to reduce GVHD have frequently been associated with a higher incidence of relapse, preliminary results of prophylactic CD4+ donor lymphocyte infusions after CD6 T cell depletion appeared promising.30 But it has been recently shown that response to donor lymphocyte infusions is statistically related to the occurence of GVHD, suggesting that in MM, like in other hematological malignancies, targets for graft versus host and graft versus myeloma are identical or that antitumor activity is related to GVHD.29
Finally, results of a Dutch prospective evaluation of T cell–depleted allogeneic SCT as part of frontline therapy were very disapointing.31 In this series of 53 patients up to the age of 55 with an HLA-identical sibling, the CR rate was only 19% and median OS from transplantation was only 25 months. The cause of death was either disease progression or transplant-related toxicity.
Reduced-intensity allogeneic transplantation
Nonmyeloablative or reduced-intensity allogeneic SCT (so-called miniallogeneic transplantation) represents a new hope and a new way of taking advantage of the graft-versus-myeloma effect related to the donor immune system while reducing transplant-related toxicity. However, it has been shown that relapses are frequent when reduced-intensity regimens are used in patients with relapsed/refractory disease.32,33 In the past few years, reduced-intensity regimens have been widely used after tumor burden reduction with HDT plus ASCT. The feasibility of reduced-intensity allogeneic transplantation after one or two ASCT has been shown.34
HDT with melphalan 200 mg/m2 supported by ASCT yields a high response rate with a low toxicity and a low transplant-related mortality. Allogeneic SCT with nonmyeloablative conditioning regimens provides a tumor-free stem cell source and reduces transplant-related mortality while harnessing the graft-versus-myeloma effect. Although the risk of acute GVHD and of infections related to chronic GVHD and its treatment remains, this approach appears to be safer than allogeneic BMT and can be proposed to older patients (up to 65 years of age). Two groups have published their preliminary experience35,36 and their results are in Table 3 .
In both studies, all patients had sustained allogeneic engraftment, and at 100 days transplant-related mortality was low. However, due to acute or or chronic GVHD complications, 1-year nonrelapse mortality was 15% and 11%, respectively. Allogeneic transplantation with reduced-intensity regimens can be safety proposed to patients older than 50 years. This approach is highly effective, with a CR rate over 50%. Progression free survival and OS rates are encouraging, but the follow-up time is still short. Compared to allogeneic transplantation with a myeloablative conditioning regimen, miniallotransplantation is obviously associated with a much lower transplant-related mortality. The incidence of acute GVHD remains relatively high and is probably influenced by the conditioning regimen itself. Transplantation from unrelated donors is feasible37 and could yield results comparable to those achieved with HLA-identical siblings.
Conditioning regimens with high doses of antithymocyte globulin or with Campath 1-H could reduce the incidence of severe acute GVHD. The optimal procedure (conditioning regimen, type and duration of posttransplant immunosuppression, interval between auto- and allogeneic transplantation, and the role of donor lymphocyte infusions) remains to be determined. Longer follow-up is needed to evaluate the impact of this strategy on EFS and OS.
Prospective multicenter trials comparing tandem autologous transplantation and tandem autologous/allogeneic transplantation are ongoing in the US and in Europe. Preliminary results of the IFM 9903-04 trial were presented at the 2003 ASH meeting.38 This trial recruited patients with newly diagnosed MM up to the age of 65 and with 2 adverse prognostic factors (β2M level > 3 mg/L, presence of chromosome 13 deletion by FISH analysis). After four courses of VAD (vincristine, doxorubicin, dexamethasone), these patients received Mel 200 with ASCT. Then, according to the availibility of an HLA-identical sibling, they received either an autograft with reduced-intensity conditoning or a second allograft prepared by Mel 220 mg/m2 (± anti-interleukin [IL]-6 antibody). There was no significant difference between the two strategies in terms of EFS or OS (both on an intention-to-treat analysis and when comparing actually received treatments). Although the follow-up is still short (median follow-up time 18 months), these results can be interpreted since the outcome in this subgroup of patients is usually poor even with tandem ASCT.20 Moreover it has been recently shown that deletion 13q detected by FISH remains a negative prognostic factor after allograft with reduced-intensity conditioning.39 In this retrospective multicenter study, patients with 13q deletion (n = 31) had a lower 2-year EFS rate (18% vs 42%) and a lower 2-year OS rate (18% vs 47%).
The major question regarding the use of allotransplantation in patients with newly diagnosed MM remains: Should it be proposed to all patients with an HLA-identical sibling (or even an HLA-identical unrelated donor) or should it be proposed to selected patients? For instance, considering the very good results obtained with tandem ASCT in patients with no adverse prognostic factors, is it justified to propose a therapy that carries a high risk of chronic GVHD and a 10%–15% incidence of toxic death? This question remains to be answered by ongoing prospective multicenter trials.
III. The Emerging Role of Targeted Therapies in the Management of Relapsed and Refractory Myeloma
Paul Richardson, MD,* Teru Hideshima, MD, PhD, Constantine Mitsiades, MD, PhD, and Kenneth Anderson, MD
Dana-Farber Cancer Institute, 44 Binney St., Dana D1B20, Boston MA 02115-6084
Multiple myeloma is diagnosed annually in approximately 15,000 new patients in the United States, with a prevalence of approximately 50,000, and remains an incurable neoplasia, despite treatment with conventional and/or high-dose chemotherapy.1 In order to overcome resistance to conventional therapies and improve patient outcome, novel therapeutic strategies for this disease have been and/or are currently being developed. Such therapies are based on the premise that a comprehensive suppression of MM tumor burden cannot be achieved solely by aiming at classical targets of cytotoxic chemotherapeutics (e.g., DNA or cytoskeleton) but also require targeting of other molecular pathways implicated in regulation of the proliferation and survival of MM cells, including the molecular mechanisms underlying the protective effects of the bone marrow (BM) microenvironment on MM cells.1
The recent expansion of the therapeutic armamentarium for MM can be attributed, at least in part, to a constellation of in vitro and in vivo preclinical models for this disease. These models have elucidated how MM cells specifically home to the BM microenvironment and the mechanisms whereby they locally proliferate, survive, resist the effects of conventional drugs, recruit new blood vessels and perturb the process of bone remodeling to cause lytic bone lesions.1 These model systems have facilitated the development of promising biologically based treatments that can target the MM cells, disrupt their interaction with the BM microenvironment, and thereby overcome classical drug resistance in vitro. Novel therapies that have already been translated into clinical applications include thalidomide (Thal) and its more potent immunomodulatory analogs (IMiDs),2 the proteasome inhibitor bortezomib (also known as PS-341, Velcade®),3 and arsenic trioxide (As2O3)4 (Figures8 and 9; see Color Figures, page 513). Furthermore, several other agents that can also target the MM cell itself and/or bypass the protection that it enjoys from BM stromal cells have been identified, including farnesyl transferase inhibitors,5 histone deacetylase inhibitors,6,7 inhibitors of vascular endothelial growth factor (VEGF) or its receptor, heat shock protein inhibitors8 and inhibitors of insulin-like growth factors and their proliferative/anti-apoptotic receptor (IGF-1R).9,10
The in vitro preclinical studies that led to the identification of these classes of drugs as candidates for clinical development in MM involve both classical drug-sensitivity studies of cultured tumor cells as well as drug-sensitivity studies of MM cells co-cultured with BM stromal cells. These latter assays can identify which drugs can overcome the protective effects of BM on MM cells. Those agents that demonstrate promising in vitro results are subsequently tested in murine models of MM. IMiDs11 and bortezomib12 have been shown to inhibit human MM cell growth, decrease tumor-associated angiogenesis, and prolong host survival in models of human MM xenografts implanted subcutaneously in severe combined immunodeficient (SCID) mice. Subsequent Phase I, II and III clinical trials of IMiDs13 and bortezomib14 have already demonstrated marked clinical activity even in patients with refractory and relapsed MM. Corresponding in vitro gene array studies of MM tumor cells before and after treatment with novel anti-MM agents7,10,15,16 have helped to identify in vivo targets and mechanisms of novel drug action as well as mechanisms of drug resistance. This helps to determine whether in vivo targets of these novel therapies correlate with their in vitro anti-MM activities in relapsed and refractory disease.
The Role of the BM in MM
Extensive studies have focused on the mechanisms whereby MM cells home to the host BM and adhere to BM stromal cells (BMSCs) and extracellular matrix proteins, and the functional sequelae of these interactions (Figure 10; see Color Figures, page 514). Such studies have identified a series of cell adhesion molecules mediating MM cell binding to fibronectin and BMSCs, thereby increasing MM cell proliferation and survival.17 This proliferative and anti-apoptotic advantage conferred by stromal adhesion is largely due to BMSC-derived cytokines, such as IL-617 and insulin growth factor 1 (IGF-1).9,10 In addition, the MM cell-BMSC binding interaction modulates the expression of other cytokines, such as VEGF, which in turn stimulates increased local angiogenesis,18 thereby further facilitating MM cell proliferation and viability. Importantly, these aforementioned cytokines/growth factors can cooperate, in additive and/or synergistic manners, to stimulate MM cell proliferation and survival.9,10,19
Antitumor Activity of Novel Agents in Animal Models
The severe combined immunodeficiency (SCID)-hu model of human MM has been developed to allow studies of the dynamic interaction between MM cells and the BM stroma, and to examine the efficacy of novel therapies in that setting. Human bone grafts are implanted bilaterally in the flanks of SCID mice. Human MM cells implanted into these grafts proliferate, secrete MM idiotypic protein detectable in mouse serum, and can migrate predominantly to the contralateral human BM graft.20 This in vivo model of human MM has provided a means to evaluate the mechanisms mediating the specific homing of human MM cells to the human BM microenvironment, as opposed to the murine BM microenvironment, as well as for studying the role of microenvironmental factors (host-MM cell interactions, cytokines, angiogenesis) in MM pathogenesis. In vivo assays of drug efficacy have also used a beige-nude-xid mouse model of subcutaneous human plasmacytoma xenografts. These models were critical for preclinical evaluation of Thal, IMiDs, bortezomib and other novel agents.
Clinical Trials of Agents Targeting MM and the BM Microenvironment
Translation of preclinical studies from the bench to the bedside has resulted in clinical trials for Thal/IMiDs,2,11,21,22 bortezomib,3,15 and As2O3.4 Thal was originally proposed as a potential therapy for refractory MM because of its known anti-angiogenic activity and the demonstration of increased angiogenesis in MM BM.2,23 In addition, Thal and IMiDs induce either apoptosis or G1 growth arrest of MM cells by multiple other mechanisms.2 They block MM cell adhesion to BMSCs and the resultant secretion of MM growth, survival, and migratory factors (IL-6, VEGF); and enhance natural killer (NK) cell number and function against human MM cells21 (Figure 8; see Color Figures, page 513).
Clinical trials have investigated Thal either as a single agent or in combination regimens to treat relapsed, refractory MM and as first-line therapy in untreated MM. Using response criteria defined as ≥ 25% reduction in serum M-protein concentration, response rates for Thal as monotherapy in relapsed, refractory multiple myeloma and typically dosed in the range of 100–600 mg per day have ranged from approximately 30% to 70%, with a median time to response ranging from 3 to 8 weeks.24,25 In relapsed, refractory disease, the combination of Thal with melphalan and melphalan plus dexamethasone (Dex) yielded response rates of 82% (> 25% reduction in M-protein) but significant rates of myelotoxicity, with 87% and 62% of patients experiencing leukopenia and neutropenia, respectively.26,27 In a study testing Thal in combination with liposomal doxorubicin, vincristine, and Dex (DVd-T) for relapsed/refractory multiple myeloma, an encouraging overall response rate of 74% was seen, but an increased incidence of neutropenia, bacterial and viral infection, and deep vein thrombosis was observed, prompting a protocol amendment to allow concomitant treatments, which proved largely successful in abrogating these toxicities.28
The safety and efficacy of Thal in the first-line treatment of MM has been clinically investigated and reported.29,30 In the Mayo Clinic Phase II trial, patients received Thal 200 mg per day and Dex 40 mg on days 1–4, 9–12, and 17–20 (odd cycles) and 40 mg/d on days 1–4 (even cycles); a 64% rate of major response, defined as ≥ 50% reduction in serum and urine M-protein, was reported. The most common grade 3/4 toxicities were deep vein thrombosis (12%), constipation (8%), rash (6%), and dyspnea (4%).29 The MD Anderson Cancer Center (MDACC) Phase II trial enrolled patients with previously untreated symptomatic disease to receive Thal 100 mg per day, escalated to 400 mg per day, plus Dex 20 mg. Partial and complete responses were observed in 63% of patients, and grade 3 toxicities included infection (23%), thrombotic/embolic events (15%), constipation (3%), and fatigue (3%).19 The ECOG recently completed a randomized Phase III study investigating Thal 200 mg per day plus Dex 40 mg or Dex 40 mg alone in patients with newly diagnosed myeloma for four cycles (28 days per cycle), followed by stem cell collection in patients eligible for high-dose chemotherapy (ECOG-EA100). A recent interim analysis of 109 patients showed an overall response rate of 80% (≥ 50% reduction in serum and urine M-protein) in the Thal/Dex arm, significantly higher than 53% for patients on Dex alone (P = .0023; one-sided Fisher’s exact test). The most important side effect reported was deep vein thrombosis (including pulmonary embolus), occurring in 16% of patients receiving the Dex/Thal combination, compared with 3% receiving Dex alone.31
Promising preclinical data prompted a Phase I IMiD CC-5013 (also known as lenalidomide or Revlimid®) dose escalation (5 mg/day, 10 mg/day, 25 mg/day and 50 mg/day) study in 27 patients (median age 57, range 40–71 years) with relapsed and refractory relapsed MM.13 Patients had received a median of 3 (range 2–6) prior regimens, including autologous stem cell transplantation and Thal in 15 and 16 patients, respectively. In 24 evaluable patients, no dose-limiting toxicity was observed in patients treated at any dose level within the first 28 days; however, grade 3 myelosuppression developed after day 28 in all 13 patients treated with 50 mg/day CC-5013. In 12 patients, dose reduction to 25 mg/day was well tolerated and therefore considered the maximal tolerated dose. Importantly, no significant somnolence, constipation, or neuropathy has been seen in any cohort. Best responses of ≥ 25% reduction in paraprotein occurred in 17 of 24 (71%) patients (90% confidence interval [CI]: 52%, 85%), including 11 (46%) patients who had received prior Thal. Stable disease (< 25% reduction in paraprotein) was observed in an additional 2 (8%) patients. Therefore, 17 of 24 (71%) patients (90% CI: 52%, 85%) demonstrated benefit from treatment.13 This study, and a second Phase I study by Zangari and colleagues which corroborated these findings,32 prompted several Phase II studies of CC-5013, either alone or in combination with Dex, for patients with first relapse and more advanced relapsed, refractory MM. These studies, and two recently completed large multicenter trials testing CC-5013 show promising response and tolerability. Other studies already ongoing or to begin soon in MM include CC-5013 as initial therapy, and as maintenance post HDT and ASCT. A second IMiD (CC-4047, also known as Actimid® ) has been tested in a Phase I study of patients with relapsed MM, and results have shown a favorable toxicity profile (although deep vein thrombosis occurred in approximately 10% of patients) and high response rates.33
Proteasome inhibitors are a novel class of therapeutics targeting the MM cell in its BM microenvironment by blocking NF-κB–mediated IL-6 production. Bortezomib has been shown to induce apoptosis of MM cells resistant to known conventional therapies. It overcomes the protective effects of IL-6, and adds to the anti-MM effects of Dex3 (Figure 9; see Color Figures, page 513). It acts in the BM microenvironment to inhibit the binding of MM cells to BMSCs, the transcription and secretion of IL-6 triggered by MM to BMSC adhesion, and BM angiogenesis. Gene microarray profiling studies demonstrate that bortezomib induces transcriptional downregulation of growth/survival signaling pathways and upregulates apoptotic cascades, ubiquitin/proteasome pathways, and heat-shock proteins.15 In a multicenter Phase II trial of bortezomib in patients with advanced and heavily pretreated MM, CR was seen by Blade criteria in 10% of patients, with 4% immunofixation negative and 6% with residual MM paraprotein detected only by immunofixation.14 The overall response rate (MR + PR + CR) was 35% with an additional 24% experiencing stable disease. In a recently updated analysis of time-to-event parameters, median time to progression was 7 months and median duration of survival was 17 months, with the median duration of response 12.7 months in those patients with PR and CR.34 Responses were associated with improved hemoglobin levels, decreased blood transfusion requirements, improvement in renal function and normalization of uninvolved immunoglobulins. Drug-related gastrointestinal toxicity and fatigue were in most cases manageable; significant thrombocytopenia and neuropathy occurred primarily in patients in whom these conditions were pre-existent, with treatment-emergent neuropathy seen in about a third of patients, and serious adverse events were relatively uncommon.14 Based upon preclinical studies showing an additive anti-MM activity of Dex and bortezomib,3 Dex was added in those patients who either progressed or achieved only stabilization of disease on bortezomib alone, with additional responses seen in 18% of those evaluable.14 As a result of these encouraging data, US Food and Drug Administration (FDA) approval for bortezomib in the treatment of relapsed and refractory MM was given in 2003. An interim analysis of a Phase III international, randomized trial of bortezomib versus high-dose Dex in relapsed MM showed significant benefit in the bortezomib arm as assessed by time to progression and OS, resulting in the closure of the Dex arm and permitting all patients to cross over to bortezomib.35 Trials of bortezomib in newly diagnosed MM patients are ongoing. Combination trials in which bortezomib has been administered with other agents, such as pegylated liposomal doxorubicin, melphalan, and Thal, have shown considerable promise, with manageable toxicity and responses observed in patients otherwise resistant to each agent alone.36– 39
As2O3 has been identified as a third agent targeting the MM cell interacting with its BM microenvironment (Figure 8; see Color Figures, page 513). As2O3 at clinically achievable levels induces apoptosis of drug-resistant MM cell lines and patient cells via caspase-9 activation, adds to Dex, and can overcome the anti-apoptotic effects of IL-6.4 It also decreases MM cell binding to BMSCs, inhibits IL-6 and VEGF secretion in BMSCs induced by MM cell adhesion, and blocks proliferation even of those MM cells adherent to BMSCs. Clinical trials of As2O3 are ongoing.
VEGF inhibitors (including PTK787/ZK222584) have been identified as a promising therapy in MM as VEGF is expressed and secreted by MM cells and BM stromal cells with VEGF secretion by MM cells augmenting IL-6 secretion in BM stromal cells.18 VEGF receptor tyrosine kinase inhibitor PTK787/ZK222584 blocks VEGF-induced tyrosine phosphorylation of Flt-1, MEK/MAPK activation, and proliferation as well as protein kinase C (PKC) activation-dependent migration.18 These studies both define VEGF as a novel therapeutic target and provide the basis for a current Phase II clinical trial of the oral agent PTK787/ZK222584 in patients with relapsed MM.
The cytosolic enzyme farnesyl transferase moves the farnesyl group from farnesyl diphosphate to the CAAX motif of Ras, thereby facilitating its attachment to the inner plasma cell membrane and initiating signal transduction. Inhibition of farnesylation therefore blocks Ras activity, and several farnesyl transferase inhibitors (FTI) inhibit tumor cell growth both in vitro and in vivo. Cytokine (IL-6, VEGF, IGF-1)-induced proliferation of MM cells is mediated, at least in part, via the Ras/Raf/MAPK signaling, which provides the basis for both ongoing and recently reported Phase I/II clinical trials of two promising FTIs, SCH-66336 and R115777, in relapsed and refractory MM.5
Histone acetylation modulates gene expression, cellular differentiation and survival; it is regulated by the opposing activities of histone acetyltransferases and histone deacetylases (HDACs). Novel hydroxamic acid-based hybrid polar compounds, such as suberoylanilide hydroxamic acid (SAHA), are histone deacetylase inhibitors that induce differentiation and/or apoptosis selectively in transformed and neoplastic cells. SAHA is an orally bioavailable HDAC inhibitor, which induces in vitro growth arrest and apoptosis of primary tumor cells from MM patients and cell lines, irrespective of their status of resistance to other agents.6,7 Phase I studies in other malignancies have documented that SAHA is well tolerated, and a Phase I trial of this promising novel agent for the treatment of relapsed MM is underway.
Heat shock protein 90 (hsp90) is a molecular chaperone that facilitates the intracellular trafficking, conformational maturation, and proper 3-dimensional folding of a broad range of intracellular proteins required for cell proliferation, survival and drug resistance. Selective inhibitors of hsp90, such as ansamycin antibiotic geldanamycin (GA) and its analogs (e.g., 17-allylamino-17-demethoxy-geldanamycin or 17-AAG) have profound pro-apoptotic consequences on MM cells, regardless of their resistance to other agents. Gene microarray profiling has shown that bortezomib treatment induces a stress response in MM cells, which includes the compensatory upregulation of hsp90, in an effort of the tumor cells to escape the effect of proteasome inhibition; conversely, blocking this protective stress response with GA or 17AAG enhances bortezomib-triggered MM cell apoptosis.15 A Phase I study of single agent 17-AAG in relapsed and relapsed, refractory MM patients is now ongoing.
P38a MAPK mediates the production of various pro-inflammatory cytokines such as IL-1B, IL-6 and tumor necrosis factor alpha (TNFα), which play a critical role in MM, and the targeting of P38a MAPK has been shown to inhibit MM cell growth and survival in the BM milieu.40 An orally bioavailable P38a MAPK inhibitor (SCIO-469) is now entering Phase II study in relapsed, refractory MM in combination with bortezomib.
Evolution of a New Treatment Paradigm for Relapsed and Refractory MM
In vitro and animal model studies have demonstrated the importance of the BM in promoting MM cell growth, survival, drug resistance, and migration in the BM microenvironment and have already inspired promising therapies based upon targeting the MM cell in its BM milieu. These studies provide the framework for development of a new treatment paradigm in MM, targeting both the tumor cell and its microenvironment. Such an approach is urgently needed since microenvironment-derived protection of tumor cells is a key reason for the lack of curative outcome in relapsed and refractory MM patients treated with conventional therapies. Many of these novel agents, which target the MM cell in its BM milieu, have multiple biologic activities, which may be advantageous, since MM lacks ubiquitously shared fundamental molecular targets, such as those that have been therapeutically exploited in chronic myelocytic leukemia or acute promyelocytic leukemia. Ongoing microarray and proteomic studies of these novel agents in MM are delineating molecular mechanisms of drug sensitivity and drug resistance. These studies will generate more selective therapies for validation in animal models and translation to the bedside in clinical trials. Conversely, gene microarray and proteomic studies of tumor, blood, and BM samples from patients treated on clinical trials of novel agents will potentially both define in vivo targets conferring drug sensitivity and resistance, and provide the framework for the development of more selective, potent, and less toxic targeted therapies. These studies will also establish the preclinical rationale for combining novel and conventional therapies, and allow for selection of those patients most likely to respond.
Autologous stem cell transplantation versus conventional chemotherapy. Results of randomized trials.
| Study (reference) . | No. of Patients . | Age . | CR Rate (%) (CC vs ASCT) . | p value . | Median EFS (months) (CC vs ASCT) . | p value . | Median OS (months) (CC vs ASCT) . | p value . |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: ASCT, autologous stem cell transplantation; CC, conventional chemotherapy; IFM, Intergroupe Francophone du Myelome; MRC, Medical Research Council; MAG, Myelome Auto Greffe; PETHEMA, Spanish Cooperative Group; USIG, US Inter Group; IMMSG, Italian Multiple Myeloma Study Group; CR, complete remission; EFS, event-free survival; NS, not significant | ||||||||
| IFM90 (1) | 200 | ≤ 65 | 5 vs 22 | < 0.001 | 18 vs 28 | 0.01 | 44 vs 57 | 0.03 |
| MRC7 (2) | 401 | ≤ 64 | 8 vs 44 | < 0.001 | 19 vs 31 | < 0.001 | 42 vs 54 | < 0.001 |
| MAG91 (3) | 190 | 55–65 | 19 vs 25 | 0.05 | 45 vs 42 | NS | ||
| PETHEMA (4) | 164 | ≤ 65 | 11 vs 30 | 0.002 | 34 vs 42 | NS | 67 vs 65 | NS |
| USIG (6) | 50 | 15 vs 17 | NS | 21 vs 25 | 0.05 | 53 vs 58 | NS | |
| IMMSG (5) | 195 | 50–70 | 7 vs 26 | < 0.0001 | 16 vs 28 | 0.0036 | 43 vs 58+ | 0.0008 |
| Study (reference) . | No. of Patients . | Age . | CR Rate (%) (CC vs ASCT) . | p value . | Median EFS (months) (CC vs ASCT) . | p value . | Median OS (months) (CC vs ASCT) . | p value . |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: ASCT, autologous stem cell transplantation; CC, conventional chemotherapy; IFM, Intergroupe Francophone du Myelome; MRC, Medical Research Council; MAG, Myelome Auto Greffe; PETHEMA, Spanish Cooperative Group; USIG, US Inter Group; IMMSG, Italian Multiple Myeloma Study Group; CR, complete remission; EFS, event-free survival; NS, not significant | ||||||||
| IFM90 (1) | 200 | ≤ 65 | 5 vs 22 | < 0.001 | 18 vs 28 | 0.01 | 44 vs 57 | 0.03 |
| MRC7 (2) | 401 | ≤ 64 | 8 vs 44 | < 0.001 | 19 vs 31 | < 0.001 | 42 vs 54 | < 0.001 |
| MAG91 (3) | 190 | 55–65 | 19 vs 25 | 0.05 | 45 vs 42 | NS | ||
| PETHEMA (4) | 164 | ≤ 65 | 11 vs 30 | 0.002 | 34 vs 42 | NS | 67 vs 65 | NS |
| USIG (6) | 50 | 15 vs 17 | NS | 21 vs 25 | 0.05 | 53 vs 58 | NS | |
| IMMSG (5) | 195 | 50–70 | 7 vs 26 | < 0.0001 | 16 vs 28 | 0.0036 | 43 vs 58+ | 0.0008 |
Single versus double autologous stem cell transplantation (ASCT). Results of randomized studies.
| . | . | Preparative Regimen . | CR Rate (%) . | EFS (months) . | OS (months) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (Ref) . | No. of Pts . | Age . | Single . | Double . | Difference . | Single vs Double . | p value . | Single vs Double . | p value . | Single vs Double . | p value . |
| Abbreviations: CR, complete remission; EFS, event-free survival; OS, overall survival; IFM, Intergroupe Francophone du Myelome; MAG, Myelome Auto Greffe; GMMG, German Multiple Myeloma Group; Mel, melphalan; TBI, total body irradiation; BU, busulfan; Cy, cyclophosphamide; VP16, etoposide; | |||||||||||
| IFM94 (10) | 399 | < 61 | Mel140+TBI | Mel280+TBI | Mel140 | 42 vs 50 | 0.10 | 25 vs 30 | 0.03 | 48 vs 58 | 0.01 |
| MAG 95 (14) | 227 | < 56 | Mel140+ multidrug + TBI | Mel280+ VP16+TBI | < Mel140 | 39 vs 37 | NS | 31 vs 33 | NS | 49 vs 73 | 0.14 |
| Bologna (11) | 220 | < 61 | Mel200 | Mel320+ BU12 | Mel120+ BU12 | 31 vs 43 | NS | 21 vs 31 | 0.02 | 56 vs 60 | NS |
| GMMG (15) | 261 | < 66 | Mel200 | Mel400 | Mel200 | – | – | 23 vs NR | 0.03 | – | – |
| Hovon (13) | 303 | < 66 | Mel140 | Mel140+ Cy120+TBI | Cy120+TBI | 13 vs 28 | 0.002 | 20 vs 22 | 0.01 | 55 vs 50 | NS |
| . | . | Preparative Regimen . | CR Rate (%) . | EFS (months) . | OS (months) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (Ref) . | No. of Pts . | Age . | Single . | Double . | Difference . | Single vs Double . | p value . | Single vs Double . | p value . | Single vs Double . | p value . |
| Abbreviations: CR, complete remission; EFS, event-free survival; OS, overall survival; IFM, Intergroupe Francophone du Myelome; MAG, Myelome Auto Greffe; GMMG, German Multiple Myeloma Group; Mel, melphalan; TBI, total body irradiation; BU, busulfan; Cy, cyclophosphamide; VP16, etoposide; | |||||||||||
| IFM94 (10) | 399 | < 61 | Mel140+TBI | Mel280+TBI | Mel140 | 42 vs 50 | 0.10 | 25 vs 30 | 0.03 | 48 vs 58 | 0.01 |
| MAG 95 (14) | 227 | < 56 | Mel140+ multidrug + TBI | Mel280+ VP16+TBI | < Mel140 | 39 vs 37 | NS | 31 vs 33 | NS | 49 vs 73 | 0.14 |
| Bologna (11) | 220 | < 61 | Mel200 | Mel320+ BU12 | Mel120+ BU12 | 31 vs 43 | NS | 21 vs 31 | 0.02 | 56 vs 60 | NS |
| GMMG (15) | 261 | < 66 | Mel200 | Mel400 | Mel200 | – | – | 23 vs NR | 0.03 | – | – |
| Hovon (13) | 303 | < 66 | Mel140 | Mel140+ Cy120+TBI | Cy120+TBI | 13 vs 28 | 0.002 | 20 vs 22 | 0.01 | 55 vs 50 | NS |
Autologous stem cell transplantation (ASCT) followed by allogeneic transplantation with reduced-intensity conditioning.
| Author (Ref) . | Patients . | Age (median) . | Auto- logous SCT . | RIC . | GVHD . | TRM . | CR Rate . | Median Follow- Up . | Survival . |
|---|---|---|---|---|---|---|---|---|---|
| * plus fludarabine in 7 cases | |||||||||
| ** 23 unrelated donors | |||||||||
| Abbreviations: Mel, Melphalan; Flu, fludarabine; TBI, total body irradiation; ATG, antithymocyte globulin; aGVHD, acute graft-versus-host disease; cGVHD chronic graft-versus-host disease; SCT, stem cell transplantation; TRM, transplant-related mortality; CR, complete remission; EFS, event-free survival; OS, overall survival | |||||||||
| Maloney (36) | 54 (48% relapsed or refractory MM) | 29–71 (52) | Mel 200 | low-dose* TBI (2 Gy ) | 38.5% aGVHD ≥ II 46% cGVHD | 100 days 2% 1-yr 15% | 52% | 18 m | 2-yr EFS 55% 2-yr OS 78% |
| Kroger (35) | 47** | 31–64 (52) | Mel 200 | Flu/Mel/ATG | 32% aGVHD ≥ II 32% cGVHD | 100 days 6% | 55% | 15 m | 3-yr EFS 54% 3-yr OS 70% |
| Author (Ref) . | Patients . | Age (median) . | Auto- logous SCT . | RIC . | GVHD . | TRM . | CR Rate . | Median Follow- Up . | Survival . |
|---|---|---|---|---|---|---|---|---|---|
| * plus fludarabine in 7 cases | |||||||||
| ** 23 unrelated donors | |||||||||
| Abbreviations: Mel, Melphalan; Flu, fludarabine; TBI, total body irradiation; ATG, antithymocyte globulin; aGVHD, acute graft-versus-host disease; cGVHD chronic graft-versus-host disease; SCT, stem cell transplantation; TRM, transplant-related mortality; CR, complete remission; EFS, event-free survival; OS, overall survival | |||||||||
| Maloney (36) | 54 (48% relapsed or refractory MM) | 29–71 (52) | Mel 200 | low-dose* TBI (2 Gy ) | 38.5% aGVHD ≥ II 46% cGVHD | 100 days 2% 1-yr 15% | 52% | 18 m | 2-yr EFS 55% 2-yr OS 78% |
| Kroger (35) | 47** | 31–64 (52) | Mel 200 | Flu/Mel/ATG | 32% aGVHD ≥ II 32% cGVHD | 100 days 6% | 55% | 15 m | 3-yr EFS 54% 3-yr OS 70% |
Outcome in gene expression profiling (GEP)–defined myeloma subgroups.
Kaplan-Meier plots of overall survival (OS) (left panel) and event-free survival (EFS) (right panel), dated from initiation of Total Therapy II, according to the GEP subgroups. Numbers in brackets indicate 95% confidence interval. Note that risk of relapse is significantly different between the groups. As expected, Group IV has the shortest EFS, Group III exhibits an intermediate risk, and Groups I and II, which are essentially the same, have the best prognosis. Although the OS is not currently significant, a trend similar to the EFS is evident. Groups I–IV are defined as above. The cumulative number of patients does not equal 221 due to removal of patients who had a diagnostic gene array but failed to start the protocol.
Outcome in gene expression profiling (GEP)–defined myeloma subgroups.
Kaplan-Meier plots of overall survival (OS) (left panel) and event-free survival (EFS) (right panel), dated from initiation of Total Therapy II, according to the GEP subgroups. Numbers in brackets indicate 95% confidence interval. Note that risk of relapse is significantly different between the groups. As expected, Group IV has the shortest EFS, Group III exhibits an intermediate risk, and Groups I and II, which are essentially the same, have the best prognosis. Although the OS is not currently significant, a trend similar to the EFS is evident. Groups I–IV are defined as above. The cumulative number of patients does not equal 221 due to removal of patients who had a diagnostic gene array but failed to start the protocol.
High-risk myeloma defined by expression of three genes.
Cumulative incidence of event-free survival defined by the various combinations of expression of three genes. Note that only high RAN/low ZHX-2/low CHC1L shows dismal prognosis. It is important to note that the three-gene combination accounts for 66% of patient outcome variability. The best current models are near 30%.
High-risk myeloma defined by expression of three genes.
Cumulative incidence of event-free survival defined by the various combinations of expression of three genes. Note that only high RAN/low ZHX-2/low CHC1L shows dismal prognosis. It is important to note that the three-gene combination accounts for 66% of patient outcome variability. The best current models are near 30%.
Gene expression profiling (GEP) model defines a high-risk disease and is not affected by presence of abnormal cytogenetics.
Left panel presents Kaplan-Meier curves for high-risk myeloma (high RAN/low ZHX-2/low CHC1L) and low-risk myeloma (all others). The right panel shows Kaplan-Meier curves for high-risk and low-risk disease with respect to the presence or absence of any cytogenetic abnormalities (CA). Note that the model is not influenced by CA, implying that GEP high-risk myeloma is a more robust indicator of poor prognosis than the current best prognostic variable.
Gene expression profiling (GEP) model defines a high-risk disease and is not affected by presence of abnormal cytogenetics.
Left panel presents Kaplan-Meier curves for high-risk myeloma (high RAN/low ZHX-2/low CHC1L) and low-risk myeloma (all others). The right panel shows Kaplan-Meier curves for high-risk and low-risk disease with respect to the presence or absence of any cytogenetic abnormalities (CA). Note that the model is not influenced by CA, implying that GEP high-risk myeloma is a more robust indicator of poor prognosis than the current best prognostic variable.
Elevated expression of cell cycle- and proliferation-associated genes is associated with poor event-free survival (EFS) (left) and overall survival (OS) (right).
Kaplan-Meier curves based on the four quartiles of CCNB1 (top), PCNA (middle), and BUB1B (bottom) gene expression. Q1 is the lowest quartile and Q4 is the highest. Note that all Q4 patients tend to do poorly.
Elevated expression of cell cycle- and proliferation-associated genes is associated with poor event-free survival (EFS) (left) and overall survival (OS) (right).
Kaplan-Meier curves based on the four quartiles of CCNB1 (top), PCNA (middle), and BUB1B (bottom) gene expression. Q1 is the lowest quartile and Q4 is the highest. Note that all Q4 patients tend to do poorly.