Abstract
Venous thromboembolism (VTE), manifested as either deep venous thrombosis (DVT) or pulmonary embolism (PE), is an extremely common medical problem, occurring either in isolation or as a complication of other diseases or procedures. Yet, despite its frequency, much remains to be learned regarding the pathogenic mechanisms that initiate VTE, about tailoring its treatment to the individual with her/his specific set of risk factors for recurrence, and about its medical management when associated with specific disease entities, such as cancer. These three topics are addressed in this chapter.
In Section I, Drs. López and Conde discuss the mechanisms by which venous thrombi may be initiated on the vessel wall in the absence of anatomically overt vessel wall injury. The authors propose a model whereby tissue factor (TF)–bearing microvesicles that arise from cells of monocyte/macrophage lineage can fuse with activated endothelial cells in regions of vessel activation or inflammation and initiate blood coagulation. Key components of this model include docking of the microvesicles to the stimulated endothelium through P-selectin glycoprotein ligand–1 on their surfaces binding to either P-selectin or E-selectin on the endothelium, and the role of hypoxia during blood stasis in initiating local endothelial activation. Elevations in the levels of TF-bearing microvesicles associated with inflammatory conditions would help to explain the increased risk of thrombosis associated with infections and inflammatory states such as inflammatory bowel disease.
In Section II, Dr. Clive Kearon discusses the risk factors for recurrent thrombosis and strategies for determining length of therapy and tailoring specific therapies through risk stratification. Those patients who experience VTE in association with a major reversible risk factor such as surgery are much less likely to experience a recurrence when anticoagulation is discontinued than are patients with a persistent risk factor, such as thrombophilia or cancer unresponsive to therapy. Those with a minor reversible risk factor, such as prolonged air travel, have an intermediate risk of recurrence after discontinuance of anticoagulant therapy. The author provides an algorithm for using risk assessment as a means of determining the length and type of therapy to be used to minimize the rate of recurrence while simultaneously diminishing the risk of bleeding associated with anticoagulation.
In Section III, Dr. Agnes Lee updates the topic of VTE associated with malignancy. Patients with cancer make up approximately 20% of those presenting with first time VTE, and the presence of VTE forebodes a much poorer prognosis for patients with cancer, likely because of the morbidity associated with VTE itself and because VTE may herald a more aggressive cancer. Recent evidence indicates that low-molecular weight heparins (LMWHs) improve survival in patients with advanced cancer through mechanisms beyond their effect as anticoagulants. Because of their improved efficacy and safety and potential anti-neoplastic effect, the LMWHs have become the anticoagulants of choice for treating VTE associated with cancer.
I. Pathophysiology of Deep Venous Thrombosis
Ian D. Conde, MD, and José A. López, MD*
Baylor College of Medicine, Thrombosis Research Section, BCM 286, N1319, Houston TX 77030
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are major causes of morbidity and death. This year, approximately two million Americans will suffer DVT, and more than 600,000 of them will also develop PE.1 In spite of this enormous disease burden, surprisingly little is known about the pathophysiology of DVT. This is in marked contrast with arterial thrombosis, in which the general outline of its mechanism is well understood, even to the molecular level.2
One of the most important advances in our understanding of venous thrombosis was also one of the first. In 1859, Rudolph Virchow deduced the major pathogenic determinants for DVT and PE. Based on exquisitely detailed and insightful pathologic observations, Virchow concluded that (1) blood stasis, (2) changes in the vessel wall, and (3) hypercoagulability were the major factors responsible for the development of venous thrombosis.3 This triad still applies, with essentially all prothrombotic factors, whether systemic or molecular, influencing one of these three mechanisms.
The clear clinical utility of Virchow’s triad notwithstanding, novel means of prevention and therapy of DVT will be facilitated tremendously by a more detailed understanding of the mechanisms of venous thrombosis. Risk factors identified by clinical observation and epidemiologic studies are useful for estimating a person’s risk of DVT, but they provide little insight into the mechanisms initiating venous thrombosis.
What causes a clot to develop in a vein? The aim of this short review is not to present a comprehensive overview of information already available in medical textbooks, but to speculate about the pathophysiology of venous thrombosis using available evidence. First, we will discuss an obvious yet somewhat neglected topic that is central to understanding DVT: the mechanisms of its initiation. We will then discuss the potential mechanisms by which known risk factors may contribute to DVT.
Initiation of Venous Thrombosis
The hemostatic system is faced with the complex task of maintaining the blood in a fluid state so that it can circulate, while simultaneously being able to convert the blood into an insoluble gel at sites of vascular injury. The hemostatic system is made up of two distinct but interlocking systems: platelets and the coagulation proteins. In the absence of vessel injury or inflammation, platelets do not adhere to the endothelium primarily because unstimulated endothelium has no receptors for unstimulated platelets and because the endothelium produces substances such as nitric oxide and prostacyclin that maintain the platelets in the unactivated state and impair their adhesion. When the endothelial layer is lost, however, platelets are exposed to subendothelial ligands for which they have specific receptors. The earliest of contacts between flowing platelets and the subendothelium is mediated by the platelet glycoprotein (GP) Ib-IX-V complex binding von Willebrand factor (VWF) in the subendothelium.2 Through this interaction, platelets roll and decelerate, allowing other platelet receptors with slower on- and off-rates to bind subendothelial proteins. As platelets adhere to the injured vessel wall, transmembrane signaling effected by ligated receptors, such as GPIb-IX-V and the collagen receptor GPVI, activate the platelets, leading to conformational activation of integrins, most prominently αIIbβ3 and α2β1. Calcium currents generated during platelet activation induce α-granule release, with the secretion of various procoagulant molecules, such as factor (F) V, VWF, and fibrinogen. Activated platelets also undergo the so-called “flip-flop” reaction, where phosphatidylserine is exposed on the outer membrane leaflet. Phosphatidylserine provides the surface for the assembly of coagulant enzyme complexes, which generate thrombin and enable fibrin deposition. The histopathologic structure of arterial thrombi is consistent with this model, with several studies describing the core of arterial thrombi as composed almost exclusively of platelets directly overlying the site of vessel injury, with the platelet core wrapped by a thick fibrin mesh extending both upstream and downstream with numerous trapped erythrocytes.4,5 This model also explains the clinical efficacy of antiplatelet drugs in the treatment of arterial thrombosis.
The sequence of events leading to venous thrombosis is less clear. In contrast to arterial thrombosis, deep vessel wall injury does not appear to be a common feature in DVT. For example, Sevitt found no evidence of vein wall injury in 49 of 50 venous thrombi that he obtained from the lower extremities of 41 patients during necropsy.6 One caveat, however, is that the resolution of the imaging techniques may have precluded observations of subtle vessel wall injuries. Nevertheless, those injuries did not include endothelial denudation, which could be observed by the techniques employed. Similar to the earlier observations of Paterson and McLachlin,7 Sevitt found that most venous thrombi consisted of two regions: ones that were composed predominantly of fibrin and trapped erythrocytes (red thrombi), and others that were composed mainly of aggregated platelets (white thrombi). Interestingly, it was the fibrin-rich regions that attached the thrombi to the vessel wall, while the platelet-rich regions localized further from the site of attachment. These findings suggest that activation of the coagulation system precedes platelet activation and aggregation during the formation of venous thrombi, and help to explain the limited efficacy of antiplatelet drugs in venous thrombosis. This being the case, the question arises: how does coagulation initiate in an intact vein?
Tissue Factor–Bearing Microvesicles and Venous Thrombosis
Coagulation in vivo is initiated by a complex of tissue factor (TF), a type I transmembrane protein, and the serine protease FVIIa to convert the zymogen FX to the active enzyme, FXa. Activated FX then joins its co-factor, FVa, on the phosphatidylserine-rich surface of activated platelets to form the prothrombinase complex, which converts prothrombin to thrombin. Tissue factor is expressed primarily in extravascular tissues, such as the brain, renal glomeruli, and vessel adventitia, forming a “hemostatic envelope” surrounding the vasculature. Within the vascular space, only monocytes have been shown conclusively to express TF, and then only in special circumstances, such as sepsis. Whether endothelial cells express TF in vivo has been a matter of controversy, but the weight of evidence thus far indicates that they generally do not.8,9 Endothelial TF expression has been documented, but only in rare cases, such as in the splenic endothelium of a baboon injected with a lethal dose of endotoxin,10 and in one case in vessels adjacent to a breast carcinoma.11 If endothelial cells do not generally express TF, then how do clots form in veins?
In recent years, evidence has accumulated indicating that TF circulates in normal plasma,12,13 both associated with cell-derived membrane microvesicles14 and as a soluble, alternatively spliced form.15 Given that TF-bearing microvesicles express several surface proteins specific for cells of the monocyte/macrophage lineage, such as CD14 and CD11b, the general consensus is that they arise from these cells.16 Hemostatic roles have been proposed for both forms of TF, especially the microvesicle-associated form. Endogenous TF-bearing microvesicles have been found to contribute to experimental thrombosis in vivo in the cremaster microcirculation,16 and were recently shown to improve hemostasis in hemophilic mice.17 In these experimental systems, TF-bearing microvesicles appear to participate in thrombosis by binding platelets at sites of injury, a process dependent on the interaction between P-selectin glycoprotein ligand-1 (PSGL-1) on microvesicles and P-selectin on activated platelets.16 The microvesicles not only bind activated platelets, they also fuse with them in a PSGL-1– and phosphatidylserine-dependent manner (I Conde et al, manuscript submitted). By fusing with the platelets, the microvesicles transfer TF and other proteins to the platelet membrane, in the process increasing TF-VIIa activity, thrombin generation, and fibrin deposition at the site of thrombosis. Failure of this hemostatic mechanism may explain why agents that block the PSGL-1–P-selectin interaction markedly inhibit platelet-dependent arterial thrombosis in animals.18,19
A steadily growing body of evidence suggests that TF-bearing microvesicles may also play important roles in DVT. In a mouse model of venous thrombosis, Myers and colleagues have shown that elevated levels of leukocyte-derived microvesicles in plasma are associated with greater thrombus masses.20 Further, several animal studies have shown that agents that block the P-selectin–PSGL-1 interaction dose-dependently inhibit experimental venous thrombosis.21,22 Although direct evidence that TF-bearing microvesicles induce DVT in humans is still lacking, circumstantial evidence suggests that they do. TF-bearing microvesicles may participate in thrombosis associated with malignancy. Cancer has long been known to be a major risk factor for DVT,23 and DVT is frequently the first clinical manifestation of malignancy.24 Many authors have found increased TF antigen levels and TF-VIIa activity in the plasmas of patients with cancer, and the association has been made with various types of cancer.25 We recently analyzed the blood of a patient with giant-cell lung carcinoma who suffered eleven major venous and arterial thromboembolic events over a 5-month period. The levels of microvesicle-associated TF in his plasma were extremely high (3764 pg/mL vs 90.8 pg/mL ± 62.2 pg/mL in 16 age- and sex-matched controls). We thus postulate that TF-bearing microvesicles are central in the pathogenesis of DVT in disease states in which monocytes are stimulated to express TF and to microvesiculate. Examples of such diseases include inflammatory bowel disease and chronic congestive heart failure, diseases that are associated not only with an increased risk of DVT but also with high levels of tumor necrosis factor-α (TNF-α), a potent inducer of monocyte-derived TF-bearing microvesicles. In these disorders, and others, increased numbers of TF-bearing microvesicles may contribute to the associated hypercoagulability. Supporting this conjecture are autopsy studies showing that DVT in the absence of vessel trauma is frequently bilateral.26 If the thrombotic trigger were truly local, one would expect thrombosis to be unilateral.
How might TF-bearing microvesicles initiate coagulation in the absence of deep vessel injury and platelet deposition? One possibility is that they interact with activated endothelium in a manner similar to their interaction with activated platelets. Like platelets, endothelial cells contain large amounts of P-selectin stored within their intracellular granules and express it on their surface upon activation, providing a receptor for docking the TF-bearing microvesicles. Also like platelets, activated endothelial cells express phosphatidylserine on their surfaces and may therefore be capable of supporting both the binding and fusion of TF-bearing microvesicles, in the process decrypting TF and initiating coagulation. Importantly, Tracy has shown that activated endothelial cells provide a catalytic surface for coagulation that is as efficient as that provided by activated platelets.27 Thrombin generation and fibrin deposition could therefore proceed readily once the microvesicle-derived TF has been transferred to the membrane of endothelial cells. How endothelium-associated anticoagulants, such as heparin sulfate proteoglycans, thrombomodulin, and tissue factor pathway inhibitor (TFPI), are neutralized so as to allow coagulation is unknown. Nevertheless, the histopathologic evidence is that in DVT, coagulation occurs on (or very near) the endothelial surface. Once coagulation initiates on the endothelial surface, platelets may be recruited to the fibrin clot rich in thrombin via adhesive interactions involving GPIb-IX-V and αIIbβ3,28 and later contribute to further thrombus growth. Consistent with this idea are (1) the observations that platelet aggregates localize to regions of the clot that are far away from its site of attachment, and (2) the small but statistically significant reduction in the risk of DVT afforded by antiplatelet drugs such as aspirin.29
The scheme described above requires that endothelial cells become activated to support the development of venous thrombi. What, then, is the activation stimulus? There are many stimuli that can activate the endothelium, among them infections, intravascular catheters, and local mediators such as TNF-α (see below and Table 1 ). Much more commonly, though, blood stasis is what precipitates venous thrombosis. In autopsy studies, the prevalence of venous thrombosis was markedly increased in those who had been bed-ridden for more than 1 week before their death.30 Also, venous thrombosis was found to be more common in the immobilized limb of hemiplegic stroke patients,31 but equally common in the two legs of paraplegic patients.32 The observation that the incidence of DVT in hospitalized patients drops as patients begin to walk33 further supports the idea that immobility and stasis precipitate DVT. Consistent with the concept that blood stasis is important in the development of venous thrombosis, strategies that prevent stasis are extremely effective in preventing DVT.34
What might link venous blood stasis and endothelial cell activation? Much evidence indicates that stasis can result in hemoglobin desaturation, leading to a hypoxic insult to the endothelium. Hamer et al showed that in a dog limb, venous oxygen tension dropped to almost undetectable levels when blood flow was halted.35 Because the endothelium is primarily oxygenated and perfused directly by the blood in the vessel lumen, hypoxia can result in cellular responses that range from no effect at all, to cell activation, and even to cell death, depending on the degree and duration of the hypoxia. Ischemia has been shown to rapidly activate endothelial cells to express P-selectin and is a hallmark of ischemia/reperfusion injury.36 Endothelial P-selectin expression in ischemia is essential for leukocyte infiltration of the vessel wall and target tissues, and for that matter for the binding of TF-bearing microvesicles. Accordingly, post-ischemic inflammation is markedly reduced in mice deficient in P-selectin or in animals treated with P-selectin–blocking agents.37 The similar requirements of leukocytes and TF-bearing microvesicles for binding activated endothelium may account for the frequently observed association of thrombosis and inflammation, so-called “thrombophlebitis.”
While the experiments performed by Hamer et al represent an extreme example of stasis-induced hypoxia, it is conceivable that similar scenarios of prolonged blood stasis may occur in clinically relevant settings. An example of systemic venous blood pooling is right-sided heart failure, which is clearly associated with the development of DVT. At a more local level, venous stasis may occur because of immobility or by vein compression by a mass such as an enlarged lymph node or tumor.
Therefore, in venous stasis, endothelial cells may become activated and express P-selectin, allowing TF-bearing microvesicles to initiate coagulation and thrombosis. A substantial amount of data supports this model. For example, induced thrombi in P-selectin knock-out mice were smaller and contained about 35% less fibrin than those of their wild-type counterparts.38 In this case, P-selectin deficiency failed to completely prevent thrombosis, but this finding has several potential explanations. First, it is likely that other receptors can substitute P-selectin as a receptor for monocyte microvesicles on the endothelium. A good candidate for this function is E-selectin. Like P-selectin, E-selectin binds PSGL-1 and is able to capture leukocytes from flowing blood but, unlike P-selectin, requires de novo protein synthesis for expression on activated endothelium.39 Consistent with a role for E-selectin as a receptor for TF-bearing microvesicles, E-selectin–deficient mice exhibited significantly less fibrin deposition and lower thrombus masses than their wild-type counterparts in an inferior vena cava (IVC) thrombosis model.20 Also supporting this model are the studies by Wakefield et al.21 In a baboon model of IVC thrombosis, these investigators showed that soluble, recombinant PSGL-1-immunoglobulin (rPSGL-1-Ig) markedly inhibited thrombosis. In addition to P-selectin, rPSGL-1-Ig binds and blocks most if not all of the natural PSGL-1 ligands, including E-selectin. Baboons that received rPSGL-1-Ig before induction of thrombosis averaged 50% fewer thrombi in the inferior vena cava than untreated animals.21 Another reason for the failure of P-selectin deficiency or rPSGL-1-Ig to prevent thrombosis may involve the experimental models of venous thrombosis. The techniques employed to induce venous thrombosis (e.g., ligation, clamping, or balloon-occlusion of veins) usually produce significant vein wall injury. The injury is likely to expose TF in the vein wall to flowing blood, which would diminish the role of blood-borne TF in initiating thrombosis.
Animal models that more accurately mimic the pathophysiology of DVT in humans are needed not only to provide insight into the mechanisms of venous thrombosis, but also to identify future therapies. In this regard, it is noteworthy that soluble rPSGL-1-Ig has already been studied in Phase I clinical studies involving more than 500 patients and that it appears to be safe.40 However, whether this agent will be clinically effective in the prevention and/or treatment of DVT will depend on the outcome of large clinical trials.
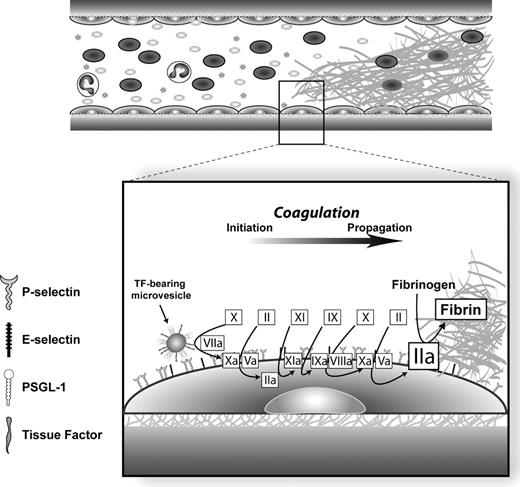
Based on the preceding arguments, we propose a model for venous thrombosis which is depicted in Figure 1 . In this model, endothelial stimulation or injury results from either blood stasis–induced hypoxia and/or from direct vein wall injury (e.g., trauma). TF-bearing microvesicles from monocyte/macrophage cells attach to and fuse with stimulated endothelial cells. This interaction involves PSGL-1 on the microvesicle and P-selectin and/or E-selectin on the endothelium. Transfer of TF to the endothelial cell initiates the enzymatic cascade of coagulation reactions, which then occur on the endothelial surface, leading to thrombin generation and fibrin deposition.
Potential Mechanisms of Venous Thrombosis in Different Clinical Scenarios
Deep-vein thrombosis is associated with many diverse clinical conditions, suggesting that the inciting stimulus for thrombosis varies depending on the underlying clinical condition. For example, it is likely that vessel injury contributes significantly to catheter-related DVT but is unnecessary in DVT in a patient with antithrombin deficiency. Using Virchow’s triad as a framework to understand venous thrombosis, we propose mechanisms by which different diseases and risk factors may facilitate DVT (Table 1 ). In this scenario, two factors determine the development of venous thrombi: (1) an individual’s baseline propensity for thrombosis, and (2) the insult, injury, or condition that precipitates thrombosis acutely.41 From this perspective, an individual’s risk for DVT would be determined by the combination of his or her baseline propensity for thrombosis and the magnitude of the acute insult. This concept may be extrapolated to arterial thrombosis, where an increased baseline propensity for thrombosis (e.g., gain-of-function polymorphisms of platelet receptors42) may pave the way for the development of a thrombus in the setting of vessel wall damage.
We exemplify the concept of the interaction between baseline propensity for thrombosis and magnitude of the acute insult for the development of DVT in the following brief clinical vignettes.
Case #1:
A 24-year-old male with no significant past medical history and no known prothrombotic risk factors sustains severe trauma to his right knee, destroying the joint. He undergoes total knee arthroplasty, and a tight tourniquet is placed above the affected knee during the surgical procedure. He receives no anticoagulation. Twenty-four hours after the surgery, the patient reports pain in his right leg, which is noted to be red and swollen. A Doppler ultrasound is performed and a large thrombus is visualized in the popliteal vein. What was the likely mechanism of DVT?
Analysis:
The tight tourniquet placed around the patient’s leg during surgery likely injured the leg veins and also caused blood stasis. Despite the fact that the patient is young and probably has a low baseline propensity for thrombosis, the vessel injury/stasis in this setting is an acute insult of sufficient magnitude as to precipitate DVT. Consistent with this, approximately 80% of patients who undergo total knee arthroplasty and do not receive thromboprophylaxis develop DVT.
Case #2:
A 51-year-old white male with no significant past medical history, but who is heterozygous for the factor V Leiden mutation, takes a flight from Houston to Melbourne, Australia (approximately 9300 miles). The patient ambulates minimally during the entire 20-hour flight. One day after his arrival in Melbourne, he suddenly becomes short of breath and tachycardic. He is taken to an emergency room, where DVT/PE is suspected, and is later confirmed by a ventilation/perfusion nuclear scan. What was the likely mechanism of DVT/PE?
Analysis:
The patient described in this case appears to have developed DVT/PE as a consequence of a long flight. The vessel injury in such cases is likely to be relatively minor. Consistent with this, the incidence of DVT/PE in individuals taking long flights is only 4.8 cases of 1,000,000 traveling more than 6000 miles.43 If the magnitude of the injury were sufficient to produce significant vein wall injury, the incidence of DVT would conceivably be much higher. In this case, the patient’s heterozygosity for factor V Leiden renders him resistant to the inactivating effects of activated protein C, a major endogenous anticoagulant that normally limits uncontrolled activation of coagulation. Thus, in the face of increased baseline hypercoagulability (e.g., factor V Leiden), even a relatively weak insult (e.g., blood stasis during the flight) can be sufficient to precipitate DVT.
Case #3.
A previously healthy 60-year-old female with no significant past medical history presents to the hospital with right upper-extremity DVT. Over the next 4 months she develops six additional DVTs in different sites. A chest computed tomography is suggestive of lung carcinoma, which is later confirmed by biopsy to be of the small-cell variety. Analysis of the patient’s blood in a specialized thrombosis research laboratory reveals that the patient’s blood had 45-fold higher levels of tissue factor compared to sex- and age-matched controls. What caused the multiple DVTs in this patient?
Analysis:
The precipitating factor for thrombosis in this case appears to have been the extremely elevated TF levels in the patient’s blood, probably secondary to the lung malignancy. The normal balance between coagulation and anticoagulation is lost with such high levels of TF. In this setting, DVT and other thrombotic events are bound to occur even at the slightest vessel wall stimulation/injury.
II. Risk Factors for Recurrent Venous Thromboembolism and Their Implications for Treatment
Clive Kearon, MB, MRCPI, FRCPC, PhD*
McMaster University, Head Clinical Thrombosis Service, Henderson General Hospital, Room 39, 70 Wing, 711 Concession Street, Hamilton ONT L8V 1C3, Canada Grant Support: Dr Kearon is an Investigator of the Canadian Institutes of Health Research. Referencing in this section has been largely confined to publications from the past 5 years; a more extensive reference list is provided in a related review by the author.5
At least 25% of episodes of acute venous thromboembolism (VTE) occur in persons who have had a previous event even though the average lifetime risk of a first VTE is only about 2%.1 In addition, previous thromboembolism is usually identified as the strongest single predictor for VTE in high-risk situations, such as after major surgery.1 Clearly, therefore, an episode of VTE identifies patients who have a much higher risk of subsequent thromboembolism than the general population. The clinical implications of this observation depend on the magnitude of the increase in risk of thrombosis associated with an initial episode, how risk of thrombosis changes with interval from the initial episode, how subsequently encountered risk factors for thrombosis interact with this heightened risk, and if efficacy of treatment varies among patients with thrombosis (Table 2 ). These attributes may vary systematically according to the circumstances that were associated with the initial episode of thrombosis or because of other differences between patients. This review starts with a description of risk factors for recurrent episodes of VTE. Subsequently, I will describe how evaluation of such risk factors can guide treatment decisions.
Risk of Recurrent Venous Thromboembolism
Reversibility of risk factor for VTE
Probably the most important advance in assessment of risk of recurrent VTE after anticoagulant therapy is stopped is recognition that patients whose thrombosis was provoked by a major reversible risk factor, such as surgery, have a low risk of recurrence (i.e., about 3% in the first year and 10% over 5 years), whereas this risk is high (i.e., about 10% in the first year and 30% over 5 years) in patients with an unprovoked (“idiopathic”) episode of VTE and in those who have a persistent risk factor without a reversible component (Table 3 ).2–,6 Patients whose thrombosis was provoked by a minor reversible risk factor, such as leg trauma, estrogen therapy, or prolonged air travel (i.e., a flight over 10 hours), have an intermediate risk of recurrent VTE after stopping anticoagulant therapy (i.e., approximately 5% in the first year and 15% over 5 years2,4,7,8).
Cancer
Cancer is associated with about a 3-fold increased risk of recurrent VTE both during9,10 and after4–,7,9,11,12 anticoagulant therapy, and among patients with cancer, the risk of recurrence is about 3-fold higher in those with metastatic disease10 (Table 3 ). Poor mobility, associated venous obstruction, and ongoing chemotherapy may further increase risk of recurrence. Conversely, risk of recurrence may be less if the cancer responds to therapy or if the initial VTE was provoked by an additional reversible risk factor, such as surgery or chemotherapy. While acknowledging that the risk of recurrent VTE after stopping anticoagulant therapy differs among patients with cancer (as above), on average, this risk appears to be higher than after an episode of unprovoked VTE, perhaps of the order of 20% within the next year.9,12 However, as VTE tends to be associated with advanced and aggressive cancers (see Section III), average life expectancy is poor in this group of patients (e.g., ~40% mortality at 6 months after diagnosis of VTE13).
Pulmonary embolism versus deep vein thrombosis
Patients who present with pulmonary embolism (PE) appear to have the same risk of recurrent VTE as those who present with proximal DVT.5–,7,14 However, after a PE, about 60% of recurrent episodes of VTE are also PE, whereas only about 20% of recurrent episodes of VTE are a PE after an initial DVT.14–,18 This pattern of recurrence, with about a 3-fold higher risk of PE after an initial PE than after an initial DVT, appears to persist long-term.14 About 10% of symptomatic PE are thought to be rapidly fatal and another 5% of those whose PE is diagnosed and treated also die from PE.5,6,14,17 Thus, after 3 or more months of treatment for DVT or PE, recurrent VTE that presents as PE probably has a case-fatality rate of about 15%. The risk of dying from acute DVT, because of early subsequent PE or other complications (e.g., bleeding, precipitation of myocardial infarction), appears to be 2% or less.4–,6,14 Based on these estimates, the case-fatality rate associated with late recurrent VTE after a preceding PE is expected to be about 10%, whereas that after a preceding DVT is expected to be about 5%. Therefore, although the risk of a recurrence is the same after PE and proximal DVT, the case-fatality rate for a recurrence is expected to be 2-fold higher after PE than after DVT.
Isolated distal deep vein thrombosis
Multiple previous episodes of VTE
Thrombophilia
Antiphospholipid antibodies:
Factor V Leiden and the G20210A prothrombin gene mutation:
Heterozygosity for the factor V Leiden or the G20210A prothrombin gene mutation does not appear to be a clinically important risk factor for recurrent VTE.2,3,5,8,12,23 However, patients who are heterozygous for both of these mutations5,12 or homozygous for the factor V Leiden mutation23 may have a higher risk of recurrent VTE (Table 3 ).
Deficiency of protein C, protein S, and antithrombin:
There is little prospective information on the risk of recurrent VTE in patients with antithrombin, protein C, or protein S deficiency. One prospective study identified a hazard ratio of 1.4 for recurrent VTE in patients with one of these abnormalities or a lupus anticoagulant.11 Another study reported relative risks for recurrent VTE of 1.0 for protein S, 1.8 for protein C, and 2.6 for antithrombin deficiency.2 A third prospective study found no increase in recurrence among 15 patients with one of these deficiencies.12 Therefore, although there is uncertainty, these abnormalities do not appear to be clinically important risk factors for recurrent VTE.
Elevated factor VIII levels:
Hyperhomocysteinemia:
Hyperhomocysteinemia, which can be caused by hereditary or acquired conditions, was associated with a 2.7-fold increased risk of recurrent VTE in one study of patients with unprovoked VTE.25 Lowering plasma homocysteine levels with vitamin therapy did not convincingly reduce the frequency of recurrent VTE in a subsequent trial.26
Vena caval filters
In a randomized trial that evaluated routine placement of vena caval filters as an adjunct to anticoagulant therapy in patients with proximal DVT, filters were shown to reduce the frequency of PE during the first 12 days, but to almost double the long-term risk of recurrent DVT.27 Despite increasing the risk of recurrent DVT, filters did not appear to be associated with more frequent PE. These findings are supported by a large epidemiological study of linked hospital discharge records which found that a caval filter was an independent risk factor for recurrent DVT (odds ratio 1.8), but was not a risk factor for PE (odds ratio 1.0)14; the filter-associated increase in DVT was largely confined to patients who initially presented with PE.19
D-dimer levels after stopping treatment
Laboratory evidence of activation of coagulation after withdrawal of anticoagulants appears to stratify patients’ risk of recurrent VTE. A low or negative D-dimer level about 1 month after stopping anticoagulant therapy, a finding that was present in one-third28 and more than one-half12 of patients, has been reported to be associated with less than half the risk of recurrent VTE. In these two studies, D-dimer levels predicted risk of recurrence in patients with and without provoking risk factors for VTE or hereditary thrombophilia.12,28
Residual deep vein thrombosis
It is uncertain if residual DVT is an independent predictor of recurrent VTE. Piovella and colleagues found that residual proximal DVT on ultrasound after 3 months of treatment was associated with more than a 3-fold increase in recurrent thrombosis, with three-quarters of DVT occurring in the same leg.9 Prandoni and colleagues reported more than a 2-fold increase in the frequency of recurrent VTE when there was persistent DVT on ultrasound.29 However, it is unclear if this association persists after controlling for differences in the time elapsed since the initial DVT (i.e., it takes time for the ultrasound to return to normal, and the risk of recurrence is also expected to decrease during this interval). Agnelli and colleagues reported a statistically significant 1.4-fold increase in recurrent VTE among patients with PE who had a concomitant DVT on ultrasound after 3 months of initial treatment compared to those without DVT.15 However, three studies by our own group did not find that residual proximal DVT was predictive of recurrent VTE during anticoagulant therapy (hazard ratio 0.9; target International Normalized Ratio [INR] 1.5 to 1.9 for half of the patients30) or after treatment was stopped (hazard ratio 1.331 and 1.022). Similarly, abnormal impedance plethysmography after 1 month of treatment (suggesting persistent proximal vein obstruction) was not found to be predictive of recurrence after treatment was stopped at 3 months (relative risk 1.3).32 Furthermore, with the exception of early recurrences that are associated with inadequate initial treatment (e.g., for only 6 weeks33), recurrent DVT is equally distributed between the initially affected and unaffected legs.12,29,33 Current evidence, therefore, suggests that residual DVT may be weakly associated with recurrent VTE (less than a 2-fold increase), but venous obstruction is not the underlying mechanism.
Age and gender
Other factors
The influence of a number of other factors on the risk of recurrent VTE is summarized in Table 3 .
How Risk Factors for Recurrent VTE Influence Treatment
In order to consider how risk of recurrence may influence or change management, it is first necessary to identify “usual treatment” of an undifferentiated (“average”) episode of VTE. In this context, “usual treatment” of VTE is considered to be therapeutic-dose unfractionated or low-molecular-weight heparin (LMWH) for at least 5 days, overlapped with and followed by treatment with a vitamin K antagonist (VKA) targeted to an INR of 2.5 for 6 months. In addition, assumptions will be made about two other variables that strongly influence management decisions. First, that risk of major bleeding is 2% per year while on long-term anticoagulant therapy, corresponding to that which is expected in the absence of risk factors for bleeding (e.g., previous stroke or gastrointestinal bleeding; active peptic ulcer disease; renal impairment; anemia; thrombocytopenia; liver disease; diabetes mellitus; use of antiplatelet therapy; poor patient compliance; poor control of anticoagulation; active cancer).5,30 Second, that patients do not find anticoagulation a major burden and, therefore, do not have a strong preference to stop therapy.
During the following discussion of how risk factors for recurrence influence treatment decisions, priority is given to factors that can be assessed clinically (e.g., from the patient’s history) and that have the strongest predictive value.
First level of assessment: Reversibility of risk factor
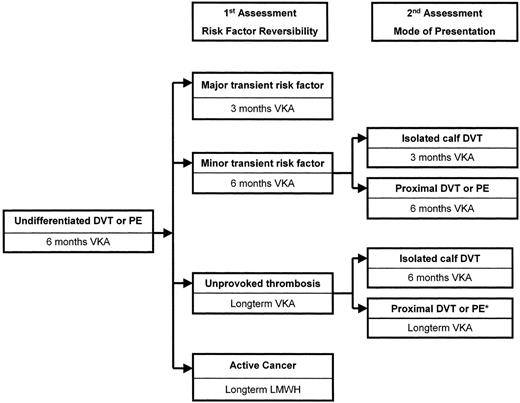
Because the presence of a reversible risk factor for VTE, lack of a provoking factor, or cancer at the time of thrombosis is the strongest predictor of risk of recurrence, this assessment carries most weight (Figure 2 ).
Major transient risk factor:
For patients whose VTE is associated with a major transient risk factor, such as recent surgery, stopping anticoagulant therapy after 3 months of treatment is expected to be associated with a subsequent risk of recurrent VTE of only about 3% in the first year and 10% over 5 years.4,5,11,15,20,22,32,36 This rate is not high enough to justify treatment for longer than 3 months.
Unprovoked VTE:
For patients with unprovoked VTE, stopping anticoagulant therapy after 6 or more months of treatment is expected to be associated with a subsequent risk of recurrent VTE of about 10% in the first year and about 30% over 5 years.3,16,36,37 This rate is high enough to justify long-term anticoagulation in the majority of such patients.3,30,31
Active cancer:
Patients with active cancer generally should remain on long-term anticoagulant therapy because the risk of recurrent VTE is expected to be higher than 10% within a year of stopping treatment.4–,7,9,11,13 As cancer is a risk factor for recurrent VTE while on a VKA, and as randomized trials support superiority of long-term treatment with LMWH compared to use of a VKA, LMWH is the preferred approach to treatment.13,38
Minor transient risk factor:
Patients who do not clearly fall into one of these three categories (i.e., VTE provoked by a minor reversible risk factor) are expected to have a risk of recurrence that is higher than that in patients with a major reversible risk factor and lower than that in patients with an unprovoked VTE after anticoagulant therapy is stopped.2,4,7,8 My preference is to treat such patients with proximal DVT or PE for 6 months, although 3 months may be adequate in these circumstances.36
Second level of assessment: Mode of presentation
Isolated distal DVT:
As these patients have about half the risk of recurrence as those with proximal DVT or PE, 6 months of treatment is adequate for patients with an unprovoked episode of isolated calf DVT, and 3 months is adequate for those with a minor transient risk factor for VTE and isolated distal DVT. While it is reasonable to reduce the duration of treatment of isolated calf DVT that was provoked by a major transient risk factor to 6 weeks,36,37 that is not my practice as (1) the shorter duration of treatment may be associated with a higher risk of recurrence22; (2) the associated gain in convenience and reduced bleeding is likely to be small; and (3) advocating a separate category of treatment duration exclusively for such a small segment of patients with VTE makes treatment recommendations for VTE more complex.
Pulmonary embolism versus deep vein thrombosis:
As a recurrent episode of VTE is estimated to be twice as likely to be a fatal PE after an initial PE than after a DVT, I encourage long-term anticoagulant therapy more strongly after a first unprovoked PE than after a first unprovoked proximal DVT.
Second versus first episode of VTE:
As recurrent VTE is more likely when there has been more than one preceding episode, I strongly encourage patients to remain on long-term anticoagulant therapy after a second episode of unprovoked VTE. The rationale for long-term therapy probably increases as the interval without treatment between preceding episodes of thrombosis decreases. If the current and all preceding episodes of VTE were associated with a major transient risk factor such as surgery, I do not routinely extend treatment beyond 3 months. Instead, I emphasize the importance of using aggressive prophylaxis during subsequently encountered high-risk situations.
Role of thrombophilia testing as a guide to duration of treatment
As there is little evidence to suggest that (1) patients with VTE provoked by a major risk factor who have thrombophilia should be treated with anticoagulant therapy for longer than 3 months,22 or (2) that patients with unprovoked VTE who have thrombophilia should be treated with a higher than usual intensity of long-term anticoagulantion,3,30 I generally do not perform testing for thrombophilia to guide the duration of anticoagulant therapy.
Qualifying Remarks
This discussion focuses on how risk of recurrent VTE influences choice of duration of anticoagulant therapy. However, risk of bleeding while on anticoagulant therapy, and patient preference, also strongly influences this decision. Furthermore, it is acknowledged that interpretation of the importance of initial steps in the risk stratification process influences whether subsequent risk factor evaluation will change management decisions. For example, I have proposed that most patients with a first episode of unprovoked proximal DVT should remain on anticoagulant therapy long term. If this is accepted, identifying additional risk factors for recurrence (e.g., an antiphospholipid antibody) will not change management of such patients. However, if 6 months of anticoagulation is judged to be adequate treatment for most first episodes of unprovoked proximal DVT, a search for additional risk factors that predict recurrence may change this decision.
III. Venous Thromboembolism and Cancer: New Insights and Therapy
Agnes Y. Y. Lee, MD, MSc, FRCP(C)*
McMaster University, Hamilton Health Sciences Henderson General Hospital, Dept. of Medicine, 711 Concession Street, Hamilton ONT L8V 1C3, Canada Acknowledgment: Dr. Lee is a recipient of a New Investigator Award from the Canadian Institutes of Health Research/Rx & D Research Program.
It has long been recognized that venous thromboembolic events are common in patients with cancer. VTE can also complicate and potentially compromise their cancer treatment, and it also predicts for a worse prognosis. Furthermore, the morbidity of acute thrombotic events, the limitations of standard anticoagulant therapy, and the high frequency of treatment failure in this population make VTE an important quality-of-life issue in cancer patients. However, despite these sobering observations, most oncologists still underestimate the prevalence of VTE and its negative impact on their patients, and few advances in the clinical management of VTE have been made in the past decades. Recent studies that suggest low-molecular weight heparins (LMWH) may improve survival in cancer patients have renewed interest and stimulated new research in studying the potential antineoplastic properties of this class of anticoagulants. This section will describe briefly the epidemiology of VTE in cancer and provide a more detailed discussion of the recent clinical studies in the primary prevention and treatment of VTE in patients with malignancy.
VTE in Patients with Cancer
Patients with cancer represent approximately 15%–20% of all new cases of venous thromboembolism occurring in the community.1 This is reflective of the prevalence of cancer in the general population and that active malignancy, with or without chemotherapy, increases the risk for VTE by 4- to 6-fold. Cancer is also an independent risk factor for death within 7 days after VTE, with up to an 8-fold increase risk of death in patients receiving chemotherapy. Moreover, cancer patients with VTE have worse survival than cancer patients free of this complication. In a population-based study, the 1-year survival of patients diagnosed with cancer and VTE at the same time was 12%, as compared with 36% in cancer patients without VTE, who were matched for gender, age at the time of the diagnosis of cancer, and year of cancer diagnosis.2 The poor prognosis may indicate that patients are dying prematurely of VTE, that VTE is a harbinger of aggressive malignancies, or both. VTE often presents late in the course of malignancy but it can also be the first manifestation of occult cancer. Approximately 10% of patients with unprovoked or idiopathic VTE are diagnosed with cancer within the first year after their diagnosis of VTE.1 The standardized incidence ratio during this first year has been estimated at 2.2–4.4.3,4 This risk is about 3-fold higher than that in patients without VTE and about 4- to 8-fold higher than in patients who have VTE secondary to a known risk factor.1 However, performing extensive investigations routinely to look for underlying cancer in patients with idiopathic VTE is not recommended given the lack of evidence that shows screening improves cancer-related survival.
While the risk of VTE by tumor type remains uncertain for the majority of cancers, the risk appears to be highest for patients with malignant brain tumors and cancer of the ovary, pancreas, and lung.5 On the other hand, the most common tumor types found in patients with VTE are cancers of the lung, colon, breast, and prostate, which largely reflects the prevalence of these cancers in the general population. After surgery, cancer patients have twice the risk of DVT and over 3 times the risk of fatal pulmonary embolism (PE) compared with patients free of cancer.6 The risk of thrombosis is increased with the use of chemotherapy or hormonal therapy, as well as indwelling central venous catheters.1 Recent clinical trials also report a high incidence of VTE associated with the use of antiangiogenic agents, such as thalidomide and inhibitors of the vascular endothelial growth factor/receptor pathway.
Multiple and interdependent mechanisms are responsible for the hypercoagulable state in patients with cancer. Tumor procoagulant activity, host inflammatory responses and extrinsic factors, which are frequently iatrogenic, are involved. Furthermore, recent evidence has shown that tumor-induced coagulation activation is intrinsically involved with tumor cell growth, angiogenesis and metastasis.
Prophylaxis of VTE
General surgery
Anticoagulant prophylaxis is recommended routinely for patients undergoing major surgery because the risk of postoperative thrombosis is substantial. Many trials have been done to compare unfractionated heparin (UFH) and LMWH in this setting but few have studied prophylaxis specifically in patients undergoing surgery for cancer. The ENOXACAN investigators conducted the first randomized trial that compared LMWH with UFH in patients undergoing general surgery for colorectal cancer.7 No difference in efficacy was detected between enoxaparin 40 mg injected once a day and UFH 5000 U administered 3 times daily in preventing venographically detected DVT and symptomatic VTE. Differences in major bleeding and mortality were not observed. Subgroup analyses of other trials are consistent with these findings.
More recently, the same investigators conducted the ENOXACAN II trial to examine the efficacy and safety of extending prophylaxis with LMWH beyond hospitalization in cancer patients.8 In this multicenter, double-blind, placebo-controlled trial, patients undergoing elective, curative colorectal surgery for cancer received enoxaparin 40 mg once daily for the first 6–10 days after surgery and then were randomized to continue with enoxaparin 40 mg once daily or placebo injections until mandatory bilateral venography was performed 25–31 days after surgery. During the treatment period, 12.0% (20/167) of the placebo patients compared with 4.8% (8/165) of the enoxaparin patients had a confirmed thrombotic event (P = 0.02). Therefore, extended prophylaxis with enoxaparin significantly reduced the rate of VTE by 60% (95% CI, 10%–82%) and this benefit was maintained at 3 months. The absolute risk reduction of 7% found in this trial means that 14 patients must be treated to avoid 1 case of venographic DVT. Overall, there was no detectable difference in any or major bleeding during the treatment period and no difference in mortality up to 1 year of follow-up.
Rasmussen also reported on extended prophylaxis after cancer surgery.9 In this open-label randomized trial, 117 patients received dalteparin 5000 U once daily for the first 7 days after abdominal surgery for cancer and then were randomized to continue dalteparin at the same dose or no further treatment for the next 21 days. All patients used graduated compression stockings throughout the study period. Preliminary results showed that prolonging prophylaxis with dalteparin significantly reduced the incidence of proximal DVT, from 15.9% to none (P < 0.005). Accordingly, 6 patients must be treated to avoid 1 episode of proximal DVT.
Fondaparinux, a selective inhibitor of activated factor X that was recently approved for prophylaxis in orthopedic surgery, has been evaluated in a Phase III, double-blind, double-dummy trial (PEGASUS trial) in patients undergoing high-risk abdominal surgery.10 Patients with and without cancer were randomized to receive once daily injections of fondaparinux 2.5 mg or dalteparin 5000 U. Based on a composite outcome of DVT detected with bilateral venography performed on day 5–10 after surgery and symptomatic VTE up to day 10, a difference in thromboembolic events was not observed (4.6% vs 6.1%, respectively; P = 0.14). Major bleeding was also comparable between the groups. However, in the subgroup of 1408 patients with cancer, fondaparinux was associated with a statistically significant reduction in VTE, with 4.7% of patients having VTE, as compared with 7.7% of patients in the dalteparin group (P = 0.02). Given the potential for bias in subgroup analyses, any conclusion regarding the relative efficacy of fondaparinux and dalteparin in cancer surgery prophylaxis is premature.
Central venous catheters
Early studies indicated that the risk of thrombosis with long-term central venous catheters (CVCs) was as high as 60%, or 1 event per 1000 device days. Consequently, prophylaxis with either low-dose warfarin or LMWH was recommended based on two small, open-label randomized trials that used venography to screen for catheter-related thrombosis. However, recent studies have reported discrepant results. In a study of 425 cancer patients receiving chemotherapy through a CVC who were randomized to LMWH prophylaxis or placebo, the incidence of symptomatic catheter-related thrombosis was 3.7% and 3.4%, respectively.11 In a similarly designed study, a difference in symptomatic events was not detected between patients randomized to warfarin 1 mg daily and those assigned to placebo.12 In addition, low-dose warfarin can produce supratherapeutic anticoagulant levels in patients receiving fluorouracil-based chemotherapy.13 Therefore, based on recent data, the overall thrombotic risk of catheter-related thrombosis is low and probably insufficient to warrant routine prophylaxis.6 Moreover, these trials suggest that prophylaxis with low-dose LMWH or low-dose warfarin is not effective in reducing symptomatic events.
Treatment of VTE
Anticoagulants are the mainstay therapy for the prevention and treatment of acute VTE. Although these agents are highly efficacious and have an acceptable safety profile in most patients, cancer patients have a higher risk of recurrent VTE and anticoagulant-related bleeding compared with patients without cancer.1 These complications likely reflect the heightened hypercoagulable state associated with malignant diseases and the multiple co-morbidities in cancer patients that may alter their response to anticoagulant therapy and their risk of bleeding. LMWHs are convenient, efficacious and safe compared with UFH and coumarin derivatives and are becoming the anticoagulant class of choice in surgical and medical oncology patients.
Initial therapy
To date, multiple randomized trials and meta-analyses of these trials have confirmed that for initial therapy LMWHs are at least as efficacious as UFH in reducing recurrent thrombosis and are likely to be associated with a lower risk of major bleeding. Furthermore, LMWHs can be given safely in an outpatient setting without the need for laboratory monitoring and have a lower risk of heparin-induced thrombocytopenia. However, whether LMWHs and UFH perform comparably in patients with cancer and acute VTE has not been formally investigated.
Long-term therapy
Despite their pharmacological and practical limitations, coumarin derivatives have been the mainstay of long-term anticoagulant treatment for VTE. Although vitamin K antagonists are highly effective in reducing recurrent thrombosis in the general population, treatment failures, serious bleeding and difficulties with maintaining the international normalized ratio (INR) within the therapeutic range are common problems in patients with cancer. A prospective cohort study reported that the 12-month cumulative incidence of recurrent VTE in cancer patients was 20.7% versus 6.8% in patients without cancer, while the corresponding estimate for major bleeding was 12.4% versus 4.9%, respectively.14 Based on the available literature, the risk of recurrent VTE is 2- to 3-fold higher and the risk of major bleeding is 3- to 6-fold higher in cancer patients than in patients without cancer.1 Patients with cancer also experience recurrent VTE despite having therapeutic INR levels and suffer serious bleeding complications without receiving excessive anticoagulation.15
To date, two published clinical trials have examined the use of long-term LMWH as an alternative to warfarin therapy in cancer patients with acute VTE. A number of other randomized studies also have compared LMWH with oral anticoagulant therapy for long-term treatment but they included primarily patients without cancer. The CANTHANOX trial compared 3 months of standard warfarin therapy with enoxaparin therapy in cancer patients with proximal DVT, PE or both.16 All patients were treated initially for at least 4 days with therapeutic doses of enoxaparin at 1.5 mg/kg once daily and were randomized to either continue with enoxaparin at the same dose or warfarin therapy. By 3 months, 15 of 75 patients had recurrent VTE or major bleeding in the warfarin group compared with 7 of 71 patients assigned to enoxaparin. The difference was not statistically significant (P = 0.09). Major bleeding was reported in 17 patients; of these, 6 patients in the warfarin group died of bleeding. Based on these results, the investigators concluded that warfarin is associated with a high bleeding risk in cancer patients with VTE and that prolonged treatment with LMWH may be as effective and safer than warfarin therapy.
In a similar patient population, the CLOT trial evaluated the use of long-term dalteparin.17 In this multicenter, randomized, open-label study, 676 cancer patients with proximal DVT, PE or both were randomized to usual treatment with dalteparin initially followed by 6 months of oral anticoagulant therapy or dalteparin alone for 6 months. In the dalteparin group, patients received therapeutic doses at 200 U/kg once daily for the first month and then 75%–80% of the full dose for the next 5 months. The cumulative risk of recurrent VTE at 6 months was reduced from 17% in the oral anticoagulant group to 9% in the dalteparin group, resulting in a statistically significant risk reduction of 52% (P = 0.002). Accordingly, 1 episode of recurrent VTE is prevented for every 13 patients treated with dalteparin. Overall, there were no differences in major or any bleeding between the groups. By 6 months, 39% of the patients had died in each group; 90% of the deaths were due to progressive cancer.
Antineoplastic Effects of LMWH
Accumulating experimental and indirect clinical evidence have suggested that anticoagulants, particularly LMWHs, may have antineoplastic effects. The FAMOUS study is the first randomized, placebo-controlled trial to examine the influence of LMWH on survival in patients with advanced solid tumors.18 In this trial, 385 patients were randomly assigned to dalteparin 5000 U once daily or placebo for 1 year. Overall, 64% of the patients had stage IV disease and the groups were balanced in other prognostic markers of survival. According to an intention-to-treat analysis, the survival estimates for patients receiving placebo at 1, 2 and 3 years after randomization were 41%, 18%, and 12%, respectively, while the corresponding rates for patients in the dalteparin group were 46%, 27%, and 21%. The trend for survival benefit, however, was not statistically significant (P = 0.19). A post-hoc analysis suggested that dalteparin was associated with improved survival in those patients who lived beyond 17 months. This unexpected observation that LMWH may have a greater impact on survival in patients with better prognosis or early disease is consistent with the findings of another post-hoc analysis of from the CLOT trial. In this study of cancer patients with VTE, dalteparin was associated with a statistically significant 50% reduction in mortality in patients who did not have metastatic disease at the time of randomization.19 Lastly, the MALT trial recently reported that cancer patients without VTE who were randomized to nadroparin for 6 weeks had an improvement in median survival compared to those assigned to placebo, and that the improvement was greater in those who had a life expectancy of greater than 6 months.20
Future Directions
The recent advances in the management of VTE in cancer patients are exciting. Based on the results of the ENOXACAN II and FAME trials, it is clear that extended prophylaxis with LMWH following major abdominal surgery for cancer reduces the risk of VTE without significantly increasing the risk of bleeding. However, it remains unknown whether the reduction in asymptomatic DVTs in these trials is clinically relevant. Therefore, it is still premature to recommend routine extended prophylaxis in all cancer patients after surgery.
The CLOT trial presents compelling evidence that LMWHs should become the standard of care as monotherapy for the treatment of VTE in cancer patients. The major obstacle in changing clinical practice is the cost of the drug, particularly in North America. Further studies are needed to evaluate whether patients with certain tumor types are more or less likely to benefit, and when anticoagulant therapy can be reasonably discontinued.
To date, the studies evaluating new anticoagulants have included few or no patients with cancer. The PEGASUS trial provides preliminary evidence that fondaparinux may be more effective than LMWH for prophylaxis in the surgical oncology setting but further studies are needed to confirm this finding. New oral agents are potentially the most attractive because of the route of administration and the elimination of laboratory monitoring. However, these drugs, including ximelagatran, a pro-drug of the direct thrombin inhibitor melagatran, are still in various developmental phases and have not been evaluated formally in patients with cancer. Their safety profiles and costs will be critical in determining how they compare with traditional anticoagulants.
Although there are still many unanswered clinical questions in thrombotic management in oncology patients, the introduction of LMWHs has improved and simplified both prophylaxis and treatment regimens. More studies are required in this population to look at antithrombotic therapy, especially on issues regarding quality of life, bleeding, cost-effectiveness and the influence of anticoagulants on cancer survival. Whether novel anticoagulants will offer even better alternatives to LMWHs awaits further study.
Potential mechanisms by which various clinical conditions may facilitate deep-vein thrombosis. Risk factors or clinical conditions that increase the risk of DVT can be classified as either increasing the baseline propensity for thrombosis, or precipitating the thrombotic event acutely. According to Virchow’s triad, these conditions promote thrombosis through one (or more) of three major mechanisms: (1) inducing hypercoagulability, (2) directly injuring the vein wall, and (3) causing blood stasis.
| . | Increased Baseline Propensity for Thrombosis . | Acute Insult . |
|---|---|---|
| Abbreviations: AT, antithrombin; HRT, hormone replacement therapy; OCT, oral contraceptives | ||
| Hypercoagulability | Genetic | Increased Coagulants |
| Increased coagulants | Blood-borne tissue factor | |
| Prothrombin mutation G20210A | Malignancy (Trousseau’s syndrome) | |
| Decreased anticoagulants | Congestive heart failure (?) | |
| AT deficiency | Systemic infection (?) | |
| Protein C deficiency | Exogenous administration of clotting factors | |
| Protein S deficiency | rVIIa | |
| Factor V Leiden | rVIII | |
| Acquired | Acute Loss of Anticoagulants | |
| Malignancy | Nephrotic syndrome (loss of AT) | |
| Hyperhomocysteinemia | Initial warfarin therapy without heparin | |
| HRT/OCT (?) | ||
| Pregnancy (hormone-related) | ||
| Nephrotic syndrome (loss of AT) | ||
| Antiphospholipid syndrome | ||
| Increased levels of clotting factors | ||
| Direct Vessel Injury | Direct vessel injury would most often represent an acute insult | Intravascular catheters |
| Trauma | ||
| Examples of low-grade, chronic vessel injury that increase the baseline propensity for thrombosis may include: | Surgery | |
| Endothelial injury secondary to chemotherapy | ||
| Hyperhomocysteinemia | ||
| Vasculitis | ||
| Antiphospholipid syndrome | ||
| Blood Stasis | More commonly functioning as an acute insult precipitating thrombosis, rather than increasing the baseline propensity for thrombosis: | Hospitalization/bed ridden |
| Pregnancy (stasis) | ||
| Limb paralysis (e.g., stroke, plaster casts) | ||
| Age | Right heart failure | |
| Obesity | Long-haul flights | |
| Pregnancy (gradual immobility/stasis) | Vein compression (e.g., enlarged lymph node) | |
| Sedentarism | ||
| . | Increased Baseline Propensity for Thrombosis . | Acute Insult . |
|---|---|---|
| Abbreviations: AT, antithrombin; HRT, hormone replacement therapy; OCT, oral contraceptives | ||
| Hypercoagulability | Genetic | Increased Coagulants |
| Increased coagulants | Blood-borne tissue factor | |
| Prothrombin mutation G20210A | Malignancy (Trousseau’s syndrome) | |
| Decreased anticoagulants | Congestive heart failure (?) | |
| AT deficiency | Systemic infection (?) | |
| Protein C deficiency | Exogenous administration of clotting factors | |
| Protein S deficiency | rVIIa | |
| Factor V Leiden | rVIII | |
| Acquired | Acute Loss of Anticoagulants | |
| Malignancy | Nephrotic syndrome (loss of AT) | |
| Hyperhomocysteinemia | Initial warfarin therapy without heparin | |
| HRT/OCT (?) | ||
| Pregnancy (hormone-related) | ||
| Nephrotic syndrome (loss of AT) | ||
| Antiphospholipid syndrome | ||
| Increased levels of clotting factors | ||
| Direct Vessel Injury | Direct vessel injury would most often represent an acute insult | Intravascular catheters |
| Trauma | ||
| Examples of low-grade, chronic vessel injury that increase the baseline propensity for thrombosis may include: | Surgery | |
| Endothelial injury secondary to chemotherapy | ||
| Hyperhomocysteinemia | ||
| Vasculitis | ||
| Antiphospholipid syndrome | ||
| Blood Stasis | More commonly functioning as an acute insult precipitating thrombosis, rather than increasing the baseline propensity for thrombosis: | Hospitalization/bed ridden |
| Pregnancy (stasis) | ||
| Limb paralysis (e.g., stroke, plaster casts) | ||
| Age | Right heart failure | |
| Obesity | Long-haul flights | |
| Pregnancy (gradual immobility/stasis) | Vein compression (e.g., enlarged lymph node) | |
| Sedentarism | ||
Characteristics of recurrence risk and management implications: a theoretical framework.
| Characteristic of Recurrence Risk Associated with the Risk Factor* . | Potential Treatment Implications . |
|---|---|
| * Risk factors often have multiple associated characteristics (e.g., cancer is associated with high acute and long-term risk of recurrence, and reduced efficacy of VKAs) | |
| High risk acutely | Higher intensity of initial treatment or use of supplemental therapies (e.g., vena caval filter) |
| Delayed decline of risk | Longer duration of treatment |
| High risk persists indefinitely | Indefinite treatment |
| Risk factors associated with reduced treatment efficacy (e.g., vitamin K antagonists [VKAs]) | Use of an alternative therapy (e.g., low-molecular weight heparin) |
| Risk factor is additive or multiplicative with other transient risk factors (i.e., non-selective) | Intermittent treatment when exposed to other risk factors |
| Risk factor is additive or multiplicative with some, but not other, risk factors (i.e., selectively) | Intermittent treatment when exposed to specific risk factors only (avoidance may also be practical [e.g., hormonal therapy]) |
| Characteristic of Recurrence Risk Associated with the Risk Factor* . | Potential Treatment Implications . |
|---|---|
| * Risk factors often have multiple associated characteristics (e.g., cancer is associated with high acute and long-term risk of recurrence, and reduced efficacy of VKAs) | |
| High risk acutely | Higher intensity of initial treatment or use of supplemental therapies (e.g., vena caval filter) |
| Delayed decline of risk | Longer duration of treatment |
| High risk persists indefinitely | Indefinite treatment |
| Risk factors associated with reduced treatment efficacy (e.g., vitamin K antagonists [VKAs]) | Use of an alternative therapy (e.g., low-molecular weight heparin) |
| Risk factor is additive or multiplicative with other transient risk factors (i.e., non-selective) | Intermittent treatment when exposed to other risk factors |
| Risk factor is additive or multiplicative with some, but not other, risk factors (i.e., selectively) | Intermittent treatment when exposed to specific risk factors only (avoidance may also be practical [e.g., hormonal therapy]) |
Risk of recurrent venous thromboembolism (VTE) after stopping anticoagulant therapy.
| Variable . | Relative Risk . |
|---|---|
| For supporting references, see text and Kearon5 | |
| Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism | |
| Transient risk factor | ≤ 0.5 |
| Persistent risk factor | ≥ 2 |
| Unprovoked VTE | ≥ 2 |
| Protein C, protein S and antithrombin deficiency | 1–3 |
| Heterozygous for factor V Leiden | 1–2 |
| Homozygous for factor V Leiden | 4.1 |
| Heterozygous for G20210A mutation in the prothrombin gene | 1–2 |
| Heterozygous for both factor V Leiden and G20210A prothrombin gene | 2–5 |
| Factor VIII level > 200 IU/dL | ~6 |
| Antiphospholipid antibodies | 2–4 |
| Mild hyperhomocysteinemia | 2.7 |
| D-dimer elevation after stopping therapy | ~2 |
| Family history of VTE | ~1 |
| Cancer: | ~3 |
| Metastatic vs non-metastatic | ~3 |
| Chemotherapy | ~2 |
| Discontinuation of estrogen | < 1 |
| Proximal DVT versus PE | ~1 |
| Distal DVT versus proximal DVT or PE | 0.5 |
| Residual thrombosis | 1–2 |
| Vena caval filter | ~1.8 |
| Second versus first episode of VTE | ~1.5 |
| Age at diagnosis of VTE | ≥1 |
| Male gender | ~1.5 |
| Asian | ~0.8 |
| Variable . | Relative Risk . |
|---|---|
| For supporting references, see text and Kearon5 | |
| Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism | |
| Transient risk factor | ≤ 0.5 |
| Persistent risk factor | ≥ 2 |
| Unprovoked VTE | ≥ 2 |
| Protein C, protein S and antithrombin deficiency | 1–3 |
| Heterozygous for factor V Leiden | 1–2 |
| Homozygous for factor V Leiden | 4.1 |
| Heterozygous for G20210A mutation in the prothrombin gene | 1–2 |
| Heterozygous for both factor V Leiden and G20210A prothrombin gene | 2–5 |
| Factor VIII level > 200 IU/dL | ~6 |
| Antiphospholipid antibodies | 2–4 |
| Mild hyperhomocysteinemia | 2.7 |
| D-dimer elevation after stopping therapy | ~2 |
| Family history of VTE | ~1 |
| Cancer: | ~3 |
| Metastatic vs non-metastatic | ~3 |
| Chemotherapy | ~2 |
| Discontinuation of estrogen | < 1 |
| Proximal DVT versus PE | ~1 |
| Distal DVT versus proximal DVT or PE | 0.5 |
| Residual thrombosis | 1–2 |
| Vena caval filter | ~1.8 |
| Second versus first episode of VTE | ~1.5 |
| Age at diagnosis of VTE | ≥1 |
| Male gender | ~1.5 |
| Asian | ~0.8 |
Model for venous thrombosis.
Abbreviations: Factor II, prothrombin; factor IIa, thrombin; PSGL-1, P-selectin glycoprotein ligand-1
Model for venous thrombosis.
Abbreviations: Factor II, prothrombin; factor IIa, thrombin; PSGL-1, P-selectin glycoprotein ligand-1
A staged approach to selecting duration of anticoagulant therapy based on assessment of risk factors for recurrent venous thromboembolism (VTE).
Abbreviations: DVT, deep-vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; LMWH, low-molecular-weight heparin.
A staged approach to selecting duration of anticoagulant therapy based on assessment of risk factors for recurrent venous thromboembolism (VTE).
Abbreviations: DVT, deep-vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; LMWH, low-molecular-weight heparin.
Referencing has been largely confined to publications from the past 5 years; a more extensive reference list is provided in a related review by the author.5