Abstract
Obtaining research funding is of utmost importance for academic hematologists, especially those engaged in basic science research. Several funding mechanisms are available to researchers working in the fields of Hematology and/or Oncology. This chapter will discuss various issues relating to applications for National Institutes of Health (NIH) R01 grants, the classic funding mechanism for investigator-initiated research. It will also discuss other opportunities for funding from other government agencies, including the Department of Veterans Affairs and the Department of Defense. We will also provide a summary of the process by which NIH R01 applications are reviewed and some guidelines and tips for the successful writing of R01 grants or their equivalents from other agencies.
I. Mechanisms of Funding for Hematologists: Writing an R01 Application
Leonidas C. Platanias, MD, PhD*
Jesse Brown VA Medical Center, Chicago; Robert H. Lurie Comprehensive Cancer Center and Division of Hematology-Oncology, Northwestern University Medical School, 8250 Olson, 710 North Fairbanks, Chicago IL 60611
Classic funding mechanisms for hematologists via the National Institutes of Health (NIH) include R01 and R21 grant applications. R01 applications can be submitted several times throughout the year, while R21 applications are only submitted in response to a specific initiative by NIH, followed by a solicitation of applications. R01 applications are for larger grants, up to 5 years, while R21 applications are usually for 2 years with a budget typically limited to $75,000–$150,000 per year.1 Due to the limitations in the funds that are available, in combination with the continuous growth and expansion of many universities and medical centers, obtaining research funding is difficult and highly competitive. This fact is important for junior faculty applicants who need to obtain funding in order to develop their independent research careers. Being persistent and responsive to the suggestions of grant reviewers may be characteristics that can make the difference. Nevertheless, despite such difficulties and the decline in the number of funded applications in recent years, there is no doubt that good science and innovative research are fundable in the end. Below we discuss the review process at NIH for R01 grant applications and we provide some suggestions and tips on the writing of a grant application. Obviously, there is no standard method to ensure success; the most important factor remains the quality of the work and the proposed scientific plan of the investigator.
Review Process for NIH R01 Grant Applications
All submitted applications arrive at the Center for Scientific Review (CSR) at the National Institutes of Health. The deadlines for new R01 applications are October 1, February 1, and June 1; for a revised application these dates are November 1, March 1, and July 1. There applications can be assigned to several of the study sections that exist at NIH. A list of the NIH study sections and their rosters can be found at the CSR home page (www.csr.nih.gov/committees/rosterindex.asp). An application that is focused on a hematology research subject can be assigned to any of the biomedical research study sections. However, study sections that usually review grants related to Hematology and/or Oncology include the Hematopoiesis Study Section (HP), the Cancer Molecular Pathobiology Study Section (CAMP), the Tumor Cell Biology Study Section (TCB), the Clinical Oncology Study Section (CONC), the Cancer Etiology Study Section (CE), the Developmental Therapeutics Study Section (DT), the Cancer Biomarkers Study Section (BSS), the Cancer Genetics Study Section (CG), and the Cancer Immunopathology/Immunotherapy Study Section (CII), as well as several basic science study sections (e.g., CDF1-4, DEV1-2), which may also be appropriate for very basic hematology research grant applications.
After a grant is assigned to a specific study section, the Scientific Review Administrator (SRA) and the Chairman of the Study Section assign it to reviewers. Usually, each reviewing member of the study section receives anywhere from 8 to 10 applications to review, but this can vary from study section to study section. The reviewers are usually senior researchers with university appointments at the rank of Associate Professor or Professor and are usually funded in the corresponding research area. One primary and one or two secondary reviewers are assigned to each application. In addition, the application may be assigned to another member of the study section who is designated as a reader. Readers review and score the application but do not write a full review. If an application requires expertise not already present in the study section, ad-hoc reviewers may be asked to participate. Such reviewers may be present at the time that the study section will ultimately meet or they may participate via teleconference. Each reviewer is expected to write a preliminary report for each assigned grant application and provide a preliminary score prior to the meeting of the study section. Such preliminary critiques and scores are posted on a secure website that can be accessed by all members of the study section (except those who have a conflict with a certain application) prior to the meeting of the panel. That way, reviewers can see what others wrote for a given application and prepare for the meeting.
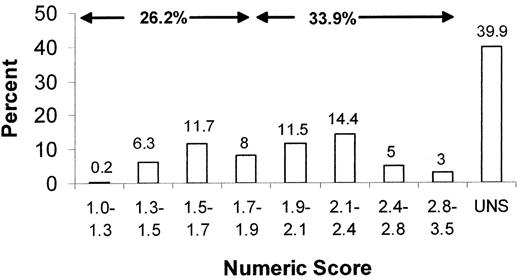
Instead of giving a numerical value, the reviewers have the option to give an unscore (UNS) rating for grants that they think belong to the lower half of the applications. The numerical scale is between 1 and 5, but scores above 3–3.5 are rarely given, as such grants receive an unscore rating. Such a preliminary recommendation for an application to be unscored (triaged) is re-evaluated during the meeting of the study section as discussed below.
At the study section meeting, the first action of the panel is to decide which applications will be discussed or will be unscored. Every member of the study section who has reviewed an application is called by the Chairperson to vote whether a given application should receive an unscore rating or be discussed. If all primary and secondary reviewers agree that an application should be unscored, there is no further discussion: the application gets triaged, and the applicant will ultimately receive the original critiques posted by the reviewers. If one of the reviewers of that application feels it should be discussed and is not persuaded by his peers otherwise, then the application is discussed in the second phase of the meeting.
The study section then starts discussing the scored applications. The chairperson first asks each of the reviewers to give their preliminary score range. After each reviewer does so, a more detailed discussion begins. The primary reviewer provides a brief description of the application, makes comments regarding strengths and weaknesses, and explains the reasons for the numerical score that was given. The secondary reviewers and readers follow and also provide their criticisms of the application. Depending on whether there is controversy or not, the discussion can be either brief or prolonged. It is not unusual for reviewers to change their original score after the discussion. In fact, if the primary and the secondary reviewer disagree on the merit of an application, the most persuasive among them may convince the other one to come closer to his or her proposed score. The chairperson also usually solicits the opinion of other members of the study section who are present and may have read the application. After the discussion is completed, the chairperson asks the members of the study section to make their final numerical recommendations. All members of the study section then vote. It should be noted that study section members are allowed to vote with a deviation range of 0.5 from the highest or lowest scores given by the primary and secondary reviewers or readers. For instance, if the primary reviewer recommends a score of 1.7, the secondary recommends a score of 2.1 and the reader recommends 1.9, the members of the study section can give a number between 1.2 and 2.6. This can lead to huge variations, as in many study sections a score of 1.2 corresponds to a percentile of < 2–4%, while a score of 2.6 puts the application close to the 40th–45th percentile. Study section members can deviate even further than that; however, in such a case they have to write a separate report of their own to justify the “dissention” from the rest of the study section. This only rarely occurs. In general there is some sort of final consensus among reviewers, and scores come close.
The applicant will ultimately receive a summary statement of the discussions and final score from the study section, as well as critiques written by each individual reviewer. Figure 1 shows the distributions of scores in a hypothetical study section, including scores for both new and revised applications. To assure funding in the current era, a percentile score of 20% or less may be necessary. These are grants in the excellent or outstanding range. It should be emphasized that study sections do not make funding decisions. The decisions for funding are made by the Institute to which a given grant is assigned. The cut-off percentages for funding may also vary between Institutes. Thus, it is possible that a grant that received a percentile close to the funding level (i.e., 19%–20%) may be fundable by one Institute but not another. For this reason, it is always to the advantage of the applicant if their applications have dual assignments, a primary assignment to one Institute, and a secondary to another.
Suggestions for the Preparation of an R01 Application
It is very important to remember that the record and experience of the principal investigator plays a major role in the funding of an R01 application. A successful grant application is usually based on a solid and important hypothesis, which requires the presence of preliminary data. It is therefore of utmost importance, especially for young investigators, that the application is based on significant background work. The publication of this work in highly regarded peer review journals is one of the factors that plays a major role. Therefore, it is extremely important for young investigators to do good work and publish it in highly ranked journals before they apply for an R01 grant application. It is not unusual to see junior faculty who attempt to write multiple grant applications without having published papers in the area and/or on the specific subject. A better approach might be to focus their initial efforts toward obtaining strong preliminary data and publishing them in top ranking journals, instead of spending their time in the preparation of applications that are not based on strong backgrounds.
A key to a successful R01 grant application is to start early. It is particularly useful to make a plan for an application 6 months in advance and have a target date in mind. During that time an agenda can be developed and a skeleton of the application can be designed. Allowing such time will help to identify gaps and deficits in the preliminary data that need to be corrected prior to the submission of the application. Also, allowing sufficient time will permit the investigator to obtain comments from colleagues about the grant. It is always a good idea to get the opinion of other experienced investigators on the proposal.
When writing the grant it is important to remember that the reviewers are not necessarily people working in the same field. Therefore, the background section should be written clearly and carefully. In the preliminary data section, it is important to show the data in a clear way, and it is always helpful if parts of the preliminary data are linked to each of the hypotheses that are present in the grant. A very important component of the application is the description of the significance of the proposed work. The application should emphasize why the grant is important and what makes this application different from others. One of the key criteria by which reviewers evaluate grants is the presence or not of innovative ideas or concepts. Sometimes the perception that the proposal is “highly innovative” may compensate for deficiencies in other parts of the proposal. Surprisingly, many study sections take pride in emphasizing their willingness to support innovative ideas that go against the mainstream. Thus, the applicant should not to be afraid to propose new concepts that might go against established models in that research area. An approach like that should not “hurt” the application, and on the contrary it can make the difference.
Another essential part of writing a good NIH grant application is to be fair to other investigators and to acknowledge their contributions in the field. It is also vital to identify potential problems and pitfalls in the proposed experiments, and to propose alternative methodologies. If the principal investigator has no expertise on a particular methodology or technical approach, he or she should not compromise the proposal by avoiding proposing studies that require such expertise. Instead, the principal investigator should identify collaborators or consultants for such investigations, and document the relationship with letters of support from these individuals. This is something that study section members always welcome. After the application is fully written, it is always a good idea to ask a senior colleague to review it and to make suggestions.
When submitting the application it is valuable to remember that you can request the Institute and the study section that the grant will be assigned to. Writing a cover letter that briefly describes the main hypothesis of the grant and the reasons for which it should be assigned to a particular institute or study section should be sufficient. In most cases, the Center for Scientific Review will honor such requests. The members of the different study sections can be found on the Web (www.csr.nih.gov/committees/rosterindex.asp). The principal investigator can review the membership of the various study sections and decide which section would be best for his or her grant application. It should be also noted that approximately a month before the study section meeting, the full composition of the group for that cycle (including ad-hoc reviewers) is published on the web. If at that point, the principal investigator realizes that a competitor or someone who conceptually disagrees with that application is part of the panel, he/she can always call the SRA and ask for exclusion of that person from the panel for the review of the grant or for reassignment of the application. There is no guarantee that the request will be honored, although SRAs are usually willing to give the investigator the benefit of the doubt.
The applicant receives his/her priority score within a couple of weeks after the study section meets. The principal investigator has to wait approximately 4–6 weeks to receive the summary statements and written comments of the reviewers. If the grant was unscored or did not receive a priority score that would bring it within the fundable range, the applicant will have to make a decision as to whether revision and resubmission of the proposal is best, or whether to aim for a completely new and different proposal. NIH allows for submission of the same grant application a total of three times. If the grant received a relatively “good” score, and the comments of the reviewers reflect an overall positive view of the application, it is always worth working toward resubmission. It is important to remember that even if an application receives priority scoring very close to the funding range, it does not mean that a revised application with only minor/cosmetic changes will be funded in the next round. For instance, an applicant may have received a priority percentile of 24%, while only the top 20% of the applications were fundable that year. This applicant should make every effort to sincerely attend to all the comments made by the reviewers and make major changes in the grant. Making minimal changes, based on the assumption that there will be a proportional improvement of the score, would be a mistake. If it is not possible to address all the comments, it is a wise idea to acknowledge that and re-focus the application. For instance, a reviewer may be critical of a specific aim of the application and request preliminary data that further supports the studies of that objective. If the principal investigator cannot obtain such data, it may be a good idea to drop that specific aim and develop a different one. Reviewers tend to appreciate applicants who make sincere efforts to revise their applications according to the recommendations of the review panel. In such a case they tend to react more positively to the resubmitted proposal. On the other hand, if they sense that the investigator is resistant to their recommendations/suggestions this may have “catastrophic” consequences for the application.
It is difficult to define a magic recipe for the funding of grant applications. There is no question that good work and new innovative concepts have a much higher chance of receiving funding than sloppy work that lacks innovation. Applicants should remember that in some cases receiving a bad review does not mean that their science is bad. It may simply reflect inability to write well and present their thoughts in a cohesive manner. Getting the input and suggestions of more senior and experienced colleagues may help significantly. The system is designed in a way that ultimately recognizes good science. The best assurance for funding is to develop a strong research program, make important contributions in the field and be persistent. This will eventually lead to funding, one way or another.
II. Mechanisms of Funding for Hematologists: Application for VA or DOD Research Support
Elizabeth A. Eklund, MD*
Robert H. Lurie Comprehensive Cancer Center; Division of Hematology-Oncology, Northwestern University Medical School, 8250 Olson, 710 North Fairbanks, Chicago IL 60611; Jesse Brown VA Medical Center, Chicago
Application to the Veterans Administration for Research Support
Overview of VA Research and Development Services
The mission of the of Veterans Health Administration (VHA) Office of Research and Development (ORD) is “to discover knowledge, develop VA researchers and health care leaders, and create innovations that translate into advances in health care for veterans and the nation.”1 Over the past year, there have been some changes in the leadership of VHA ORD. This resulted in re-organization of the research services and some changes in the research proposal submission process. Currently, the VHA ORD is organized into four services: Biomedical Laboratory Research and Development (BLR&D), Health Services Research and Development (HSR&D), Rehabilitation Research and Development (RR&D), and Clinical Science Research and Development (CSR&D).
The BLR&D service focuses on the “causes, progression, diagnosis, and treatment of virtually the full range of diseases and disorders affecting veterans.”2 A key to application for BLR&D support is direct, specific connection between the proposed investigations and a human disease. For example, it is not adequate to state that investigations of cell cycle regulation are “relevant to cancer.” The investigator should demonstrate that the specific aspect of cell cycle regulation being studied is relevant to a specific mechanism involved in oncogenesis. Although a translational research component is not a necessity for BLR&D funding, inclusion of such a component may be beneficial. Translating laboratory discoveries into treatments for VA patients is a prominent emphasis of the BLR&D mission literature.2
The CSR&D service “conducts research that focuses on intact human beings as the unit of examination.”2 Proposals that fall under the auspices of CSR&D include clinical trials, effectiveness and intervention trials, technological studies, and epidemiology. Studies of outcomes, health systems administration, and cost effectiveness are submitted to HSR&D. In the past, clinical trials proposals required an approved letter of intent (LOI), prior to submission of a complete application. With re-organization of the CSR&D, LOIs will not presently receive scientific evaluation and triage. The changing role of the LOI is an issue that should be discussed on a frequent basis with the investigator’s local VHA ORD office.
Past VA research and development support
The annual budget for Veterans Health Administration research increased from $316 million in 1999 to $400 million in 2003.1 Of this budget, ~70% is used to support investigator-initiated research through the Merit Review program, ~10% for Centers of Excellence, and less than 10% each for Multi-center Clinical Trials and Career Development Awards.1 Originally, a VA Merit Review was considered adequate to support an investigator’s entire research effort. However, the goal of the Merit Review program has changed. At present, there is increasing emphasis on Merit Review–funded investigators competing successfully for research support from additional sources.
Eligibility for VA research funding
Because VA views its research program as an intramural activity, physicians may apply for VA research funding if they have a VA appointment with salary support. VA salary support is incremental, divided into 8ths of the individual’s total salary. In order to be eligible for VA research support, a physician must have at a minimum a 5/8ths appointment (although there is a process for requesting an exception—check with your local VHA ORD Administrative Office for details). An exception to this is the career development award, discussed below. It is expected that physicians with VA research support will participate actively in VA clinical care, since the salary support is derived from the clinical program and is not included as a direct cost in the merit review budget. Non-clinician VA investigators are also eligible for research support. There are four classifications of non-clinician VA investigators: Assistant Research Scientist, Research Scientist, Research Career Scientist (RCS), and Senior Research Career Scientist (SRCS).3
An Assistant Research Scientist is a recently trained non-clinician who has not yet obtained funding through the VA Merit Review Entry Program (MREP, see below). The title Research Scientist is applied to non-clinician investigators upon obtaining a VA Merit Review. Salary support for a Research Scientist is derived from the Merit Review budget.2 In contrast, an RCS or SRCS obtains salary support through a specific, highly competitive application process that is separate from the Merit Review application. RCS and SRCS are renewable appointments requiring Merit Review funding and contribution to VA research including participation in mentoring junior scientists, functioning as a “resource for the research community,” directing a core facility, or serving on local and/or national VA research committees.2
Specific programs for VA research funding: Career Development Awards
The VA provides support for individual investigators through the Career Development Program (referred to as CADE).4 Generally, CADE provides salary and research support for investigators early in their careers. These awards are designed to provide a period of intensive research, with limited clinical responsibilities, for individuals with a clinical doctoral degree. The most recent handbook4 describes three Career Development Awards: Research Career Development (RCD), Advanced Research Career Development (ARCD), and Career Development Enhancement Award (CDEA).
The RCD award is for individuals who are less than 5 years from completion of clinical training. This is a mentored, non-renewable, 3-year award providing Principal Investigator (PI) salary and $20,000/year. The intent is development of an independent investigator, who successfully competes for a VA Merit Review. The applicant cannot be the recipient of an NIH K series award and must not have additional significant funding. Since the general form of the applications is similar, it is advisable to submit an NIH K08 and an RCD award simultaneously, although only one can be accepted. At the completion of an RCD award, the investigator can apply for an ARCD award. This is a 3-year non-renewable award, which provides PI salary and $50,000 per year for research support. This is a mentored award for individuals with 3–5 years of research experience and post-clinical training.4 The exclusion criteria are receipt of a non-mentored research grant or an NIH K series award. The CDEA is for a senior VA investigator to spend 6 months on “a new area of research specialization.”4 The major requirement is identification of a sponsor who will “direct or facilitate the training.” The PI must have at least 6 years of previous VA research support and obtain 6 months leave. The limit is one in the entire VA career.
Specific programs for VA research funding: Merit Review Awards
The Merit Review program supports investigator initiated research through the Merit Review Entry Program (MREP or type II Merit Review) and the Merit Review (or type I Merit Review).5 The MREP is a non-renewable, mentored, 3-year award. The intent is that the individual be competitive for a type I Merit Review at the end of the funding period. Qualification for an MREP application requires that the recipient be less than 5 years from clinical or postdoctoral training and not be independent.5 Unlike RCD and ARCD awards, MREP proposals are evaluated by the same subcommittees (merit review boards) that evaluate type I applications. This provides an advantage, since the relatively small MREP budget, in comparison to a type I award, alters the expectations of the merit review board in favor of the applicant. Additionally the VHA ORD emphasizes training of young faculty, which also provides an advantage to individuals applying for this funding mechanism. Successful MREP applicants tend to have a competitive advantage for a successful type I application, at the conclusion of the MREP.
A successful MREP has four components: a specific mentoring plan, an applicant with a strong background, a well-organized research plan, and evidence the work will lead to independence.6 As with any mentored application, it is important to demonstrate involvement of the mentor. A clue to mentor involvement is the quality of the application; an amateurish application is generally interpreted by the subcommittee as denoting a lack of mentor interest. A successful mentoring plan includes course work, attending mentor lab meetings, attending relevant clinical conferences (especially for PhD applicants), and expertise of the mentoring team in the area of the applicant’s research. The strength of the applicant is judged primarily by publication record and preliminary data. In general, subcommittees are more impressed by first or senior author publications than middle author publications, since the latter do not have the same implications for potential independence. The research plan of an MREP proposal is expected to meet the same criteria as a type I Merit Review, as described below. Additionally, subcommittees are concerned that the proposed investigations demonstrate significant difference in direction from the mentor’s program. Such differences are considered evidence there is a realistic expectation the applicant will have an independent program at the end of the funding period. Since the application includes abstract pages from the mentor’s ongoing support, the subcommittee has a good idea how the applicant’s work fits into the mentor’s program.
The type I VA Merit Review is the major source of funding for investigator-initiated research.6 A type I Merit Review is renewable, but parameters dictating the award duration are currently changing. Successful applicants are generally independent investigators with a proven track record. The type I Merit Review program is not suitable for individuals without a current research effort or who recently completed a clinical training program with limited research experience. Such applicants would not be likely to succeed because productivity is a criterion in Merit Review evaluation. A year ago, the ORD proposed a specific number of publications as a benchmark for Merit Review funding. This idea met with limited enthusiasm from the research community, since publication quality was not considered. Based on these objections, specific numerical criteria were discarded, but proposals currently receive both scientific and productivity scores during the review process. Weighting these two components into an overall score is left to the discretion of the subcommittee.
Similar to an NIH RO1, a successful type I application will be hypothesis driven, with strong supporting preliminary data and mechanistically oriented specific aims. It is important to avoid constructing a proposal in which one or more aims rely upon results of data that has not yet been obtained. Additionally, reliance on a reagent or animal model not currently available to the investigator should be avoided. The format of the Merit Review application includes a section entitled “Relevance to Veteran’s Health.” This section is quite important since it provides the subcommittee with evidence the proposed investigations have a potential impact on treatment or diagnosis of a disease relevant to the veteran population. Applications that propose mechanistic investigations of potential therapeutic targets are likely to be met with enthusiasm.
Preparation and submission of a VA research proposal
Submission of a VA research proposal requires cooperation with the local VA ORD. The local office provides current forms and the timetable for submission of components of the application. This information is available on the VA ORD web site (http://www1.va.gov/resdev/funding/), but this site is not updated as rapidly as the local office. The amount of specific administrative assistance the local ORD provides will vary according to the site. However, all local VA ORD committees must approve applications prior to submission. Some local R&D committees provide a formal scientific review, employing local investigators and/or outside reviewers as an ad hoc review committee. At other sites, the review process may be less formal. It is advisable to determine early in the application process the timetable for events such as local R&D committee meetings and requirements, and the LOI submission dates.
The peer review process: VA subcommittees
At the time the application is submitted, the investigator indicates an area of research on the face page. The choice of Hematology versus Oncology will suggest which subcommittee reviews the proposal, although the actual assignment of the proposal is the prerogative of the Program Review Division of ORD. Overall, it is important to remember that VA sees its R&D program as an intramural one. It is intended to support the research interests of its VA-salaried faculty and medical centers, as well as its national programmatic priorities. Restrictions on PI eligibility and other issues reflect this.
The emphasis of the Hematology subcommittee (HEME) is red cells, coagulation, white cell function, and hematopoiesis, although proposals focused on leukemia and lymphoma are reviewed by HEME. The Oncology A subcommittee (ONCOA) reviews proposals relevant to endocrine tumors, neuro-oncology, lymphoma, leukemia and myeloma. Therefore, there is some overlap between the HEME and ONCOA. If the proposal focuses on oncogenesis or translational approaches to hematologic malignancy, ONCOA may be appropriate. If the proposal emphasizes hematopoiesis or congenital hematologic disorders, HEME would be appropriate.
Application to the Department of Defense for Research Support
Overview of the DOD research and development program
Research is sponsored by the Department of Defense (DOD) through a “Congressionally Directed Medical Research Program” (CDMRP). The mission statement of this program is to “provide hope by promoting innovative research, recognizing untapped opportunities, creating partnerships and guarding the public trust.”7 Due to the structure of the CDMRP, the disease-specific programs that are sponsored, and how much support each program receives, vary from year to year. Calls for proposals are posted on the DOD/CDMRP web site (http://cdmrp.army.mil/). New announcements may occur during the fiscal year, if supplemental funding becomes available. Therefore, it is advisable to check the DOD web site on a regular basis.
The majority of DOD/CDMRP research support has been appropriated for breast and prostate cancer ($150 million and $85 million per year, respectively).7 Additional programs supported over the past 3 years include neurofibromatosis ($20 million/yr), ovarian cancer ($10 million/yr), chronic myeloid leukemia ($4.25 million/yr), and tuberous sclerosis ($2 million/yr). Other funded areas include hepatitis, lung cancer, muscular dystrophy, fragile X syndrome, children’s hospice, arthropod bone infections, and alcoholism. Since research support is determined by Congress, programmatic priorities are subject to constituent and disease-specific interest-group pressure.
Award mechanisms available through the DOD
Each of the various disease-specific programs has a combination of award mechanisms that vary from year to year. Career Development Awards have been offered regularly in several disease programs. The goal of these awards is to support an investigator who is established in another area of research, but would like to move into the disease-specific area. The intent is to provide time for the investigator to “acquire training, data and experience to compete for more traditional awards.”7 The New Investigator Award has similar goals, but is targeted to post docs or assistant professors who would like to develop an independent program in the disease-specific area.
Investigator Initiated Awards have been offered by all disease-specific programs. Successful awards generally propose clinical trials or pre clinical investigations directly applicable to the disease. At times, a disease specific program may specifically request applications for either a Clinical Trials Award or a Therapeutic Development Award, instead. Successful Clinical Trials Awards generally propose human therapeutic trials, as opposed to studies using samples from human subjects. In contrast, Therapeutic Development Awards focus on “the preclinical assessment of therapeutics, as well as the development of tools for the preclinical evaluation in model systems.”7 This includes studies of cell lines, animal models, or specimens from human subjects. Another variation on the Investigator Initiated Award is the Idea Development Award. These have been occasionally solicited through some programs. These awards support “innovative ideas that may be viewed as high risk, but have the potential for high gain in scientific or clinical knowledge.”7 Although the instructions state that preliminary data are not required, proposals with data have a higher likelihood of success.
Specific DOD programs for hematology research
The DOD sponsors several programs relevant to hematology research. The chronic myeloid leukemia (CML) research program was established in 2002. This program has supported 10 investigators.8 Proposal applications were only accepted in 2002 and 2004, with the 2003 appropriation being used to support proposals held over from 2002. The mechanisms that have been offered through the CML program are Investigator Initiated Awards (2002 and 2004) and Therapeutic Development Awards (2004). The majority of successful applications proposed translational presclinical studies, emphasizing targeted therapeutic approaches to CML.
The Neurofibromatosis program might also apply to hematologists. This program has sponsored 118 proposals from 1999 to 2003.7 As with the CML program, the majority of successful proposals focused on experimental therapeutics. The Neurofibromatosis program has sponsored research through Career Development, Investigator Initiated, and Clinical Trials Awards. The connection between this program and hematology would be juvenile myelomonocytic leukemia, or molecular studies of neurofibromin 1. In the past, this program has enjoyed significant support, offering multiple funding mechanisms, which makes it attractive for hematologists working on regulation of ras signaling pathways.
Preparation and submission of a research proposal to the DOD
Although the general guidelines for a successful DOD proposal are similar to an NIH R01, there are some important differences. First, it is essential to specifically address goals of the program and the questions that are to be answered through the award mechanism. These are detailed in the announcement. The other difference is the presence of lay “community members” on the grant review study sections. These individuals, who are patients with the target disease or family members of such patients, are present to ensure that funded investigations address the needs of patients with the disease. These individuals are the target audience for the “lay abstract” section of the proposal. Additionally, the applicant should keep in mind that these individuals are looking for evidence that the investigations will result in treatments or advances of immediate use to patients. This emphasis is reflected in the focus of proposals receiving DOD support. The abstracts of funded proposals, which are available on the DOD/CDMRP web site, are a good source of information regarding the type of project likely to have a favorable reception.
Submission of proposals to the DOD/CDMRP is entirely online. The forms and instructions are available through the CDMRP web site. The application process involves an online letter of intent, which is due 1 month before the proposal deadline. The LOI includes investigator and budget information, a title, and an abstract. The web site is designed so that the investigator designates other individuals who can access the files and proposal information. This must include an Institutional Research Administrative Officer in the investigator’s Office of Grants and Contracts. It is also advantageous to include administrative support personnel in the list of individuals with access to the proposal, for assistance in entering contact and budget information.
The completed proposal is uploaded, as a single PDF file, by an Institutional Research Administrative Officer. This upload must be completed by 5 pm Eastern time on the deadline day, requiring submission of the proposal to the institutional Grants and Contracts Office some days in advance. Generation of a single PDF file (not a series of linked PDF files) that includes all form pages, the body of the proposal, and all submitted reprints can be a challenge. It is advisable to obtain the assistance of someone who has done this successfully in the past.
Example of distribution of numeric scores and percentages in a given study section, in a format similar to the one distributed by Scientific Review Administrators to study section members, for their information.
The numbers shown here are hypothetical and not from an actual study section.
Example of distribution of numeric scores and percentages in a given study section, in a format similar to the one distributed by Scientific Review Administrators to study section members, for their information.
The numbers shown here are hypothetical and not from an actual study section.