Abstract
Allogeneic hematopoietic stem cell transplantation (alloSCT) has been established as an effective consolidation therapy in acute myeloid leukemia (AML) in first or subsequent remission. Although it effectively prevents relapse, treatment-related mortality (TRM) associated with alloSCT may compromise that beneficial effect. As a result, alloSCT may be restricted to patients with a relatively high risk of relapse. Here, we review studies that identify categories of AML patients who may specifically benefit from alloSCT. In addition, we discuss recent developments with respect to alternative donors, stem cell sources, and supportive care. Finally, we highlight recent results obtained with reduced-intensity alloSCT, which already significantly influence our therapeutic strategy in elderly patients with AML.
During the last three decades allogeneic hematopoietic stem cell transplantation (alloSCT) has become an established therapeutic modality in patients with acute myeloid leukemia (AML). The alloreactive immunotherapeutic effects against the leukemia (graft-versus-leukemia reactivity) contribute greatly to its efficacy. No other established therapy applied during complete remission offers as strong an anti-leukemic effect. Unfortunately, the benefit of alloSCT is considerably offset by the complications following transplantation. These complications, which are related to disparity of host-recipient histocompatibility and the toxicity of high-dose chemotherapy and radiotherapy, ask a considerable price in terms of morbidity and mortality. As a result, alloSCT produces only a limited gain in overall survival despite significantly reducing relapse.1–6 Key issues in the current clinical use of alloSCT in the treatment of AML include the following: In what category of patients is alloSCT the therapy of choice? What is the overall value of newer, less cytotoxic, conditioning regimens? Do these variants maintain their anti-leukemic efficacy and generate reduced mortality? What is the current role of unrelated donor SCT? What future developments might address the common complications of infections and graft-versus-host disease (GVHD), contributing further to improved outcome?
Indications of Allogeneic SCT in Adults with AML
AlloSCT in first complete remission: intermediate risk and unfavorable risk
There is a marked diversity in the risk of relapse in patients with AML according to their (cyto)genetic profiles. After attainment of a first complete remission, intermediate- and poor-risk AML have a priori probabilities of recurrence of approximately 50% and 80%, respectively. Furthermore, in patients with AML of intermediate or unfavorable risk, the chance of salvage is generally low if the leukemia recurs.7 When applied as first-line therapy, alloSCT represents the best option for prevention of relapse in such patients. Since alloSCTs are done depending on the availability of a matched donor (and not in the setting of randomized prospective comparisons), estimates of the overall value of alloSCT can best be derived from prospective studies that allow for a donor/no donor comparison.1–6 The low rate of relapse following alloSCT has been confirmed in all donor/no donor comparisons but has not translated into a consistent survival advantage. However, significantly higher disease-free survival (DFS) has been reported in both the EORTC-GIMEMA AML-10, based on more than 1000 patients, and MRC AML-10, based on more than 1500 patients (Table 1 ). While poor-risk patients seemed to benefit most in the EORTC study, in the MRC study the benefit appeared to be restricted to patients with intermediate-risk AML. Recent results by the Dutch-Belgian-Swiss HOVON-SAKK cooperative consortium (n = 1000, not yet published) indicate comparable findings, with significantly higher DFS in patients with a sibling donor, apparent in both intermediate- and poor-risk AML (Table 1 ). Therefore, alloSCT from an HLA-matched family donor to intermediate- and unfavorable-risk patients is recommended whenever feasible (Table 2 ).4–6 Moreover, in cytogenetic poor-risk patients with long-term survival probabilities of only 20% on standard chemotherapy, it seems reasonable to offer the patient a transplant from a matched unrelated donor if an HLA-matched family donor is not available (Table 2 ). Whereas the value of alloSCT in distinct cytogenetic subsets of AML has been assessed, various molecularly defined subsets with prognostic significance have been proposed more recently. These molecularly defined entities have not yet been robustly evaluated for outcome of alloSCT. The limitation of the molecular analyses is that they have usually been done in highly selected subgroups of patients and retrospectively performed studies.8 Age is a particularly important determinant of outcome following alloSCT. Therefore, age limits have commonly been part of eligibility criteria for alloSCT. The introduction of reduced-intensity conditioning regimens, which are associated with decreased early toxicity, has challenged these traditional age restrictions (see below).
AlloSCT in first complete remission: favorable risk
Myeloablative alloSCT is generally not recommended for patients in first complete remission with cytogenetic favorable subtypes of AML where the relapse probability is 35% or less. This applies to most patients with the so-called core binding factor leukemias—AML t(8;21), AML inv(16), and acute promyelocytic leukemias with t(15;17). In those conditions the risk of procedure-related death (approximately 10%–20%) does not outweigh the potential benefit of the transplant. In the favorable-risk category, it seems reasonable to reserve the option of an alloSCT for an eventual relapse. The advantage of postponing alloSCT is that over-treating a majority of good-risk patients is avoided (Table 2 ). In addition, in case of relapse many of those patients can still be rescued with an allograft.7
AlloSCT for advanced AML
Due to its potent anti-leukemic effects, alloSCT is the treatment of choice for any relapsed patient who is eligible. If an alloSCT can be performed, it offers the best prospect for cure.7 Outcome of allografts beyond first remission, however, is inferior to that in first-remission patients, owing to an increase in both treatment-related mortality (25%–35%) and relapse (40%–45%).9,10 If, prior to relapse, the patient has had a previous allograft or autograft, the likelihood of rescue with an alloSCT is significantly reduced.7
AlloSCT for primary induction failure
Patients who fail to achieve remission after one or two courses of chemotherapy, including high-dose cytosine arabinoside, usually have a dismal prognosis. A few older studies suggested that alloSCT can rescue some of those patients with DFS rates between 15% and 40%. Updated results from the City of Hope study confirmed these earlier findings and suggested that alloSCT can cure approximately one third of patients with primary refractory AML.11 Adverse prognostic factors in that series included unfavorable cytogenetics and the use of an unrelated donor. Therefore, patients failing induction chemotherapy may still be considered candidates for an allogeneic transplant. Published reports evaluating the role of alloSCT in primary refractory AML remain limited, however, preventing a documented estimate of the value of this approach. Hence, important factors determining outcome (e.g., age, cytogenetics, type of donor, and HLA match) should be taken into account to restrict the option of alloSCT to those with a reasonable chance for cure.11
AlloSCT for AML: Alternative Donors
Matched unrelated donor transplantation
Currently, more than 8 million HLA-typed volunteer donors from approximately 50 registries around the world appear in the Bone Marrow Donors Worldwide (BMDW) file (www.bmdw.org). It has resulted in a high probability (70%–80%) of finding at least one HLA-A, -B, and -DR–matched donor for any Caucasian patient. The probability of finding a match for HLA-A, -B, -C, -DR, and -DQ, however, is considerably less (35%–40%). A recent report confirmed that the risk of GVHD, graft failure, and mortality increases progressively with the number of HLA disparities, emphasizing the importance of high-resolution HLA typing and the selection of donors with, preferably, no more than one mismatched allele out of 10.12 Despite improvements in supportive care and HLA matching, outcome following unrelated donor alloSCT is still inferior to that after HLA-identical sibling transplantation.10,13,14 While the degree of HLA match significantly affects outcome, the selection of patients for whom a search is initiated also has a significant impact on outcome. Most unrelated donor alloSCTs are currently performed in patients with AML in first remission with poor risk features, in patients in second remission, and, most recently, in elderly patients with AML (Table 3 ) after reduced-intensity conditioning.
The Bone Marrow Donors Worldwide file not only has registered adult volunteer unrelated donors, but also includes the tissue type of many umbilical cord blood cell grafts. Whereas the overall lower cell dose has hampered the use of cord blood transplants in adult patients, results of a recent study suggest that outcome after unrelated umbilical cord blood transplant may approach the results of unrelated bone marrow transplantation in acute leukemia.14
HLA-mismatched family member hematopoietic stem cell transplantation after high-dose preparative regimens
Patients with AML lacking a sibling donor, but who are transplant candidates, may benefit from an unrelated donor SCT or, alternatively, from a family mismatched donor, thereby avoiding an expensive and time-consuming donor search. The Perugia group has systematically developed a protocol based on intensified conditioning (total-body irradiation, thiotepa, fludarabine, anti-thymocyte globulin) and the infusion of a high-dose of CD34+-selected progenitor cells harvested from the peripheral blood. The approach is associated with a high rate of engraftment, low incidence of GVHD, and an event-free survival of approximately 45%–50% for patients receiving their transplant in remission.15 The anti-leukemic activity is based on both an intensified preparatory regimen and the possibility of a donor-versus-recipient NK cell alloreactivity.16 The approach has been developed and evaluated in only a limited number of patients and centers to date. It therefore requires further study before it can be implemented in clinical practice on a broader scale.
Transplantation after Reduced-Intensity Conditioning (RIC-allo-SCT)
During the last decade, several groups have explored new, less-intensive conditioning regimens for alloSCT. This development has been fuelled by the incentive to apply allografting to older patients and to other disease categories beyond the acute and chronic leukemias.17–19 It has become clear that reduced-intensity conditioning (RIC), which includes potent immunosuppressive agents in addition to (dose-reduced) anti-leukemic agents, effectively permits engraftment of donor hematopoietic stem cells. Due to a reduced anti-leukemic effect of the conditioning regimen, the procedure largely relies on the graft-versus-leukemia effect of the alloreactive lymphocytes to eradicate residual leukemia cells. Accordingly, the relapse rate in patients without GVHD appears to be considerably greater than in patients with clinical evidence of acute GVHD grades II–IV or chronic GVHD.20 Also, recipients of unrelated donor grafts may show less disease progression than recipients of matched sibling grafts,21 as was suggested in a recent comparison (Table 3 ). The favorable immunological anti-leukemic effects, however, may be counterbalanced by the morbidity and mortality associated with GVHD. Hence, long-term follow-up of transplant recipients is needed to fully assess the net result of these variables. Meanwhile, several studies strongly suggest that the morbidity (hematopoietic, pulmonary, hepatic, infections) and mortality following RIC alloSCT are less than after myeloablative conditioning,22–25 leading investigators to raise the upper age limit for alloSCT to 65–70 years. While the reduced intensity of the conditioning regimen has resulted in reduced non-relapse mortality in older patients with AML (15%–20%) (Table 3 ), some studies have suggested a concomitant increase of relapse (Hegenbart et al. in Table 3 ).21,26,27 This has provoked the question of which anti-leukemic agents might be added to the preparative regimen (and at what dose).26–31 Two approaches have been attempted to retain effective anti-leukemic cytotoxic therapy, while avoiding the toxicity and risk of high-dose conditioning: 1) the addition of tumor-targeted therapy, such as gemtuzumab (anti-CD33) or radiolabeled monoclonal antibodies, to the conditioning regimen; 2) the introduction of moderate to intensive dosages of busulfan, thiotepa, or melfalan in the conditioning regimen. These approaches are currently being tested in phase II studies. In addition, given the incidence and associated morbidity of chronic GVHD in elderly patients (Table 3 ), the prevention and control of GVHD has also become a major issue in the development of RIC alloSCT. Currently, optimized control and prophylaxis of GVHD is being investigated in several studies, including prospective randomized studies in Europe. Eventually, the appropriate comparison of autologous SCT or chemotherapy as consolidation therapies by donor/no donor studies (with sufficient follow-up) should establish the long-term value of this approach, especially in older patients.
Improved Supportive Care
Whereas myeloablative alloSCT offers the strongest anti-leukemic effect when applied as consolidation treatment in AML, the reduction of relapse may be offset by the morbidity and mortality associated with the procedure. Potential fatal complications include GVHD, opportunistic infections, and organ failure, such as venoocclusive disease and interstitial pneumonia. These complications are often interrelated. Several reports have shown that transplant related mortality has gradually decreased in the last two decades.10,31,32 Major changes in the clinical management of alloSCT recipients that have contributed to this improved survival include: 1) improved prevention and treatment of GVHD by cyclosporine and methotrexate and possibly by mycophenolate mofetil; 2) transplantation of a higher hematopoietic progenitor cell dose; 3) molecular monitoring of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) and the subsequent pre-emptive treatment of patients with viral reactivation; 4) improved detection and therapy of fungal infections; 5) the introduction of high-resolution typing for HLA matching and the increased number of potential unrelated donors; and 6) the appreciation of a number of pretransplant and procedure-related factors that affect outcome, which allow risk assessment, and thereby may guide transplant policies.33 The reduction of transplant-related mortality is especially evident in patients transplanted in first complete remission and has been less pronounced in patients transplanted beyond first remission.10 These results may argue in favor of tissue typing early after diagnosis in order to move to alloSCT without delay as an early consolidation therapy in patients with AML in first remission. A significant reduction of transplant-related mortality has been achieved during the last two decades, and ongoing developments may add to that improvement.32 In particular, the acceleration of immune reconstitution and improved ability to induce and manipulate tolerance to prevent host-versus-graft and graft-versus-host reactions are currently the focus of intense investigation.34
Disease-free survival analysis for donor versus no donor, by cytogenetic risk group.
| . | No. of Patients . | . | ||
|---|---|---|---|---|
| Study Group (ref), Cytogenetic Risk Category . | Donor . | No Donor . | Hazard Ratio . | 95% CI . |
| * Significant benefit in the donor group, (p < 0.05). | ||||
| ‡ Unpublished observations, Cornelissen J, van Putten W, Löwenberg B, on behalf of the HOVON/SAKK AML-study group | ||||
| Abbreviations: CI, confidence interval; EORTC-GIMEMA (EORTC; European Organization for Research and Treatment of Cancer, GIMEMA; Gruppo Italiano Malattie Ematologiche dell’Adulto); MRC 10 (MRC; Medical Research Council); HOVON/SAKK (HOVON; Dutch-Belgian Hemato-Oncology Cooperative Group, SAKK; Swiss Group for Clinical Cancer Research) | ||||
| EORTC-GIMEMA (6) | ||||
| Intermediate | 61 | 104 | 1.16 | (0.75–1.81) |
| Poor and very poor | 64 | 94 | 0.58* | (0.39–0.87) |
| MRC 10 (5) | ||||
| intermediate | 192 | 416 | 0.74* | (0.59–0.92) |
| poor | 48 | 121 | 0.88 | (0.61–1.28) |
| HOVON/SAKK‡ | ||||
| intermediate | 187 | 336 | 0.76* | (0.59–0.92) |
| poor | 120 | 194 | 0.65* | (0.49–0.85) |
| . | No. of Patients . | . | ||
|---|---|---|---|---|
| Study Group (ref), Cytogenetic Risk Category . | Donor . | No Donor . | Hazard Ratio . | 95% CI . |
| * Significant benefit in the donor group, (p < 0.05). | ||||
| ‡ Unpublished observations, Cornelissen J, van Putten W, Löwenberg B, on behalf of the HOVON/SAKK AML-study group | ||||
| Abbreviations: CI, confidence interval; EORTC-GIMEMA (EORTC; European Organization for Research and Treatment of Cancer, GIMEMA; Gruppo Italiano Malattie Ematologiche dell’Adulto); MRC 10 (MRC; Medical Research Council); HOVON/SAKK (HOVON; Dutch-Belgian Hemato-Oncology Cooperative Group, SAKK; Swiss Group for Clinical Cancer Research) | ||||
| EORTC-GIMEMA (6) | ||||
| Intermediate | 61 | 104 | 1.16 | (0.75–1.81) |
| Poor and very poor | 64 | 94 | 0.58* | (0.39–0.87) |
| MRC 10 (5) | ||||
| intermediate | 192 | 416 | 0.74* | (0.59–0.92) |
| poor | 48 | 121 | 0.88 | (0.61–1.28) |
| HOVON/SAKK‡ | ||||
| intermediate | 187 | 336 | 0.76* | (0.59–0.92) |
| poor | 120 | 194 | 0.65* | (0.49–0.85) |
Established indications for allogeneic hematopoietic stem cell transplantation (alloSCT) in acute myeloid leukemia.
| Prognostic Subgroup . | First CR . | Second or Higher CR . |
|---|---|---|
| In all these conditions an allogeneic stem cell transplantation from a fully matched family donor is generally the first therapeutic option of choice. | ||
| † If a matched family donor is not available, an allotransplant from a matched unrelated donor as salvage therapy is the second choice in situations of high risk. | ||
| Abbreviations: CR, complete remission; AML, acute myeloid leukemia. | ||
| Favorable-risk AML | ||
| Acute promyelocytic leukemia | No | Yes† |
| Core binding factor AML | No | Yes† |
| Intermediate-risk AML | Yes | Yes† |
| Poor-risk AML | ||
| Age ≤ 60 years | Yes† | Yes† |
| Age > 60 years | Investigational | Investigational |
| Prognostic Subgroup . | First CR . | Second or Higher CR . |
|---|---|---|
| In all these conditions an allogeneic stem cell transplantation from a fully matched family donor is generally the first therapeutic option of choice. | ||
| † If a matched family donor is not available, an allotransplant from a matched unrelated donor as salvage therapy is the second choice in situations of high risk. | ||
| Abbreviations: CR, complete remission; AML, acute myeloid leukemia. | ||
| Favorable-risk AML | ||
| Acute promyelocytic leukemia | No | Yes† |
| Core binding factor AML | No | Yes† |
| Intermediate-risk AML | Yes | Yes† |
| Poor-risk AML | ||
| Age ≤ 60 years | Yes† | Yes† |
| Age > 60 years | Investigational | Investigational |
Outcome of reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia (AML).
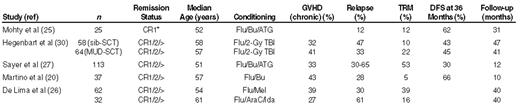
*Poor risk
Abbreviations: AraC, cytosine arabinoside; ATG, antithymocyte globulin; Bu, busulfan; CR, complete remission; DFS, disease-free survival; Flu, fludarabine; GVHD, graft-versus-host disease; Ida, idarubicin; Mel, melphalan; TBI, total-body irradiation; TRM, treatment-related mortality.
