Abstract
The evolution of indolent lymphomas to aggressive histologies, known as histologic transformation (HT), is a frequent occurrence for all subtypes of low grade B cell lymphoproliferative disorders. The risk of developing HT is approximately 3% per year for patients with indolent lymphoma. Clinically these present with a rapid change in the behavior of the disease, with evidence of a highly proliferative malignancy with a propensity to involve extranodal sites. The prognosis of patients following transformation is generally poor, with median survival of about 12 months. Recent studies suggest that the development of HT is very complex with the acquisition of multiple cytogenetic abnormalities in the low-grade lymphoma cells prior to HT. To date, there are no biologic or genetic parameters predictive of the development of HT. A myriad of genetic lesions have been identified in HT, and provide insight into its pathogenesis. These include genes regulating proliferation (C-MYC and C-MYC-regulated genes); control of the cell cycle (CDKN2a and CDKN2B); and programmed cell death (TP53, C-MYC, and BCL2). Gene expression profiling has been applied to the study of HT and has increased our understanding of the transformation process. There has been limited progress in the treatment of patients with HT. Conventional chemotherapy is generally of limited benefit, although a subset of patients are long-term survivors following high-dose therapy and autologous stem cell transplantation. The use of radioimmunotherapy and new agents targeting specific lesions or aberrant pathways may impact on the management of these aggressive diseases.
Overview
Histologic transformation from an indolent to a more aggressive lymphoma is a common pathway of virtually all histologic subtypes of indolent non-Hodgkin lymphomas (NHL). The most frequently described and studied entity is histologic transformation (HT) of follicular lymphoma (FL) to diffuse large B cell lymphoma (DLBCL). However, HT is also observed in other low grade B cell lymphoproliferative disorders including marginal zone lymphomas (MZL) including the MALT lymphomas, lymphoplasmacytic lymphoma (LPL) including Waldenström macroglobulinemia, and small lymphocytic lymphoma (SLL)/B cell chronic lymphocytic leukemia (Richter’s syndrome). Clinically HT should be considered in patients with rapid progression of lymphadenopathy, infiltration of uncommon extranodal sites (excluding the bone marrow), development of systemic symptoms, elevated serum lactate dehydrogenase (LDH), or hypercalcemia. In LPL, additional clinical findings accompanying HT are worsening cytopenias and organomegaly.1
The largest body of information on HT is related to FL, and will be the focus of this review. HT is generally defined in FL as loss of the follicular architecture and evolution to large cells, implying development of DLBCL or much less commonly Burkitt lymphoma. Histologically these tumors resemble de novo DLBCL or other aggressive histologies, and have high Ki-67 staining and transferrin receptor-related protein, indicators of a high proliferative index.2 The loss of CD21- and CD23-positive follicular dendritic cells (FDCs) within neoplastic follicles may precede HT.3 Using tissue microarrays of paired samples of FL pre- and post-HT,4 by immunohistochemistry, 89% of cases expressed a germinal center (GC) phenotype as defined previously by gene expression profiling studies in DLBCL, CD10+ (80%) or CD10–, bcl-6+, MUM1– (9%). DLBCLs that have evolved from MALT lymphomas are typically BCL-2 and CD10 negative, but BCL-6 positive.5
The frequency of HT in FL varies from 10%–70%, and is dependent on the definition of HT, length of follow-up, and whether biopsies or autopsies were performed. Estimates of the frequency of HT may be incorrect, since patients with aggressive-behaving indolent NHL are often treated as presumed HT, without histologic confirmation. In autopsy series cases were selected for fatal outcome and HT has been observed in up to 69% of patients with FL.6–8 In patients with FL the probability of HT is 22% at 5 years and 31% at 10 years, with a trend toward plateau at 6 years.6 A recent series from the British Columbia Cancer Agency reported approximately equal likelihood of HT in FL, SLL and LPL with incidences of 20%, 15% and 10%, respectively, in 406 patients with a median follow-up of 84 months.7 In splenic MZL 13% of patients have been reported to undergo HT9 and the 5-, 10- and 15-year risks for all indolent NHLs were 14%, 21% and 39%, respectively.7 This suggests that the risk of HT is about 3% per year. Risk factors at initial presentation of FL for the development of HT have been reported to be β2-microglobulin greater than 3 mg/L, no complete response (CR) with initial treatment, and serum albumin less than 3.5 g/dL.6 The risk of HT in patients who receive immediate treatment versus patients who are observed appears to be similar,8 and the type of initial treatment had no impact on risk.7 A recent analysis of 30 patients with HT reported that grade III histology and high FL International Prognostic Index (IPI) score were risk factors at presentation of FL.10
Cytogenetics in HT
Analogous to other models of malignant transformation, the acquisition and accumulation of additional genetic abnormalities are responsible for HT of indolent lymphoma to a diffuse aggressive histology (see Dr. Gascoyne’s paper in this volume). The overwhelming majority of cases of DLBCL evolving from FL, as well as MZL and LPL, are clonally related. In the case of FL, the DLBLC has been shown to arise from a single FL cell in the overwhelming majority of cases studied.11 In contrast, in histologic (Richter’s) transformation of chronic lymphocytic leukemia to DLBCL, the CLL clone and the DLBCL clone are related in only 60% of cases.12 In FL, by using PCR-single strand polymorphism analysis of immunoglobulin VHDHJH gene sequences, intraclonal variation is observed in FL.13 However, the related DLBCL does not show intraclonal divergence but still suggests antigenic selection for the VHDHJH mutational events.11 Given the amount of genomic instability leading to HT, microsatellite instability has been examined in HT samples. Although generally rare in NHL, 2 of 8 cases examined showed a high level of microsatellite instability. However, no mutations of the MLH1 and MSH2 DNA mismatch repair genes were noted.14
In FL, in addition to t(14;18) (bcl-2/IgH) a large number of other non-random secondary cytogenetic abnormalities have been described.15 The use of multicolor fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH) has expanded the insight into these abnormalities.16 Although many of these cytogenetic abnormalities accompany disease progression where follicular architecture is retained as well as following HT, detailed analysis has demonstrated widespread secondary chromosomal abnormalities at initial diagnosis and during the course of FL.17 Following HT, the bcl-2/Ig rearrangement persists in virtually all cases. No single cytogenetic abnormalities appear to be associated with HT. Chromosomal abnormalities associated with HT of FL include loss of 17p; 9p21; 6q; 13q; 4q; 11q; 1p. Chromosomal gains are reported of 6p12-p21; 3q; 2p16; 7; 7q; 12 (12q12-q14); X (Xq); 17q; 18q21;10p1 and 20p13.18 Less commonly, abnormalities involving 8q24 have been reported. Although limited by sample availability loss of heterozygosity has been identified on 12p, 6q, and 9p in 43% of cases studied.19
In splenic MZL with HT, 7q deletion has been observed in 3 of 7 cases, and loss of one extra copy of 3q, and abnormalities of 7p22 and 19p13 were also seen.20 In gastric MZL it has been suggested that in the t(11;18) negative cases that a number of allelic imbalances involving 3q26-27, 6q23-25, 7q31, 11q23-24 and 18q21 may be associated with transformation to DLBCL.21
Specific Gene Alterations in HT
Significant insight into specific gene alterations that are associated with some of the many cytogenetic abnormalities in HT have been reported. Genomic abnormalities detected by array CGH have been correlated with cDNA microarray analysis.18 Over 300 genes that were either over-or underexpressed could be related to areas of genomic gain (or amplification) or loss, including a number of C-MYC target genes. However, in spite of amplification of specific regions containing known genes involved in lymphoma pathogenesis such as BCL-2 and REL, increased expression of many genes were not seen using cDNA microarrays.
Specific genetic lesions have been identified in HT and provide insight into its pathogenesis.22 These include alterations in genes regulating: proliferation (C-MYC and C-MYC-regulated genes); control of the cell cycle (CDKN2a and CDKN2B); and programmed cell death (TP53, C-MYC, and BCL2). C-MYC gene rearrangements are generally uncommon in HT, reported in up to 8% of cases. Lossos et al found a mutation of C-MYC in only 1 of 12 cases using paired samples, pre- and post-HT, and no C-MYC amplification.23 Alterations in CDKN2a and CDKN2B at 9p21, encoding the cdk inhibitors p15 and p16, are inactivated in HT. This occurs by deletion, hypermethylation, or mutation, and is reported in up to 73% of cases using paired samples.
Mutations of TP53 are reported in a subset of cases of HT of FL; mutation at diagnosis of FL is uncommon. When observed in FL, these cases generally have a more aggressive clinical behavior. Mutations of TP53 have been reported in 26% and 80% of cases of transformed FL.24,25 TP53 is similarly inactivated in about 20% of transformed MALT lymphomas by deletion or mutation.26 In a recent report by Davies et al of serial biopsies from patients with FL, TP53 mutations were observed in 28% of cases.27 They also found that although TP53 mutations were not present at diagnosis, in subsequent biopsies where the histology remained FL, 14% of cases had mutations, providing evidence that TP53 mutations can precede HT, occurring from 6 months to 4 years prior to transformation. The presence of mutated TP53 had no impact on overall survival (OS) from diagnosis of FL or from HT. Overexpression of p53 by immunostaining, as a marker of mutation, was detected in only 64% of cases with documented mutations. These investigators examined expression of MDM2, a key regulator of p53, in these specimens, and found increased expression by immunostaining with HT. This was independent of the mutational status of TP53. This suggests novel mechanisms of p53 regulation with transformation and that other regulators such as MDM2 may be novel therapeutic targets.
In the majority of cases using paired samples, the BCL-2/IgH nucleotide sequence is identical in the original FL and the DLBCL samples, with no changes in the BCL-2 sequences in the breakpoint region. However, point mutations in the open reading frame of the BCL-2 gene were noted in 3 of 6 cases studied and some of these mutations led to amino acid substitutions.28
Given the central role of the BCL-6 gene in DLBCL, including translocations and mutations, investigators have examined BCL-6 alterations in HT.29 BCL-6 translocations or deletions have been reported in 39% of cases of FL who went on to develop HT, whereas in FL that did not transform, translocations were noted in 14% of cases.30 This suggests that BCL-6 translocations in FL may increase the risk of HT. However, in an analysis of paired samples, both loss and gain of BCL-6 translocations were observed, supporting the notion that BCL-6 translocation is not required for HT. Other alterations in the BCL-6 gene including deletions and point mutations have also been reported in the majority of cases of HT examined.31 This did not strictly correlate with increased BCL-6 expression, which was dependent on the location of the mutations within the first intron of the gene. Hence, BCL6 mutations may deregulate BCL-6 mRNA in some cases of HT but do not appear to be required for the pathogenesis of transformation.
Using array CGH of paired samples several investigators have reported genomic imbalances at multiple chromosomal regions using paired samples. Amplifications and deletions by CGH have been correlated with gene expression.32 Upregulated genes included AIMIL (1p36), SCN11A (3p22), CALM3/CPL (10p15), CPN1 (10q24) and ARF4L (17q21). The identified downregulated genes included DKFZp761B107 (4p15), LOC118812 (10q24), NAP1L4 (11p15), EB-1 (12q23), C14orf135 (14q23), SIRT2 (19q13) and CHD6 (20q12).
Additional genes that may have a role in HT include the silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) gene.33 This transcription repressor maps to chromosome 12q24, a breakpoint observed in transformed B and T cell lymphomas. In four cases of paired samples, there was a loss on one allele in the transformed specimen by FISH. Interestingly, downregulation of SMRT-induced a transformed phenotype in fibroblasts and restoration of SMRT function in transformed B cell lymphoma lines induced apoptosis. Additional proteins that have increased expression following histologic transformation include activation-induced cytidine deaminase34 and the bcl-2 family member MCL-1.35 These changes may be secondary and unrelated to the transforming event.
Gene expression profiling
Gene expression profiling (GEP) is a potentially powerful tool for enhancing the understanding of HT. Lossos et al examined paired specimens from 12 patients with HT of FL.23 They identified 671 genes with at least 3-fold change in expression, including 415 known genes and 256 ESTs. Of the known genes only one oncogene, C-MYC, previously associated with HT was identified. Moreover 15 C-MYC-regulated genes were also significantly altered in expression. Further analysis found that cases segregated into groups with increased or decreased expression of C-MYC-regulated genes and a group with no significant change in these genes. Since C-MYC controls proliferation as well as programmed cell death, increased expression of C-MYC-regulated genes may enhance proliferation, whereas decreased expression may impede programmed cell death and therefore contribute to HT. A study by Elenitoba-Johnson et al identified 113 differentially expressed genes using 12 paired samples pre- and post-HT.36 They found several growth factor and cytokine receptors (hepatocyte growth factor, fibroblast growth factor receptor 3, lymphotoxin β receptor, platelet derived growth factor receptor β), N-ras and ras-related genes and p38 MAP kinase to be over-expressed following HT. These were confirmed by quantitative RT-PCR. Moreover, activated (phosphorylated) p38 MAP kinase was overexpressed in cases of HT using immunohistochemistry. This is important since cytokine and growth factor genes are targets of p38, and suggests that p38 MAP kinase may have an important role in HT. The p38 Map kinase inhibitor SB203580 decreased the level of phosphorylation and inhibited proliferation of t(14;18) lymphoma cell lines in vitro and inhibited tumor growth in NOD-SCID mouse xenografts. Proteonomic analysis of transformed FL cell lines treated with this inhibitor identified groups of proteins and signaling pathways modified by target inhibition.37 Therefore, targeting p38 MAP kinase may be a novel approach toward treatment of HT.
Analogous to studies in de novo DLBCL, investigators have utilized GEP to develop prognostic models based on analysis of tissue at initial diagnosis to identify patients with aggressive or more indolent disease. Gene expression patterns of normal tumor-infiltrating immune cells have been correlated with survival.38 These have been referred to as IR-1 (immune response), with a signature of infiltrating T cells, and IR-2, with signature of macrophages and FDCs. A follow-up study looking further at macrophage infiltration confirmed the independent adverse prognostic impact of a high level of CD68-positive macrophages.39 However, the poor outcome of those patients with high macrophage infiltration was not a consequence of HT. Glas et al utilizing GEP, developed a model that could accurately be related to clinically aggressive or indolent disease based also on biopsies at initial diagnosis;40 importantly, this model did not predict for the future development of HT. This supports the notion that HT might not be inherent at initial diagnosis of FL, but reflects the progressive accumulation of additional genetic changes leading to a disease that is both histologically and clinically distinct.
Clinical Prognostic Factors
Patients with HT generally have a very poor prognosis. The median OS historically ranges from 2.5 to 22 months, although it is generally less than 1 year. Early studies reported that patients who achieve CR had improved survival, 11 versus 40.5 months (Table 1 ). Bastion et al reported the following factors influenced survival following HT in 52 patients: prior CR, normal LDH; no marrow involvement; absence of B symptoms; stage I-III disease; response to salvage chemotherapy and treatment with a CHOP-like regimen.6 The time from diagnosis and number of prior relapses did not have an impact on survival after HT. Yuen et al have reported on 74 patients with HT.8 The following factors in a Cox regression analysis were identified to be significant predictors for survival: stage (limited versus extensive); prior therapy (none versus any); and response to treatment (CR versus partial response [PR] or no response). Attainment of CR was associated with the extent of disease at HT, while prior therapy was not. The median survival for patients in this series who attained a CR was 81 months.9 This suggests that a limited subset of patients with HT treated with CHOP-like regimens experience long survival in remission.
Treatment of HT
Conventional therapy
The majority of patients have short survival following HT. Patients with HT have generally been treated similarly to patients with de novo DLBCL. Patients treated with CHOP-like regimens had a median survival of 12 months, with a 3-year survival of 32%.6 Yeun et al have reported a median survival following HT in 74 patients of 22 months, with a subset of patients having prolonged responses. Extrapolating from the results of treating de novo DLBCL with CHOP plus rituximab or other regimens that have recently been shown to be superior to CHOP, it would appear that unless a clinical trial option is available, this regimen should probably be the chemotherapy of choice for treating patients with HT. Following recurrence, patients are treated with salvage regimens with limited benefit. EPOCH plus rituximab has been reported in 18 patients with HT previously treated patients with HT, the majority had prior CHOP-like regimens, with median EFS of 12.4 months.41 In a randomized trial where rituximab was compared to yttrium-90 ibritumomab tiuxetan, 3 of 4 patients with HT who received rituximab alone responded, but no duration of response was reported for this limited number of patients.42
High-dose therapy
Except for the patients with very limited disease who have not been previously treated for FL, essentially all patients will relapse quickly following conventional treatment. Based on the results of high-dose therapy and autologous stem cell transplantation (ASCT) in patients with relapsed DLBCL with chemosensitive disease, this strategy has also been applied to patients with HT (Table 2 ). In a series from the Dana-Farber Cancer Institute, in which 21 patients have undergone anti-B-cell purged autologous bone marrow transplantation for FL transformed into DLBCL, no acute in-hospital deaths were seen. With follow-up from 12 to 120+ months, estimated 5-year disease-free survival (DFS) was 46%.43 Patients who underwent HT within 18 months of diagnosis of FL had a significantly better OS when compared with patients who transformed later. Nineteen patients in a minimal disease state from St. Bartholomew’s Hospital received an anti-B-cell purged ABMT within 1 year of HT.44 The median survival was 4.4 years, with 3 patients (16%) in remission at over 4 years of follow-up. Of note following recurrence after ABMT, 5 of 7 rebiopsied patients had continued evidence of HT. A series from Stanford reported 17 patients with HT including 13 patients transplanted at first relapse, and only 6 patients in CR at transplant.45 The 4-year DFS and OS were 49% and 50%, respectively. Similar results were reported by the European Bone Marrow Transplant Registry.46 A subgroup of patients with residual chemosensitive disease who attained CR after high-dose therapy had an OS at 5 years of 69%. All 3 patients with chemoresistant disease at the time of high-dose therapy died of relapsed or progressive disease. A series from the Princess Margaret Hospital included 35 patients with HT.47 At median follow-up of 52 months, 5% of patients died due to progressive disease or treatment-related toxicity, and myelodysplasia/acute leukemia contributed to deaths in 8%. The OS and progression-free survival at 5 years was 37% and 36%, respectively. Age greater than 60 years predicted for poor survival in this report. Berglund et al reported 11 patients who had chemosensitive disease who underwent ASCT with chemosensitive disease, with OS and DFS 81% and 72%.48 Results of these studies suggest that aggressive therapy with ASCT is a reasonable treatment option for selected patients less than age 60 and with chemosensitive disease.
Radioimmunotherapy
Radiolabelled anti-CD20 monoclonal antibodies have significant activity in relapsed and refractory B cell NHL patients. Both 90Y ibritumomab tiuxetan and 131I tositumomab have been investigated in patients with HT. Witzig et al reported on 9 patients who received 90Y ibritumomab tiuxetan with an overall response rate (ORR) of 56%.42 A phase II CALGB trial is currently ongoing with this agent in patients with HT. A larger number of patients with HT who received 131I tositumomab have been reported. Fifty-eight evaluable patients with refractory HT who were treated with 131I tositumomab on phase I, II and III clinical trials have been reported.49 The median time from diagnosis was 80 months, and 48% had no response to their most recent treatment. The ORR was 53%, with a median response duration of 11 months. Twenty-nine patients had a CR with a median duration of 20 months, and median survival was 22 months. A follow-up study demonstrated improved quality of life with 131I tositumomab treatment in these patients. 131I tositumomab has been investigated in rituximab refractory patients including 10 patients with HT.50 When these patients were analyzed along with patients who had non-follicular grade I/II histology and/or masses greater than 7 cm, the OR and CR rates were 42% and 16%, respectively, with the 3-year progression-free survival of 11%. These studies provide evidence for activity of radioimmunotherapy in patients with HT, and further studies will better define the role of this approach in these patients.
Other treatment approaches
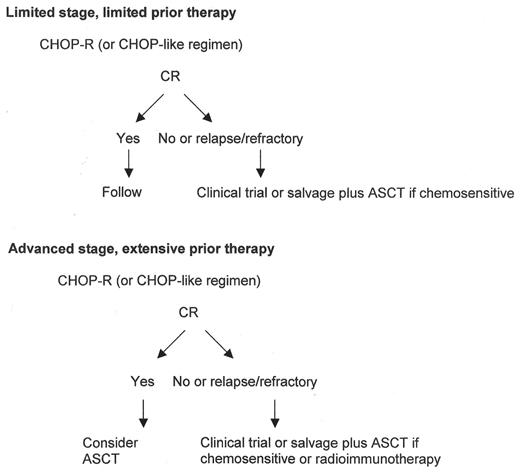
Given the generally limited benefit of current therapy, novel approaches in patients with HT are needed. The current approach towards patients with HT is shown in Figure 1 . The encouraging phase I data of combining 131I tositumomab with high-dose chemotherapy may be attractive given the responses seen in these patients with radio-immunotherapy alone.51 With this approach in relapsed and refractory patients with aggressive histologies, the CR rate was 57%, with OS and event-free survivals of 55% and 39%, respectively. Both conventional and non-myeloablative allogeneic stem cell transplants have been performed in patients with HT; however, the numbers and heterogeneity of the patients treated is too limited to draw significant conclusions. The observations from GEP studies where specific proteins and pathways may be aberrant following HT may suggest new therapies. Overexpression of Aurora kinase B4 and activation of p38 MAP kinase36 merit further clinical study as potential targets. The close similarity of DLBCL following HT to de novo germinal center-like DLBCL, with involvement of BCL-6, provides a rationale for investigation of histone deacetylase inhibitors in patients with HT. With increased understanding of the pathogenesis of HT, novel treatment strategies are likely to follow
Prognostic factors for outcome in histologic transformation.
| Abbreviations: CR, complete response, HT, histologic transformation; CHOP, cyclophosphamide, hydroxydaunomycin, vincristine, prednisone; LDH, lactate dehydrogenase |
| Prior CR for treatment of indolent lymphoma |
| Absence of prior therapy |
| CR following treatment for HT |
| Normal LDH |
| No marrow involvement |
| Absence of B symptoms |
| Stage (limited versus extensive) |
| Treatment with a CHOP-like regimen |
| Abbreviations: CR, complete response, HT, histologic transformation; CHOP, cyclophosphamide, hydroxydaunomycin, vincristine, prednisone; LDH, lactate dehydrogenase |
| Prior CR for treatment of indolent lymphoma |
| Absence of prior therapy |
| CR following treatment for HT |
| Normal LDH |
| No marrow involvement |
| Absence of B symptoms |
| Stage (limited versus extensive) |
| Treatment with a CHOP-like regimen |
Autologous stem cell transplantation for histologic transformation.
| . | # Patients . | DFS . | OS . |
|---|---|---|---|
| Abbreviations: DFS, disease-free survival; OS, overall survival | |||
| Friedberg43 | 27 | 46% @ 5 yrs | 58% @ 5 yrs |
| Foran44 | 27 | 52% @ 4 yrs | 63% @ 4 yrs |
| Berglund48 | 11 | 72% @ 6 yrs | 81% @ 6 yrs |
| Chen47 | 35 | 36% @ 5 yrs | 37% @ 5 yrs |
| Williams46 | 50 | 30% @ 5 yrs | 51% @ 5 yrs |
| Cao45 | 17 | 49% @ 5 yrs | 50% @ 5 yrs |
| . | # Patients . | DFS . | OS . |
|---|---|---|---|
| Abbreviations: DFS, disease-free survival; OS, overall survival | |||
| Friedberg43 | 27 | 46% @ 5 yrs | 58% @ 5 yrs |
| Foran44 | 27 | 52% @ 4 yrs | 63% @ 4 yrs |
| Berglund48 | 11 | 72% @ 6 yrs | 81% @ 6 yrs |
| Chen47 | 35 | 36% @ 5 yrs | 37% @ 5 yrs |
| Williams46 | 50 | 30% @ 5 yrs | 51% @ 5 yrs |
| Cao45 | 17 | 49% @ 5 yrs | 50% @ 5 yrs |
Approach to patients with histologic transformation.
Abbreviations: CHOP, cyclophosphamide, hydroxydaunomycin, vincristine, prednisone; CR, complete response; ASCT, autologous stem cell transplantation
Approach to patients with histologic transformation.
Abbreviations: CHOP, cyclophosphamide, hydroxydaunomycin, vincristine, prednisone; CR, complete response; ASCT, autologous stem cell transplantation