Abstract
The β thalassemias are one of a few medical conditions in which reactivation of a gene product that is expressed during fetal life can functionally replace a deficiency of essential proteins expressed at a later developmental stage. The fetal globin genes are present and normally integrated in hematopoietic stem cells, and at least one fetal gene appears accessible for reactivation, particularly in β° thalassemia. However, rapid cellular apoptosis from α globin chain precipitation, and relatively low levels of endogenous erythropoietin (EPO) in some β+ thalassemia patients contribute to the anemia in β thalassemia syndromes.
In clinical trials, three classes of therapeutics have demonstrated proof-of-principle of this approach by raising total hemoglobin levels by 1–4 g/dL above baseline in thalassemia patients: EPO preparations, short chain fatty acid derivatives (SCFADs), and chemotherapeutic agents. Although thalassemic erythrocytes survive only for a few days, the magnitude of these responses is similar to those induced by rhu-EPO in anemic conditions of normal erythrocyte survival. New oral therapeutic candidates, which stimulate both fetal globin gene expression and erythropoiesis, and combinations of therapeutics with complementary molecular actions now make this gene-reactivation approach feasible to produce transfusion independence in many patients. Development of the candidate therapeutics is hindered largely by costs of drug development for an orphan patient population.
The Appeal of Therapeutic Fetal Globin Gene Reactivation
The β thalassemia syndromes are caused by more than 175 molecular mutations affecting the β globin gene complex, which result in decreased synthetic ratios of non-α to α globin chains, precipitation of excess unbalanced α globin chains, and programmed cell death of erythroblasts early in their development (review1). It is well-established that affected patients do not become anemic until the fetal (γ) globin genes are developmentally silenced, and that patients with persistent high levels of fetal globin typically have less severe anemia, have milder clinical syndromes, and are often transfusion-independent.2 The β thalassemias are thus one of a few clinical conditions in which a gene that is transiently expressed during fetal life can functionally replace a mutant gene normally expressed later in development.1 Reactivation of fetal γ globin expression is appealing as a therapeutic approach to the β thalassemias, as the fetal globin genes are universally present and appropriately contextually integrated in the β-globin locus in hematopoietic stem cells in virtually all humans.
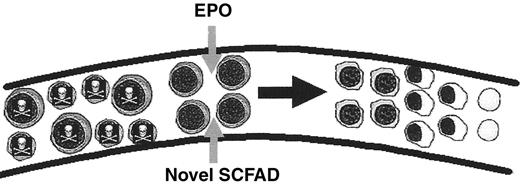
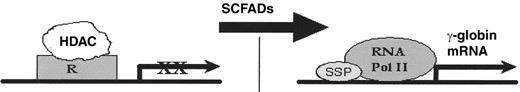
Several mechanisms for optimal correction of β thalassemia at the molecular and cellular levels are established, as illustrated in Figure 1 . Three classes of potential therapeutic agents have been investigated in β thalassemia syndromes: chemotherapeutic agents, short-chain fatty acid derivatives (SCFADs) (of which some are histone deacetylase [HDAC] inhibitors), and the recombinant growth factor erythropoietin (EPO). Fetal globin gene expression has been induced by chemotherapy and SCFADs in animal models, and proof-of-principle has been demonstrated in small clinical trials of these agents. Perhaps due to the molecular and clinical diversity of the β thalassemia syndromes, and the need for therapeutics that are tolerable long-term, large collaborative trials of agents to reactivate fetal globin gene expression to high levels have not yet been undertaken in thalassemia, unlike sickle cell disease. Nonetheless, although the clinical trials have been small, patients with diverse thalassemia syndromes have been included, and significant hematologic responses have been observed, as shown in Table 1 . In combination with preclinical molecular, cellular, and animal studies, the data from these trials now provide a critical base of information with which to design rational strategies for inducing γ globin gene expression and enhancing survival of thalassemic erythroblasts to an extent sufficient to render many subjects transfusion-independent.
Pathophysiologic Features of βThalassemia That Must Be Overcome
It has been generally assumed that reduction of the globin chain imbalance and resulting α globin excess by increasing expression of fetal or β globin, or by decreasing α globin, are all that is necessary to improve red cell survival and blood counts in β thalassemia. Recent studies, however, indicate that a number of factors collectively contribute to the anemia.15,22–27 For example, EPO levels in thalassemia subjects are surprisingly often not elevated to a degree appropriate for the degree of hypoxia.22,23 This lack of a compensatory EPO response may be especially problematic in the β thalassemia syndromes with vastly accelerated apoptosis of erythroblasts.22,25–28 In addition to stimulating proliferation of erythroid cells, EPO decreases apoptosis and prolongs erythroid cell survival through induction of the Bcl-2 family proteins, Bcl-xL and Mcl-1. 28,29 A red cell survival advantage of increased endogenous EPO in β thalassemia that may facilitate effective γ globin induction was suggested by the intriguing observation of Collins and colleagues that hematologic responses to the fetal globin inducer sodium phenylbutyrate occurred only in those subjects who had high endogenous EPO levels (> 160 mU/mL), and was unrelated to any particular pattern of globin gene mutation.11 A subset of subjects with inappropriately low levels of endogenous EPO has responded to combined therapy with butyrate plus EPO, whereas each agent alone had a lesser or minimal effect in the same time-frame.24 These findings together strongly suggest that the exogenously administered EPO might be acting as a survival factor, in addition to an erythropoietic stimulant. Prolonging erythroid precursor cell survival for a sufficient interval may allow a fetal globin inducer to be able to act to correct the pro-apoptotic chain imbalance.
Defining a Therapeutic Effect in the β Thalassemias, and Efficacy of Experimental Agents
The functional clinical endpoint necessary for a therapeutic effect of fetal globin induction in the β thalassemias is abolishing or substantially decreasing requirements for regular transfusion of red blood cells. A total hemoglobin level of 7.0 g/dL is generally adequate, and regular red cell transfusions are not required unless there are clinical complications such as poor growth or facial disfigurement from marrow expansion. However, hemoglobin levels ≥ 8 g/dL provide more exercise tolerance, and levels ≥ 9 g/dL typically render the patient asymptomatic, as in β thalassemia trait. Achieving these functional endpoints obviously requires different magnitudes of therapeutic effect for different thalassemia patients. In thalassemia intermedia patients with basal total hemoglobin levels between 6–8 g/dL, a 1–2 g/dL increase in hemoglobin levels would be quite adequate to prevent the need for a regular transfusion program and would be highly beneficial, while subjects with thalassemia major and baseline hemoglobin levels below 5 g/dL would require higher levels of fetal globin induction, sufficient to increase hemoglobin levels by 4–5 g/dL above baseline. Investigators in this area are increasingly coming to the realization that this latter type of patient will likely require combination therapy, with more than one agent acting at different molecular levels, to achieve an adequately potent and therapeutic response.
The clinical endpoints for which the efficacy of EPO was established in the treatment of anemia related to renal failure or cancer chemotherapy provide a significant point of reference. Erythropoietin raised total hemoglobin (Hb) by 1–2 g/dL and hematocrit (Hct) by 3%–6% (absolute values), within 6 weeks of treatment in individuals with normal red cell survival. Clinical trials of 5-azacytidine, the short chain fatty acids, sodium phenylbutyrate (SPB) and arginine butyrate, and EPO preparations have already demonstrated responses of similar magnitude in thalassemia patients (review1). Increases in total hemoglobin levels of 1–5 g/dL above baseline have been achieved by these agents when administered for at least 3–6 months’ duration, as shown in Table 1 . This is particularly remarkable since thalassemic cells survive for only a few days or even hours, compared to normal red cell survival of 120 days. Of the chemotherapeutic agents, hydroxyurea (HU) treatment has increased total hemoglobin (Hb) by 0.6–0.8 g/dL in HbE/β thalassemia patients, which was statistically significant.6,8 Hajjar and Pearson reported that fetal globin increased rapidly with HU treatment, but a 6-week time-frame was required for a peak hemoglobin response, and then was followed by a decline in total Hb, suggesting cellular growth inhibition.6 5-azacytidine administration has increased total Hb levels by an average of 2.5 g/dL (range 1–4 g/dL), even in end-stage patients with life-threateningly severe anemia.3–5 Of the SCFADs and HDAC-inhibitors, SPB increased hemoglobin levels by 2.1 g/dL in 50% of un-transfused subjects.11 Arginine butyrate, administered first intensively and then intermittently, has increased total Hb levels by 1–5 g/dL (mean 2.6 g/dL) when administered for at least 3 months.12,24,30 Responses to arginine butyrate have rendered patients transfusion-independent for several years with home therapy, given on an intermittent dose schedule, but was difficult to titrate and required prolonged evaluation to develop a schedule to optimize fetal globin induction while avoiding the anti-proliferative effects common to HDAC inhibitors. Isobutyramide administered briefly for 4 weeks to thalassemia intermedia patients has increased HbF and has decreased transfusion requirements in thalassemia major.14,31 EPO preparations, in several doses and regimens, have increased Hb levels by 1–3 g/dL above baseline in thalassemia intermedia patients, with highest responses in children, and have decreased transfusion requirements in thalassemia major.1,15–20 Thus, multiple trials have already shown proof-of-principle of the utility of therapeutic induction of fetal globin in the β thalassemias. These studies are particularly promising as both chemotherapeutic agents and HDAC inhibitors suppress cell growth, and this adverse activity would be particularly counter-productive in the setting of thalassemia.
Combinations of agents with complementary actions have produced higher responses than either agent alone: in β+ thalassemia patients with relatively low HbF (< 30%) and low EPO levels (< 140 mU/mL), 3 of 4 subjects responded to butyrate + EPO significantly more than to either agent alone, and in Hb Lepore, where 2 subjects responded additively to HU + sodium phenylbutyrate.21 Striking synergistic effects have been found in animal models, where combined butyrate and 5-azacytidine produced a 3-fold increase in fetal globin expression above the significant levels induced by each agent alone.32 These findings strongly suggest that combinations of agents with complementary, yet distinct, molecular mechanisms of action in inducing fetal globin expression should produce significantly higher levels of HbF than single agents. However, each therapeutic candidate must first be investigated and approved alone in β thalassemia.
Novel Fetal Globin-inducing Candidate Agents: Compounds with a Combination of Therapeutic Actions
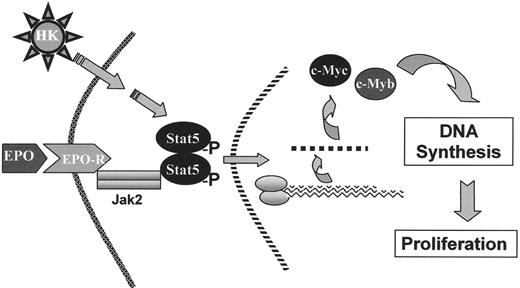
Recent studies demonstrate that select novel SCFADs and select hydroxamic acids offer strong potential for inducing fetal globin gene expression, stimulating erythroid proliferation,13,33–36 and prolonging erythroblast survival.13,33–35 Furthermore, some of these agents are active at oral doses that should be significantly more tolerable than those required for SPB or arginine butyrate.13,35 These agents activate the Aγ globin gene promoter in reporter assays, in cells cultured from β thalassemia subjects, and do not inhibit erythroid cell growth, as the HDAC inhibitors do.13,33–35 The SCFADs sodium 2,2-dimethylbutyrate and α-methylhydrocinnamate stimulate erythroid colony formation beyond optimal hematopoietic growth factors alone, and signal through phosphorylation of STAT-5, the same downstream signaling pathway as EPO and IL-3, as shown in Figure 2 ; such compounds have been designated Hemokines.33 Treatment with sodium 2,2-dimethylbutyrate or α-methylhydrocinnamate has increased fetal globin, total Hgb, and hematocrits in anemic baboons undergoing daily phlebotomy, and reticulocytes and hematocrits in transgenic and normal mice.33,35 Butyric- and propionic-hydroxamic acids have also increased red cell production in mice.36 These third-generation SCFADs should provide two therapeutic effects in one tolerable agent.
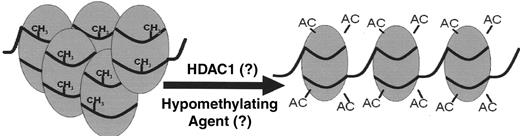
One molecular question to resolve is whether inhibition of HDAC is necessary for efficient upregulation of γ globin gene promoter activity. HDAC-inhibitory activity is associated with strong induction of the cellular growth suppressors p21 and p27, actions that are counter-productive in β thalassemia (review 37,38). Interestingly, HDAC-inhibitory activity is not present with most of the erythropoietic SCFADs discussed above, and the potency of HDAC inhibitory activity does not correlate directly with HbF inducibility.39 A complementary question to resolve is whether histone acetylation and hypomethylation are required at upstream regulatory sequences for high-level γ globin gene induction, in view of the recent report that the Aγ globin gene promoter is hypomethylated in adult marrow to a degree similar to fetal liver cells.40 Most experimental reporter gene assays by which compounds are screened have utilized the Aγ globin gene promoter. It is possible that higher expression may be achieved with induction of the Gγ-globin gene promoter in addition, perhaps using the hypomethylating agent 5-azacytidine, which has produced high-level responses in patients and synergistic responses in combination with butyrate in animal models. Decitabine, a metabolic product of 5-azacytidine, is another hypomethylating agent that is easier to administer, although still carcinogenic.41 It will be interesting to determine if these agents induce hypomethylation of the Gγ-globin gene promoter as a mechanism for additive or synergistic HbF responses.
Another potential method for inducing fetal globin expression was suggested by studies utilizing combinations of the growth factors EPO and stem cell factor (SCF), to stimulate proliferation, and transforming growth factor (TGF)-β, to induce premature differentiation (an action that hydroxyurea could theoretically provide).42 Such combinations could be considered in the more severe thalassemia phenotypes, although pricing issues may be problematic for long-term use of recombinant growth factors.
Supportive measures may affect the activity or clinical efficacy of fetal globin inducers. First, folic acid should be given and iron supplementation used in subjects with ferritin levels < 1000 ng/mL. Endogenous iron stores appear to not be readily mobilizable to support new erythropoiesis, as reflected by the loss of drug effect when ferritin levels decline by 50% on butyrate treatment. Second, patients do not respond to fetal globin inducers for 3–4 months following blood transfusions. Apparently, these agents cannot act efficiently when the erythroid marrow is suppressed by transfusion, and a period of anemia may be necessary to restore drug susceptibility. Third, removal of imperfect thalassemic red cells by the spleen can confound responses, as only splenectomized patients responded to EPO in some trials.1,15 Fluxes in hemoglobin levels are broader in thalassemia subjects than in normal subjects with normal red blood cell survival, and should be anticipated. Endogenous EPO levels typically decline when total Hb levels increase by 1–2 g/dL above baseline with butyrate therapy. Additional iron supplements or changes in dosing regimens can restore hematologic responses.
Obstacles of New Therapeutics
Proof-of-principle of fetal globin reactivation has already been demonstrated for several experimental fetal globin-inducing agents in β thalassemia: EPO preparations, SPB, arginine butyrate, and 5-azacytidine have increased total hemoglobin levels by ≥ 2 g/dL above baseline. Hydroxy-urea has produced lesser, but statistically significant, increases in hemoglobin levels in HbE/β thalassemia, and thalassemia intermedia. Yet, there are several factors contributing to the lack of development of any of these agents for FDA approval, or drug registration in other countries, for β thalassemia.
Therapeutics for fetal globin induction as long-term (life-long) treatment ideally should have the following characteristics: 1) be orally active at tolerable doses; 2) not inhibit erythroid cell proliferation; and 3) not be mutagenic. Therapeutic candidates must be evaluated and developed individually for approval in β thalassemia. Major obstacles to development of HbF-inducing agents for thalassemia include: 1) costs of new drug development, including the challenges of pharmaceutical companies weighing the potential market and academic investigators securing financing for drug development; and 2) perceptions regarding the ultimate efficacy (and competitiveness) of this therapeutic approach compared to other approaches. For example, many thalassemia patients are managed with blood transfusions and iron chelators, and the oral chelators are demonstrating therapeutic effects. Therefore, only a fraction of the thalassemia population might initially utilize a HbF-inducing type of therapeutic, even though such drugs would improve the underlying condition.
Factors mitigating these obstacles to development of HbF-inducing agents include: 1) orphan drug development requires smaller pivotal clinical trials than drug candidates for larger populations; 2) chronic blood transfusions have significant risks that chelation cannot abrogate; and, 3) thalassemia patients will require long-term treatment. Although more than one therapeutic will likely be necessary for severely affected patients, subsets of patients with β thalassemia have already been identified who respond well to individual agents (and can be identified by basal HbF and EPO levels), and clinical trials can be focused on these patients (Table 2 ).
Certain barriers to development are agent-specific. For example, both standard and long-acting preparations of EPO can be given to β thalassemia patients on a weekly basis, but differences in pricing for acute and chronic therapies may be an issue to pharmaceutical companies. For 5-azacytidine and decitabine, parenteral administration and their mutagenicity are barriers to long-term use. These considerations would not apply, however, for subjects with end-stage disease, allo-antibodies precluding transfusion, and no other therapeutic strategies as options. The first generation SCFAs, arginine butyrate and SPB, are rapidly metabolized and thus require IV infusion or large numbers of oral tablets, respectively. However, new SCFADs persist for several hours and will require markedly lower doses, similar to hydroxyurea. An additional advantage is that these agents have two therapeutic actions, both in experimental models and in non-human primates.
Outlook for Development of New Therapeutics
A number of steps could be undertaken to advance the rate of clinical progress and drug development for β thalassemia in academia. It would be useful to establish guidelines or consensus on those issues that frequently delay the funding of grant applications for prolonged periods. First, a consensus on suitable study endpoints would set uniform and reasonable goals and allow comparison of different agents. For example, an endpoint of total Hb responses of 1 g/dL over baseline (the target endpoint on which EPO was approved) represents a level of increase which would benefit many patients, although higher responses and combinations of agents would be required in more severe subjects. Second, the β thalassemia syndromes represent clinically and molecularly diverse disorders, albeit frequently fatal ones. Accordingly, what response rate in the population must be achieved for a therapeutic approach to be recognized as successful? In the treatment of cancer, a comparably lethal family of diseases, therapeutic efficacy is often recognized at response rates below 20%, and most major drugs in other conditions are effective in 25%–60% of patients due differences in drug metabolism.43 Therefore, in the highly diverse thalassemia syndromes, responses to any one agent should not be expected to be higher than 40%–50%. Moreover, each type of therapeutic may provide incremental efficacy alone, but more significant effects in combination, as occurs in cancer therapy. Third, it should be recognized that a sufficient duration of time is required on a trial for hematologic responses to occur following induction of fetal globin; thus, short-term studies may be prematurely pessimistic. Fourth, decline in total Hb levels over time should be anticipated, such as when endogenous EPO levels decline as total Hb rises and when iron stores are utilized. These physiologic “secondary” responses should not be misinterpreted as loss of drug effect. Fifth, chemotherapy and HDAC inhibitors suppress erythropoiesis, perhaps cumulatively. Changes in dosing regimen and replacement of iron may restore responses. Sixth, for inclusion in clinical trials to evaluate a new drug, patients should not have received transfusions within 3–4 months and ideally should be splenectomized. Seventh, the opportunity to conduct combination studies, where the impact on the disease is likely to be high, will not be possible until novel drug candidates are individually approved. Therefore, evaluating agents that are already FDA-approved for other uses, with safety profiles established, can be undertaken more rapidly (e.g., an EPO product and a hypo-methylating agent).
Development of an entirely new chemical entity into a medicinal preparation that can be tested in clinical trials is a costly undertaking. Several candidates identified in academic laboratories could become the mainstay of treatment, including erythropoietic SCFADs that are orally active at low concentrations, and possibly butyric and propionic hydroxamic acids, which are HDAC inhibitors. Studies required for an Investigational New Drug Application to initiate clinical trials include:
Synthesis of an ultra-pure compound, with identification of impurities, and validation of all methods of detection
Mutagenicity testing, by three methods
Formulation of the compound into a medicinal preparation
Stability testing to determine shelf life and breakdown products
Formal toxicology testing with dose-ranging and 28-day dosing in 2 species
Cardiac toxicity screening, metabolism and distribution, and other system-specific safety pharmacology tests
Such studies are typically performed at contract laboratories, at a usual cost of > $1.5–$2 M, and such expenditures are not funded by basic research grants. Obtaining financing for these FDA-required studies has been an almost impossible obstacle to translation of promising bench research into clinical trials. New NIH programs designed to facilitate translational research may provide mechanisms to accomplish some of these tasks.
Summary
The β thalassemia syndromes are among the few molecularly defined conditions in which an endogenous fetal gene can functionally replace a defective adult gene. Several candidate therapeutics have demonstrated proof-of-principle in these disorders by inducing fetal globin expression and subsequently raising hemoglobin levels in β thalassemia patients. The magnitude of responses to individual agents is similar to those induced by rhu-EPO in anemic conditions with normal red cell survival, and additive responses occur with drug combinations. New oral candidate therapeutic agents, which both induce fetal globin gene expression and stimulate erythropoiesis, and combinations of therapeutic agents with complementary molecular actions, now make this gene-reactivation approach feasible for many patients. Development of these agents is now hindered largely by costs.
Hematologic responses to fetal globin gene inducers or erythropoietin (EPO) in β-thalassemia patients.
| Therapeutic Agent . | Action . | Thalassemia Syndromes . | Increase in Total Hb (g/dL) [range] . | Responses . | Authors . |
|---|---|---|---|---|---|
| Abbreviations: SCFAD, short chain fatty acid derivative, HDACi, histone deacetylase inhibitor; AC, acetyl group; R, repressor; SSP, stage selector protein(s); BFU-E, burst forming units erythroid | |||||
| 5-Azacytidine | Hypomethylation +/− cytotoxicity | Thalassemia intermedia | 2.5 [1.5–4] | 1/1 | Ley 19823 |
| 1/1 | Dunbar 19894 | ||||
| 3/3 | Lowrey 19935 | ||||
| Hydroxyurea (HU) | Cytotoxicity and erythroid regeneration →high-F BFU-E | HbE/β-thalassemia | 2.0 [1–3.3] | 3/3 | Hajjar 19946 |
| 2.7 [2.2–3.2] | 2/2 (β globin) | Zeng 19957 | |||
| 0.6 [0–1.7] | 11/13 | Fucharoen 19968 | |||
| Thalassemia intermedia | 1.0 | 3/8 | Loukopoulos 19989 | ||
| HbE/β-thalassemia | 0.9 | 7/19 | Singer 200210 | ||
| Sodium phenylbutyrate (SPB) | ? HDACi effect | Thalassemia intermedia and major | 2.1 [1.2–2.8] | 4/8 untransfused | Collins 199511 |
| Arginine butyrate (AB) and AB + Erythropoietin (EPO) | Activates γ globin gene promoter, (SSP binding), (? HDACi effect) | Thalassemia intermedia and major | 2.7 [0.6–5] | 7/10 (AB +/− EPO) | Perrine 199312 Perrine 200313 |
| Isobutyramide | Activates γ globin gene promoter | Thalassemia intermedia | Increase in HbF, Reduced transfusion requirements | 6/10 | Cappellini, 20002 |
| Thalassemia major | 2/4 | Reich 200014 | |||
| EPO | Stimulates erythroid proliferation, promotes erythroid cell survival | Thalassemia intermedia | 2 [1–3] | 5–7/10 | Rachmilewitz 199815 |
| Thalassemia major | 2.5 | 4/4 | Bourantas 199716 | ||
| 2 | 8/10 | Nisli 199617 | |||
| Reduced transfusion requirements | 3/26 | Nisli 199718 | |||
| 5/10 | Chaidos 200419 | ||||
| Darbepoietin | Stimulates erythropoiesis | Thalassemia intermedia | 1.6 [0.7–3.8] | 4/6 | Singer 200320 |
| HU + EPO | As above | Thalassemia intermedia | 1.7 [0.5–4] | Loukopoulos 19989 | |
| HbE/β-thalassemia | 1.2 | 1/5 | Singer 200210 | ||
| HU + SPB | As above | β Lepore | 3 [2–4] | 2/2 | Olivieri 199721 |
| Therapeutic Agent . | Action . | Thalassemia Syndromes . | Increase in Total Hb (g/dL) [range] . | Responses . | Authors . |
|---|---|---|---|---|---|
| Abbreviations: SCFAD, short chain fatty acid derivative, HDACi, histone deacetylase inhibitor; AC, acetyl group; R, repressor; SSP, stage selector protein(s); BFU-E, burst forming units erythroid | |||||
| 5-Azacytidine | Hypomethylation +/− cytotoxicity | Thalassemia intermedia | 2.5 [1.5–4] | 1/1 | Ley 19823 |
| 1/1 | Dunbar 19894 | ||||
| 3/3 | Lowrey 19935 | ||||
| Hydroxyurea (HU) | Cytotoxicity and erythroid regeneration →high-F BFU-E | HbE/β-thalassemia | 2.0 [1–3.3] | 3/3 | Hajjar 19946 |
| 2.7 [2.2–3.2] | 2/2 (β globin) | Zeng 19957 | |||
| 0.6 [0–1.7] | 11/13 | Fucharoen 19968 | |||
| Thalassemia intermedia | 1.0 | 3/8 | Loukopoulos 19989 | ||
| HbE/β-thalassemia | 0.9 | 7/19 | Singer 200210 | ||
| Sodium phenylbutyrate (SPB) | ? HDACi effect | Thalassemia intermedia and major | 2.1 [1.2–2.8] | 4/8 untransfused | Collins 199511 |
| Arginine butyrate (AB) and AB + Erythropoietin (EPO) | Activates γ globin gene promoter, (SSP binding), (? HDACi effect) | Thalassemia intermedia and major | 2.7 [0.6–5] | 7/10 (AB +/− EPO) | Perrine 199312 Perrine 200313 |
| Isobutyramide | Activates γ globin gene promoter | Thalassemia intermedia | Increase in HbF, Reduced transfusion requirements | 6/10 | Cappellini, 20002 |
| Thalassemia major | 2/4 | Reich 200014 | |||
| EPO | Stimulates erythroid proliferation, promotes erythroid cell survival | Thalassemia intermedia | 2 [1–3] | 5–7/10 | Rachmilewitz 199815 |
| Thalassemia major | 2.5 | 4/4 | Bourantas 199716 | ||
| 2 | 8/10 | Nisli 199617 | |||
| Reduced transfusion requirements | 3/26 | Nisli 199718 | |||
| 5/10 | Chaidos 200419 | ||||
| Darbepoietin | Stimulates erythropoiesis | Thalassemia intermedia | 1.6 [0.7–3.8] | 4/6 | Singer 200320 |
| HU + EPO | As above | Thalassemia intermedia | 1.7 [0.5–4] | Loukopoulos 19989 | |
| HbE/β-thalassemia | 1.2 | 1/5 | Singer 200210 | ||
| HU + SPB | As above | β Lepore | 3 [2–4] | 2/2 | Olivieri 199721 |
Potential therapeutics forγ-globin gene induction in the β-thalassemia syndromes.
| Patient Characteristics . | Therapeutic Classes . |
|---|---|
| Abbreviations: EPO, erythropoietin; HDACi, histone deacetylase inhibitor | |
| Thalassemia intermedia Low HbF (< 30%) and Low EPO (< 100 mU/mL) Total Hb > 5 g/dL | • EPO • Fetal globin gene activator |
| Thalassemia intermedia/major High HbF (> 60%) and High EPO (> 160 mU/mL) Total Hb > 4–5 g/dL | • Fetal globin gene activator or • Hydroxyurea |
| Thalassemia major Total Hb > 4–5 g/dL | • EPO • Fetal globin gene activator • Hypomethylating agent/HDACi (?) |
| Patient Characteristics . | Therapeutic Classes . |
|---|---|
| Abbreviations: EPO, erythropoietin; HDACi, histone deacetylase inhibitor | |
| Thalassemia intermedia Low HbF (< 30%) and Low EPO (< 100 mU/mL) Total Hb > 5 g/dL | • EPO • Fetal globin gene activator |
| Thalassemia intermedia/major High HbF (> 60%) and High EPO (> 160 mU/mL) Total Hb > 4–5 g/dL | • Fetal globin gene activator or • Hydroxyurea |
| Thalassemia major Total Hb > 4–5 g/dL | • EPO • Fetal globin gene activator • Hypomethylating agent/HDACi (?) |
Therapeutic actions for γ-globin gene induction in the β-thalassemias
Abbreviations: EPO, erythropoietin: SCFAD, short chain fatty acid derivative, HDACi, histone deacetylase inhibitor; AC, acetyl group; R, repressor; SSP, stage selector protein(s)
Therapeutic actions for γ-globin gene induction in the β-thalassemias
Abbreviations: EPO, erythropoietin: SCFAD, short chain fatty acid derivative, HDACi, histone deacetylase inhibitor; AC, acetyl group; R, repressor; SSP, stage selector protein(s)
Hemokine (HK) signaling.
A few short chain fatty acid derivatives, referred to as “Hemokines” (HK), stimulate erythroid proliferation and prolong erythroid cell survival through the same signaling pathways utilized by erythropoietin (EPO) and interleukin-3 .
Hemokine (HK) signaling.
A few short chain fatty acid derivatives, referred to as “Hemokines” (HK), stimulate erythroid proliferation and prolong erythroid cell survival through the same signaling pathways utilized by erythropoietin (EPO) and interleukin-3 .