Abstract
A qualitative abnormality of platelet function should be considered in patients with mucocutaneous bleeding in the absence of thrombocytopenia or von Willebrand disease. Antiplatelet drugs are the most common cause of acquired platelet disorders leading to bleeding. Uremia, hepatic cirrhosis, myeloma and related disorders, polycythemia vera, essential thrombocythemia, and cardiopulmonary bypass have long been recognized as clinical situations in which platelet dysfunction may contribute to bleeding. When an acquired platelet disorder is suspected, it is useful to examine platelet function by measuring the bleeding time, examining platelet-dependent closure time in a platelet function analyzer and performing platelet aggregometry. When a specific acquired platelet disorder is diagnosed, many treatment options are available including controlling the underlying disease, giving platelet transfusions and administering a hemostatic drug.
A qualitative abnormality of platelet function can be a cause of mucocutaneous bleeding in persons with normal platelet counts. The absence of a family history—defined as no bleeding among first degree relatives—should prompt investigation for an acquired disorder. In contrast to congenital platelet disorders, which are rare, acquired disorders of platelet function are encountered commonly in hematology practice. A variety of systemic diseases, medications and procedures can be implicated (Table 1 ). The clinical significance of an acquired platelet disorder is defined primarily by the patient encounter. The impact of a platelet disorder on major bleeding may be difficult to extricate from the effect of multiple antithrombotic drugs or multi-organ failure, particularly in the intensive or coronary care unit setting.
Bedside Examination
The major shortcoming of the bedside exam is the unreliability of the history for superficial mucocutaneous bleeding. At least one fourth of persons who complain of serious bleeding do not have a bleeding disorder and at least one third of persons who have no complaints of bleeding actually have von Willebrand disease or a platelet disorder.1 It is therefore useful to be precise and exhaustive when asking about or examining for bleeding, and to try to confirm all symptoms with a physical sign or by repetitive interviews.
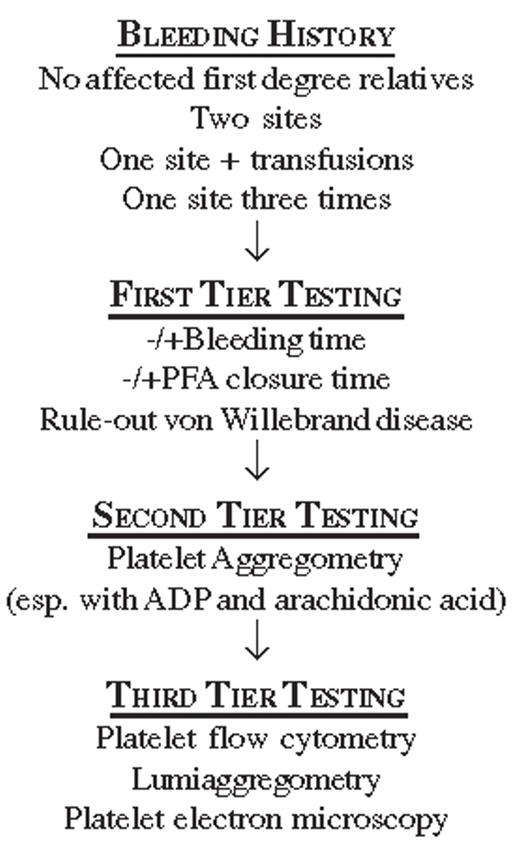
Of several criteria for a positive bleeding history,2,3 The International Society on Thrombosis and Hemostasis suggests that bleeding should be considered clinically significant when there are two or more distinct bleeding sites such as the skin, nose, gums, vagina, gastrointestinal tract, or genitourinary tract; this includes spontaneous bleeding as well as provoked bleeding, such as occurs with dental work, parturition, trauma or surgery. A bleeding history is also considered significant when there is only a single bleeding site so severe that it leads to red cell transfusions. Finally, significant bleeding is indicated by a single symptom recurring on three separate occasions.2
Laboratory Evaluation of Platelet Function
A reliably predictive “screening” test for platelet dysfunction does not exist. Neither the bleeding time (BT) nor the platelet function analyzer (PFA) is good for screening asymptomatic persons. The BT is notoriously operator-dependent and affected by a subject’s age and skin laxity, and both it and the PFA closure time are prolonged in patients with low hematocrits and normal platelet function. They can, however, be useful for narrowing diagnostic considerations among patients with a history of superficial bleeding.
The BT and PFA are not good for screening because each has limited sensitivity: when the BT and PFA were used to examine platelet function in 148 patients with known platelet disorders, their individual sensitivities were only 36% and 30%, respectively, and their combined sensitivity was 48%.4 Subset analysis suggested that the BT is better at identifying platelet storage pool deficiency and the PFA better for diagnosing type I von Willebrand disease.4 Nonetheless, a normal BT or PFA closure time does not rule out a clinically significant acquired platelet abnormality. The specificity of an abnormal BT or PFA was addressed in a study of 61 normal persons.5 In this cohort, the PFA false positive rate was observed to be nearly 16%. Among persons ultimately found to have an acquired platelet disorder, the specificity of the combined PFA (a prolongation of both epinephrine and adenosine diphosphate [ADP] closure times) was 79% and the specificity of the BT was 91%.5
An approach for diagnostic testing to evaluate a clinically significant bleeding history without any identifiable underlying condition is shown in Figure 1 . The BT and PFA closure time may be measured, although subsequent testing need not be dependent on a prolonged BT or PFA closure time. One will, however, use a panel of tests (typically the VWF:Ag and VWF:RCo assays) in order to rule out von Willebrand disease (VWD).4 After VWD is ruled out, platelet aggregation testing by light transmission should be performed. Aggregation studies can confirm the effects of aspirin, thienopyridines, β-lactam antibiotics, and paraproteins on platelet function (see below). When aggregometry fails to reveal a functional platelet disorder, more subtle functional defects may be detected using flow cytometry to measure αIIbβ3 activation or α granule secretion via P-selectin (CD62P) expression.6 Lumiaggregometry, which simultaneously measures aggregation and luciferase luminescence, provides an assay for secretion by determining the release of dense granule adenosine triphosphate (ATP). Electron microscopy will reveal structural platelet abnormalities, including a decreased number or abnormal morphology of platelet α granules and dense granules.
Medications
A large number of drugs and foods affect platelet function in vitro.7 However, only a small number predictably cause clinical bleeding and they are readily identifiable because they are anti-platelet drugs commonly used to prevent or treat arterial thrombosis.8 Aspirin is the prototype of a medication that causes clinically significant bleeding. The anti-platelet effect of aspirin is mediated via irreversible acetylation of platelet cyclooxygenase 1 (COX-1) and the resulting inhibition of thromboxane A2 (TXA2) synthesis. Single aspirin doses of 325 mg or more, or smaller doses (i.e., 81 mg) taken for several days, produce essentially complete inhibition of TXA2 production.9,10 The hemostatic risks associated with aspirin are predictable: ~5%–10% minor bleeding and ~1%–2% major bleeding (defined as bleeding requiring hospitalization or red cell transfusion). In the US Physicians Health study of 22,000 male physicians receiving 325 mg of aspirin (or placebo) every other day, hemorrhagic stroke was observed in 0.2% of the aspirin group versus 0.1% of the placebo group (not significant), but gastrointestinal bleeding requiring transfusions was significantly increased by aspirin (0.5% vs 0.3% in the placebo group). Gastrointestinal bleeding is dose-dependent, but the antithrombotic and anti-hemostatic effects of aspirin remain constant at all doses above 75 mg, indicating a direct adverse effect of aspirin on COX-1 mediated gastric mucosal cytoprotection.
Non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) reversibly inhibit platelet COX-1, and normal platelet function is usually restored rapidly after most types of NSAIDs are stopped. For example, the PFA closure times return to baseline 24 hours after completion of a 7-day course of ibuprofen (600 mg tid).11 Clinical bleeding in normal individuals due to the effect of an NSAID on hemostasis is uncommon, while gastrointestinal bleeding due to gastric ulceration is not uncommon. Moreover, there appears to be a paradoxical prothrombotic effect of ibuprofen when it is ingested within 2 hours of taking aspirin because it transiently blocks aspirin’s target acetylation site.
COX-2 is a COX-1 homologue that is rapidly synthesized in cells such as endothelial cells, fibroblasts, and monocytes in response to growth factors, cytokines, endotoxin, and hormones. Inhibitors of COX-2 were developed to avoid the gastrointestinal side effects of COX-1 inhibitors. These medications have no effect on platelets, which don’t express COX-2. Initial short-term studies investigating analgesic effects revealed no adverse cardiovascular events. These studies largely excluded patients at high risk for cardiovascular events. Recent studies using these medications in trials of colorectal adenoma prevention and after cardiac surgery have demonstrated an increased risk for cardiovascular complications.12,13 The mechanism appears to be that COX-2-mediated production of prostacyclin is inhibited while platelet production of TXA2 is unaffected. Because prostacyclin inhibits platelet function and is vasodilatory, its decrease tips the prothrombotic-antithrombotic balance in favor of thrombosis. Future investigations will determine if nonselective NSAIDs also unfavorably influence the natural history of arterial athero-thrombosis.14
The thienopyridines clopidogrel and ticlopidine are indicated for patients with vascular disease. However, clopidogrel has virtually supplanted ticlopidine because of the latter’s association with potentially life-threatening hematological side effects (thrombotic thrombocytopenic purpura, agranulocytosis, and aplastic anemia). Both medications bind irreversibly to the platelet purinergic receptor P2Y12, thereby inhibiting platelet responses induced not only by exogenous ADP, but also by ADP released from platelet dense granules.15 Steady-state inhibition of platelet function occurs after 3–5 daily doses of 75 mg clopidogrel, but it can be achieved sooner when a 300 mg loading dose is given. The effect of clopidogrel on the bleeding time is greater than that of aspirin.10 Nonetheless, there is little difference in the risk of bleeding. Combinations of clopidogrel and aspirin are being used with increasing frequency to prevent or treat arterial thrombosis16 and would be predicted to have increased hemostatic toxicity because the combination synergistically prolongs the bleeding time to almost 5 times baseline levels. After cessation of clopidogrel, platelet function is ~50% of normal at 3 days and back to normal after 7 days.17
Integrin αIIbβ3 (glycoprotein IIb/IIIa) antagonists are potent inhibitors of platelet function. Three antagonists are currently approved for use in the United States. Abciximab is a chimeric human-mouse αIIbβ3-specific Fab immunoglobulin fragment, tirofiban is an Arg-Gly-Asp-based peptidomimetic analog of tyrosine, and eptifibatide is a synthetic cyclic heptapeptide based on the Lys-Gly-Asp motif of the snake venom disintegrin barbourin. Although unique structurally, these antagonists inhibit platelet aggregation by preventing fibrinogen and von Wille-brand factor (VWF) binding to αIIbβ3. They are used in patients undergoing a percutaneous coronary intervention (PCI) concurrently with heparin and other anti-platelet therapy such as aspirin. Bleeding occurs in ~10% of patients receiving an αIIbβ3 antagonist, but intracranial bleeding and/or death due to bleeding are rare (< 0.5% and < 0.1%, respectively). There are substantial differences between the duration of the anti-platelet effects of abciximab and the two small molecule antagonists because of the slower dissociation rate of abciximab. Although unbound abciximab is cleared rapidly, elimination of the αIIbβ3-bound drug is significantly slower and some residual receptor blockade (~25%) can be detected for up to 7 days. The anti-platelet effect of the small molecules is directly proportional to their elimination half-life and typically subsides within hours of stopping the infusion.18
Clinical bleeding and abnormal platelet function occur in patients given large doses of various β-lactam antibiotics (penicillins > cephalosporins), attributable in large part to impairment of the interaction between agonist-receptor pairs.19 The clinical effect is most severe in hypo-albuminemic patients because higher levels of unbound drug interact with the platelet surface, and impaired hemostasis may be long-lasting due to a residual impairment of receptor function even after the antibiotic is discontinued. β-lactam compounds only contribute to clinical bleeding when there is a co-existing hemostatic defect, such as uremia, thrombocytopenia or vitamin K deficiency.
A number of commonly used prescription drugs that affect platelet function in vitro have little or no effects on hemostasis in vivo. The theoretical anti-platelet effects of statins,20 cilostazol,10 sildenafil,21 fluoxetine,22 and epoprostenol21 have so far not been matched by significant bleeding side effects, even with post-marketing surveillance.20 Nonprescription drugs, commonly used herbal medications and spices also affect platelet function in vitro, but there is only anecdotal evidence of clinical bleeding.7,23 Like ethanol, their predominant clinical effect is to potentiate a hemostatic defect caused by another drug or some other factor.
Acquired von Willebrand Syndrome
Acquired von Willebrand syndrome (AVWS), usually due to an acquired decrease in large VWF multimers, has been reported to cause bleeding associated with a variety of systemic diseases, most notably clonal hematological and autoimmune disorders. It has been observed in patients with myeloproliferative disorders, especially polycythemia vera and essential thrombocythemia, where substantial increases in platelet numbers appear to deplete VWF, producing a clinicopathological picture similar to type II and platelet-type von Willebrand disease. Accordingly, desmopressin (Table 2 ) or VWF concentrates may not correct the hemostatic defect, and platelet cytoreduction may be required to control bleeding. Aortic stenosis (AS) has also been associated with AVWS, likely as a result of ADAMTS-13-mediated proteolysis of large VWF multimers undergoing structural changes induced by shear stresses across the narrowed valve. In one series of 50 patients with AS, 21.4% with severe disease experienced at least one episode of mucocutaneous bleeding, and PFA closure times were prolonged in 92% and 50% of those with severe and moderate AS, respectively. The percentage of circulating large VWF multimers was negatively correlated with AS severity. Depletion of large VWF multimers was associated with significant intraoperative blood loss. Correction of AS with valve replacement normalized the assays and led to resolution of clinical bleeding.24
Systemic Disorders Associated with Abnormal Platelet Function
The most common systemic disorder associated with clinically important platelet dysfunction is uremia. In uremia, circulating substances impair platelet interactions with the vessel wall; the most important of these may be nitric oxide (NO), which diffuses into platelets, activates soluble guanylate cyclase and inhibits platelet adhesion, activation and aggregation.25 NO is present at higher levels in uremia for several reasons, one of which is accumulation of the NO donor guanidinosuccinic acid (GSA) secondary to inhibition of the urea cycle. Dialysis, which removes many potentially platelet-toxic substances including GSA, decreases the bleeding time and improves clinical bleeding. Another factor contributing to uremic bleeding is anemia, which attenuates platelet/vessel wall interactions because fewer red cells occupy the central stream of flowing blood, thereby leading to fewer platelets being displaced toward the vessel wall and available to adhere to sites of endothelial injury.
Although patients with cirrhosis may demonstrate abnormal platelet function, serious bleeding is usually caused by thrombocytopenia, coagulopathy, and vascular abnormalities. Cirrhotic patients with severe variceal bleeding may benefit from treatment with recombinant activated factor VII.26
The paraproteins associated with plasma cell dyscrasias can sometimes cause platelet dysfunction when they adsorb to the platelet surface, thereby impairing platelet-platelet interactions. Paraproteins have also been associated with the AVWS. When bleeding in a patient with a plasma cell dyscrasia is associated with impaired platelet responses in vitro, plasmapheresis may be helpful.
Myeloproliferative disorders can be associated with a number of in vitro platelet function abnormalities, as well as with the AVWS. Nonetheless, the morbidity and mortality of these disorders is more frequently due to thrombotic complications, and recent evidence suggests that aspirin may prevent clinically significant thromboses without increasing bleeding risk.27
Although there are a number of potential causes for bleeding during and after cardiopulmonary bypass (e.g., the use of heparin, complement activation and contact-induced fibrinolysis), platelet dysfunction is an important factor. Platelets are activated and degranulate in the extra-corporeal circuit, impairing their ability to function in vivo. In addition to degranulation caused by the artificial membranes, platelet function is compromised further during prolonged bypass by the use of large doses of heparin and by lengthy hypothermia.28
Platelet Storage
Because cold storage impairs platelet recovery following transfusion, platelets for transfusion are stored at room temperature. This causes platelet dysfunction, increases the risk of bacterial overgrowth and limits the shelf life of banked platelets to 5 days.29,30 Recent evidence suggests that the platelet cold storage lesion results from GpIb-IX-V clustering. Transfused platelets with clustered GpIb-IX-V are recognized by αMβ2 (Mac-1) on hepatic and splenic macrophages, which results in their removal from the circulation by phagocytosis.31 Addition of UDP-galactose to cold-stored platelets induces galactosylation of GpIbα, which in non-human models impairs macrophage recognition and increases platelet survival.32
Treatment
Effective peritoneal- or hemo-dialysis improves platelet function in most patients with renal failure. BT shortening, and perhaps improved clinical hemostasis, can also be achieved by correcting anemia with red cell transfusions or erythropoietin injections. The target hemoglobin concentration is not clearly established but should not exceed 12 g/dL, as higher hemoglobin concentrations have been associated with increased mortality in patients with co-existent coronary artery disease.25
Although the transfusion of normal platelets may be required to treat clinical bleeding due to an acquired functional platelet disorder, a number of other therapeutic agents are available that are not associated with the risks of transfusion (Table 2 ). In particular, desmospressin (DDAVP), a vasopressin analog whose pressor effects are substantially less than its antidiuretic effects, is useful by causing the release of VWF from tissue stores, predominantly endothelial cells. DDAVP has been reported to shorten the bleeding time in 50%–75% of patients with uremia. Recombinant factor VIIa (rFVIIa) has also been used effectively in this setting, although this is an off-label use.33 In fact, rFVIIa appears to have a wide spectrum of clinical uses in a variety of different hemorrhagic disorders due to both platelet dysfunction and coagulopathy.33
Special Issues Related to Surgery
Hematologists may be asked to assist in the management of patients receiving anti-platelet medication in the peri-operative setting. Although the effect of aspirin on platelet function persists for the life of the platelet, studies in which patients have taken aspirin up to the time of surgery have shown only a slight increase in bleeding complications. A retrospective study of 1900 patients who had ingested aspirin within 12 hours of coronary artery bypass grafting (CABG) versus 706 who had not, showed a 4.1% increase in the need for red cell transfusion, an increase in the need for platelet and cryoprecipitate transfusion from 2.1% to 4.5% and an increase in the re-operation rate from 2.0% to 3.7%.34 Studies of patients receiving clopidogrel within a few days of surgery have similarly demonstrated a significant increase in bleeding complications. A prospective study of 470 cardiac surgery patients, 91 of whom had received clopidogrel within 5 days of operation, found an increased relative risk of death, need for intraaortic balloon pump, arrhythmia, re-intubation and stroke in patients who had received clopidogrel, although the number of events was small. Six of the 7 deaths in patients taking clopidogrel occurred in patients who received it within 48 hours of surgery.35 Fewer data are available to assess surgical bleeding risks in patients receiving αIIbβ3 inhibitors, but they would be expected to have a much lower impact as their effects are more rapidly reversible upon cessation. Thus, the question of when to discontinue anti-platelet agents preoperatively can be difficult and must be individualized, taking into account the type of surgery and the risk-benefit ratio of perioperative bleeding versus thrombotic events. For CABG, the American College of Cardiology/American Heart Association guidelines recommend cessation of aspirin 7–10 days before and clopidogrel 5 days before any elective procedure.36 For non-cardiac and non-neurologic surgery, monotherapy with aspirin or clopidogrel does not need to be discontinued. When the two are used together, the risks of perioperative bleeding are substantially higher and consideration should be given to stopping the clopidogrel. Patients receiving abciximab should have surgery delayed for at least 12 hours if possible.36
Acquired platelet disorders.
|
|
Hemostatic drugs.
| Drug . | Indication . | Dose . | Onset . | Duration . | Caveat . |
|---|---|---|---|---|---|
| * f/b, “followed by” | |||||
| Desmopressin | Acute bleeding before biopsy or emergency surgery | 0.3 μg/kg (IV, SC) | 30–60 min | 6–12 hours | Tachyphylaxis |
| 300 μg (Intranasal) | 60–90 min | 6–12 hours | |||
| q 12–24 hour x 4–5 doses | |||||
| Conjugated estrogens | Chronic, recurrent bleeding or before elective surgery, esp. from uremia | 0.6 mg/kg IV infusion or 50 mg po q day x 5–7 days | 7 days | 2 weeks | Use for < 7 days |
| Antifibrinolytic agents | Intractactable mennorhagia, oral bleeding or GI bleeding | Aminocaproic acid: 5 g IV over 1 hour *f/b 1 g/h infusion for 8 hours | 30 min | 2–4 hours after discontinuation | Enters the extravascular or GI bleeding compartments; |
| or 5 g po x 1 f/b 1 gm po hourly for 8 hours | 1.5 hours | Renal clearance; contraindicated in DIC and with | |||
| Tranexamic acid (TA): 10–15 mg/kg body weight po or IV q 8 hours | 30–60 min | hematuria; TA not available in US | |||
| Aprotonin | Coronary bypass surgery | Test dose of 1.4 mg f/b 140 or 280 mg load f/b 140 or 280 mg pump prime dose f/b 35 or 70 mg/hr intra-operative infusion | 15 min | Hours after discontinuation | Requires central line; monitor for thrombosis |
| Recombinant activated factor VII | Hemophilia-related inhibitors; other uses are off-label | 90 (50–100) μg/kg IV bolus every 2 hours or as needed | Immediately | 2 hours | Monitor for thrombosis |
| Drug . | Indication . | Dose . | Onset . | Duration . | Caveat . |
|---|---|---|---|---|---|
| * f/b, “followed by” | |||||
| Desmopressin | Acute bleeding before biopsy or emergency surgery | 0.3 μg/kg (IV, SC) | 30–60 min | 6–12 hours | Tachyphylaxis |
| 300 μg (Intranasal) | 60–90 min | 6–12 hours | |||
| q 12–24 hour x 4–5 doses | |||||
| Conjugated estrogens | Chronic, recurrent bleeding or before elective surgery, esp. from uremia | 0.6 mg/kg IV infusion or 50 mg po q day x 5–7 days | 7 days | 2 weeks | Use for < 7 days |
| Antifibrinolytic agents | Intractactable mennorhagia, oral bleeding or GI bleeding | Aminocaproic acid: 5 g IV over 1 hour *f/b 1 g/h infusion for 8 hours | 30 min | 2–4 hours after discontinuation | Enters the extravascular or GI bleeding compartments; |
| or 5 g po x 1 f/b 1 gm po hourly for 8 hours | 1.5 hours | Renal clearance; contraindicated in DIC and with | |||
| Tranexamic acid (TA): 10–15 mg/kg body weight po or IV q 8 hours | 30–60 min | hematuria; TA not available in US | |||
| Aprotonin | Coronary bypass surgery | Test dose of 1.4 mg f/b 140 or 280 mg load f/b 140 or 280 mg pump prime dose f/b 35 or 70 mg/hr intra-operative infusion | 15 min | Hours after discontinuation | Requires central line; monitor for thrombosis |
| Recombinant activated factor VII | Hemophilia-related inhibitors; other uses are off-label | 90 (50–100) μg/kg IV bolus every 2 hours or as needed | Immediately | 2 hours | Monitor for thrombosis |