Abstract
Atherosclerosis is no longer considered a disorder due to abnormalities in lipid metabolism. In fact, the inciting event of atherosclerosis is likely an inflammatory insult that occurs decades before the disease becomes clinically apparent. Rapidly evolving knowledge of the pathogenesis of atherosclerosis, coupled with novel, target-specific therapies, is revolutionizing the treatment of atherosclerosis. As a result, a variety of treatments are now undergoing evaluation for their ability to ameliorate the inflammatory pathways likely to cause the atherosclerotic process to initiate and propagate. Once initiated, atherosclerosis progresses as a result of a well-studied series of changes in the constituent cellular make-up of the vessel wall. Specific cytokine-mediated events in this cycle are required for lesional growth. The clinical manifestations of atherosclerosis occur so late in this process that interventions such as percutaneous coronary interventions can deal with isolated areas of disease; however, they do not influence the underlying disease process.
Pathogenesis of Atherosclerosis
Marchand introduced the term “atherosclerosis” describing the association of fatty degeneration and vessel stiffening.1 This process affects medium and large-sized arteries and is characterized by patchy intramural thickening of the subintima that encroaches on the arterial lumen. Each vascular bed may be affected by this process; the etiology, treatment and clinical impact of atherosclerosis varies from one vascular bed to another.2 The earliest visible lesion of atherosclerosis is the fatty streak, which is due to an accumulation of lipid-laden foam cells in the intimal layer of the artery. With time, the fatty streak evolves into a fibrous plaque, the hallmark of established atherosclerosis. Ultimately the lesion may evolve to contain large amounts of lipid; if it becomes unstable, denudation of overlying endothelium, or plaque rupture, may result in thrombotic occlusion of the overlying artery.
Atherosclerotic lesions are composed of three major components. The first is the cellular component comprised predominately of smooth muscle cells and macrophages. The second component is the connective tissue matrix and extracellular lipid. The third component is intracellular lipid that accumulates within macrophages, thereby converting them into foam cells. Atherosclerotic lesions develop as a result of inflammatory stimuli, subsequent release of various cytokines, proliferation of smooth muscle cells, synthesis of connective tissue matrix, and accumulation of macrophages and lipid.
Roles of inflammation, endothelial perturbation and lipids
The atherosclerotic process is characterized, in its earliest stages, by perturbations in endothelial function. Atherosclerosis is likely initiated when endothelial cells over-express adhesion molecules in response to turbulent flow in the setting of an unfavorable serum lipid profile. Animals fed a pro-atherogenic diet rapidly overexpress vascular cell adhesion molecule-1 (VCAM-1). Li3 demonstrated that expression of VCAM-1 on endothelial surfaces was an early, and necessary, step in the pathogenesis of atherosclerosis. Increased cellular adhesion and associated endothelial dysfunction then “sets the stage” for the recruitment of inflammatory cells, release of cytokines and recruitment of lipid into the atherosclerotic plaque.
Inflammation and chronic endothelial injury
It is now widely accepted that the earliest stages of the development of atherothrombosis are mediated, in large part, by the inflammatory cascade.4 VCAM-1 expression increases recruitment of monocytes and T-cells to sites of endothelial injury; subsequent release of monocyte chemo-attractant protein-1 (MCP-1) by leukocytes magnifies the inflammatory cascade by recruiting additional leukocytes, activating leukocytes in the media, and causing recruitment and proliferation of smooth muscle cells (Figure 1 ). In response to signals generated within the early plaque, monocytes adhere to the endothelium and then migrate through the endothelium and basement membrane by elaborating enzymes, including locally activated matrix metaloproteinases (MMP) that degrade the connective tissue matrix (Figure 2 ). Recruited macrophages both release additional cytokines and begin to migrate through the endothelial surface into media of the vessel. This process is further enhanced by the local release of monocyte-colony stimulating factor (M-CSF), which causes monocytic proliferation; local activation of monocytes leads to both cytokine-mediated progression of atherosclerosis, and oxidation of low-density lipoprotein (LDL, Figure 3 ).
Once initiated, many mediators of inflammation have been described to influence the development of the atherosclerotic plaque. For example, CD40L elaborated within the plaque has been shown to increase the expression of tissue factor (and thus, presumably increase the likelihood of thrombosis) in atherosclerotic plaques; anti-CD40L abrogates evolution of established atherosclerotic lesions in animal models.5 Inflammatory mediators expressed by smooth cells within the atherosclerotic plaque include, but are not limited to, interleukin (IL)-1β, tumor necrosis factor (TNF) α and β, IL-6, M-CSF, MCP-1, IL-18 and CD-40L. The impact of these mediators is diverse and includes mitogenesis, intracellular matrix proliferation, angiogenesis and foam cell development (Figures 4 & 5 ).
Models within which these mediators have been “knocked-out” now exist. Most such models support a role for these mediators in the pathogenesis of atherosclerosis; for example, atherosclerosis-prone mice lacking MCP-1 or M-CSF are less likely to develop progressive atherosclerosis than wild-type mice.
Given the importance of the inflammatory cascade in pathogenesis of atherosclerosis clinical interest has focused on the development of markers of risk; predominant among these is C reactive protein (CRP) and fibrinogen. Experimental work supports an association between these markers and the pathogenesis of atherosclerosis; thus, Danenberg and colleagues6 evaluated the pro-inflammatory and prothrombotic effects of CRP on monocytes and endothelial cells in vivo by subjecting wild-type mice, which do not express CRP, and human CRP-transgenic (CRPtg) mice to two models of arterial injury. In an arterial injury model complete thrombotic occlusion of the femoral artery at 28 days was seen in 17% of wild-type mice compared with 75% of CRPtg arteries. After adjustment for lipid status, CRP levels remain independent predictors of atherosclerosis, including peripheral arterial disease.7 Activities of CRP in experimental models are protean and include decreased endothelial nitric oxide (NO) and prostacyclin secretion, increased MCP associated chemotaxis, increased IL-8 and increased MMP-1 activity. Impaired release of NO is associated with increased vascular tone and may be associated with increased platelet activation and intimal proliferation.8
Molecular targeting to address chronic inflammation
The increasing knowledge of the pathogenesis of atherosclerosis at the molecular level has led to the development of specific molecular targets for anti-atherosclerotic therapy. Peroxisome proliferator-activated receptors (PPARs)9 have emerged as important anti-atherogeneic targets; endothelial-specific roles of PPAR-α include inhibition of adhesion molecules, including VCAM-1, increased endothelial NO release, reduced foam cell formation, and reduced uptake of glycated LDL and triglyceride-rich remnant lipoproteins. Ligands of PPAR-γ include fatty acids and the oral hypoglycemic drugs belonging to the glitazone family. PPAR-γ is expressed in numerous cell types found within the atherosclerotic lesion, including endothelial cells, smooth muscle-cells, macrophages, and T cells. Ligand binding with PPAR-γ may have multiple antiatherogenic effects (Table 1 ). Studies of humans treated with PPAR agonists have been reported; glitazone treatment reduces CRP, MMP-9 and TNF-α serum levels, perhaps through PPAR-α activation.10,11
More broadly, it is likely that many of the beneficial effects of medications widely used for the treatment or prevention of atherosclerosis are mediated, in part, through their ability to modify the inflammatory cascade. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, widely known as the “statins,” appear, in clinical studies, to have anti-atherogenic effects in excess of those likely to occur due to improved lipid status. Experimental models support the supposition that HMG-CoA inhibitors act, at least in part, by reducing inflammation. Thus, statins induce release of anti-atherogenic cytokines (such as IL-4 and IL-10) and diminish expression of pro-atherogenic cytokines, such as IL-6, IFN-γ and TNF-α in macrophages.12 Statins also reduce expression of MCP-1 and other mediators in a variety of models of atherogenesis.
The role of lipid
The lipid hypothesis of atherogenesis has been dramatically modified over the last 20 years. Once viewed as the initiating agent of atherothrombosis, it is now recognized that localization and accumulation of lipid occurs in response to earlier changes in the vascular endothelium. Accumulation of lipid is, however, required for the development of the definitive plaque. Lipid deposition likely starts with the movement of LDL from the blood into the vessel wall. Once within the media three fates can befall the LDL: it may move back into the bloodstream (a hallmark of lesional regression and a process that may be facilitated by some lipid lowering strategies), it may become oxidized (through action of free radicals or direct activity of leukocytes) or it may be taken up by monocyte/macrophages which ultimately become foam cells. Oxidized LDL is particularly atherogenic and is chemotactic for monocyte-macrophages.
Macrophages bind intra-intimal LDL via a family of novel receptors known as scavenger receptors, which recognize LDL only after it has been oxidized. Uptake of oxidized LDL renders the macrophages less mobile, thereby promoting the accumulation of these lipid-laden cells in the intima. The foam cells retain their metabolic activity and secrete a variety of cytokines and inflammatory mediators. Outcomes of their activation include recruitment and proliferation of smooth muscle cells (which in turn elaborate additional locally active cytokines), further LDL oxidation, recruitment of additional monocyte/foam cells and additional impairment of endothelial function.
Evolution of the atherosclerotic plaque
Evolution of the atherosclerotic plaque is characterized by gradual enlargement over time due to the accumulation of foam cells. The gradually enlarging plaque may precipitate chronic stable angina. However, myocardial infarction oftentimes occurs within vessels with relatively unremarkable narrowing. This suggests that rapidly growing plaques cause many myocardial infarctions. This observation has led to further research into the mechanism of acute thrombotic occlusion of the epicardial vessels at sites of atherosclerotic disease.
Slowly growing plaques gradually accumulate lipid within foam cells; proliferation of smooth muscle cells and elaboration of intracellular matrix produce the definitive fibrous plaque. In general, such plaques tend to have adherent endothelial layers that are not prone to sudden disruption with associated activation of coagulation.
Some plaques grow at a much greater rate than would be predicted by simple lipid accumulation and expansion of the components of the fibrous plaque. Cholesterol accumulation within such plaques is due to both “passive” transfer of LDL from the circulation and scavenging of red blood cell membranes deposited during intraplaque hemorrhage.13 Angiogenic signaling and proliferation of microvessels within the plaque is only now beginning to be understood; however, plaque hemorrhage is likely attributable to bleeding from fragile microvessels that proliferate within the plaque itself, presumably in response to local angiogenic stimuli. Kockx et al identified intraplaque hemorrhage from microvessels triggering macrophage activation and foam cell formation in carotid lesions.14 These authors propose that intraplaque microhemorrhage may initiate platelet and erythrocyte deposition, lead to iron deposition, activate macrophages and contribute to foam cell formation. Support for the importance of angiogenesis in the pathogenesis of plaque growth was recently bolstered by the finding that intra-plaque microvessels were an independent predictor of plaque rupture.15 The potential importance of angiogenesis in the development of atherosclerosis is found in experiments that demonstrate that antiangiogenic therapy reduced atherosclerotic lesion development in a placebo controlled trial in atherosclerosis prone mice.16 Additional trials of antiangiogenic therapies in patients with atherosclerotic vascular disease are currently planned or underway.
Chronic stable angina is due to flow-limiting epicardial coronary disease. Acute myocardial infarction is usually due to acute thrombotic occlusion of an epicardial vessel. Acute thrombotic occlusion occurs as a consequence of sudden disruption of the atherosclerotic plaque associated with spontaneous fissuring or rupture when exposed to high shear stress at sites of stenosis and arterial branching. Two forms of plaque injury are recognized: superficial and deep. Superficial injury produces areas of focal endothelial denudation that can enlarge and lead to the formation of mural or even occlusive thrombi. Plaques that are capped with superficial collagen fibers separated by large number of lipid-filled macrophages tend to predispose to superficial injury.17,18 Deep intimal injury is characterized by a split or tear that extends from the luminal surface of a plaque deep down into the plaque substance. This type of injury, which tends to occur in plaques that contain a large lipid-rich pool, exposes blood to the highly thrombogenic contents of the plaque (in particular tissue factor elaborated within the plaque, a process exacerbated by local expression of CD40L). If sufficient thrombogenic stimulus is present the clot may completely occlude the epicardial vessel causing acute myocardial infarction; prevention of acute thrombotic occlusion of epicardial vessels is the primary way by which anticoagulants (such as aspirin, clopidogrel or warfarin) reduce the risk of recurrent myocardial infarction.
Non-occlusive thrombosis may also cause rapid increases in the size of atherosclerotic plaques as a result of platelet activation and elaboration of platelet-derived growth factors (PDGF) on the surface of the plaque. Platelet activation can directly influence the clinical course of atherosclerosis; it is likely that acute myocardial infarction and unstable coronary syndromes are due to thrombin and von Willebrand factor-mediated platelet activation and aggregation. Additionally, platelet activation can play a role earlier in the pathogenesis of atherosclerosis. For example, PDGF and tumor growth factor (TGF)-β are two very potent mitogenic cytokines elaborated by activated platelets that act at the site of the thrombus to promote atherosclerotic lesional development.
Damage to the vessel wall
Thrombogenesis is promoted by loss of endothelium, which may be caused by direct physical damage such as occurs with angioplasty, hemodynamic stress, use of tobacco products, high blood cholesterol levels, or enzymes released from platelets and leukocytes.19 The shedding of endothelial cells exposes the subendothelium to platelets and blood coagulation factors. Platelets that adhere to the subendothelium undergo shape change, aggregate, and secrete their granular contents, thereby recruiting more platelets. At physiologic shear rates, platelet adhesion to subendothelial collagen is mediated by von Willebrand factor and possibly other adhesive proteins, which bind to a glycoprotein receptor (GPIb) on the platelet surface20 as well as to subendothelial components.
Although endothelial cell loss represents the most severe form of vascular damage, more subtle injury may also promote thrombogenesis. Thus, endothelial cells exposed to endotoxin, cytokines such as IL-1 and TNF, thrombin, hypoxia, or increased shear stress synthesize tissue factor and internalize thrombomodulin, thereby promoting coagulation. In addition, these perturbed cells also produce plasminogen activator inhibitor-1 (PAI-1), which impairs fibrinolysis, and acquire receptors to which leukocytes and platelets adhere.21 Finally, the altered endothelial cells synthesize factors that regulate local blood flow. These include vasoconstrictors known as endothelins, as well as vasodilators such as prostacyclin and NO.8,21
Platelet activation
Platelets adhering to collagen undergo a shape change, secrete their granular contents, and aggregate. In addition to collagen, a variety of other agonists, including thrombin, epinephrine, and thromboxane A2 (TXA2), also promote platelet aggregation.22 Whereas all of these agents stimulate the synthesis of TXA2, collagen, thrombin, and TXA2 also induce the release of adenosine diphosphate (ADP) from platelet granules, which amplifies the aggregation process. In addition to these pathways, thrombin-induced platelet aggregation occurs through a third mechanism that may involve the activation of platelet calpain.23
Epidemiology and Prevention of Atherosclerosis
The most effective means of preventing arterial thrombosis is to prevent atherosclerosis. The proven risk factors for atherosclerosis are hypercholesterolemia, hypertension, cigarette smoking, obesity, physical inactivity, age, family history, diabetes and male sex. The first five of these risk factors are potentially reversible, and there is evidence that their reversal reduces the complications of atherosclerosis.
Cholesterol and lipids
The plasma level of cholesterol is determined by genetic factors, by the type and amount of fat in the diet, and by other factors such as obesity, physical activity, and disease states. Based on the results of animal studies, epidemiologic data, and interventional studies, there is good evidence for an association between hypercholesterolemia and atherosclerosis.
The association between serum cholesterol levels and the risk of coronary heart disease is continuous.24,25 Familial hypercholesterolemia, a disorder caused by an absent or defective LDL receptor, causes premature coronary heart disease.26,27 In the heterozygous form of this disorder, which occurs in 1 in 500 people, the total cholesterol concentration is usually in excess of 300 mg/dL. Approximately 5% of all patients who present with acute myocardial infarction (MI) before the age of 60 have heterozygous familial hypercholesterolemia. The homozygous form of familial hypercholesterolemia occurs in about 1 in one million individuals and presents with cholesterol levels ranging from 600–1000 mg/dL. Patients usually develop severe coronary heart disease before the age of 20.
Reduced levels of HDL cholesterol are associated with an increased risk of coronary heart disease. The main causes of reduced HDL cholesterol include cigarette smoking, obesity, physical inactivity, androgenic and related steroids (including anabolic steroids), beta-blocking agents, hypertriglyceridemia, and genetic factors. In contrast, weight reduction, exercise and some medications elevate HDL cholesterol levels.
Both the cholesterol level and the prevalence of coronary heart disease are influenced by environmental factors, including diet. Thus, individuals who immigrate from countries where the prevalence of coronary heart disease and the serum cholesterol levels are low to a country with a high prevalence of coronary heart disease will often have increases in both serum cholesterol levels and rates of coronary heart disease.
The evidence that decreasing serum cholesterol levels with cholesterol-lowering drugs or dietary modification slows or reverses the progression of coronary atherosclerosis28,29 and reduces coronary events30 comes from many randomized trials that include more than 40,000 subjects.28,30,31 Lowering the serum cholesterol level with diet or drug therapy also slows the progression of angiographically documented coronary atherosclerosis in patients with arterial bypass grafts.28 Modifying several risk factors, such as lowering the serum cholesterol level, the blood pressure, and the levels of LDL cholesterol and by cessation of smoking, reduces the risk of ischemic heart disease.32,33 Individuals with several risk factors benefit most from these measures.34
Aggressive lowering of the serum cholesterol level in patients with recent MI results in a rapid decrease in the risk of subsequent ischemic cardiac complications, the need for surgical revascularization, and death rates.35,36 This effect occurs even when the total cholesterol level falls within the upper range of normal (5.5–5.8 mmol/L, 213–310 mg/dL).
Emerging risk factors
Evidence is increasing that a variety of additional risk factors for atherosclerotic disease exist. Elevations of CRP and fibrinogen have been widely studied as novel predictors for the development and progression of atherosclerosis. These factors have been discussed earlier in the text.
Congenital or acquired hyperhomocysteinemia is associated with an increase in the risk of both arterial and venous thromboembolism.37,38 In some cases, homocysteine levels may be reduced with the administration of folic acid or vitamins B6 or B12. 39,40 Whether these interventions reduce the risk of atherosclerosis and its complications remains controversial; for example a recent randomized study demonstrated an increased risk of vascular events if patients were treated with homocysteine lowering therapy after percutaneous coronary stenting.41
Impaired fibrinolysis has been linked to atherosclerotic vascular disease in some but not all studies. Anti-phospholipid antibodies are clearly associated with premature arterial thromboembolism, and may be associated with accelerated atherosclerosis. Both thoracic radiation therapy and heart transplantation are associated with accelerated atherosclerosis and ischemic cardiac syndromes likely as a result of therapy-induced endothelial injury.
Elevated levels of lipoprotein(a) have also emerged as a potential risk factor for atherosclerosis. Interest in this biochemical abnormality is heightened by the observation that niacin therapy reduces lipoprotein(a) levels, although this therapy has not yet been shown to reduce the risk of atherothrombotic complications.
Summary
Atherosclerotic vascular disease continues to be the leading cause of death in the Western world. Our understanding of the pathogenesis of this disorder has increased rapidly over the last two decades; current advances point towards novel causes, and innovative treatments, for this common and troublesome condition. It is safe to anticipate that as our understanding of the molecular pathogenesis of this condition improves numerous novel treatments for this condition will emerge.
Potential anti-atherogenic activities of peroxisome proliferator-activated receptors (PPARs).
|
|
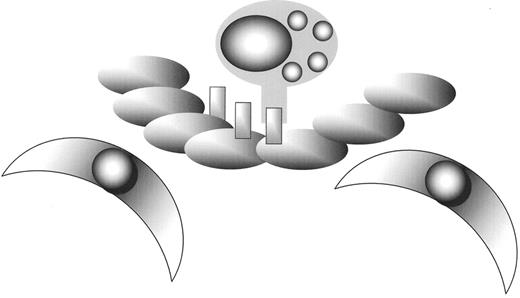
Atherosclerosis is initiated when leucocytes adhere to the endothelium as a result of expression of adhesive proteins.
Atherosclerosis is initiated when leucocytes adhere to the endothelium as a result of expression of adhesive proteins.
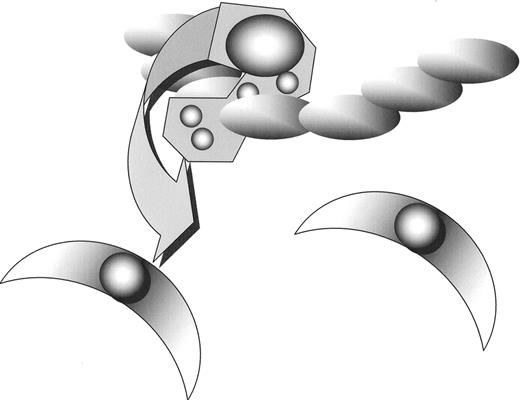
Leucocytes than cross the endothelial barrier and begin to accumulate.
Monocytes within the sub-endothelial space subsequently “orchestrate” the development of atherosclerosis through cytokine release.
Monocytes within the sub-endothelial space subsequently “orchestrate” the development of atherosclerosis through cytokine release.
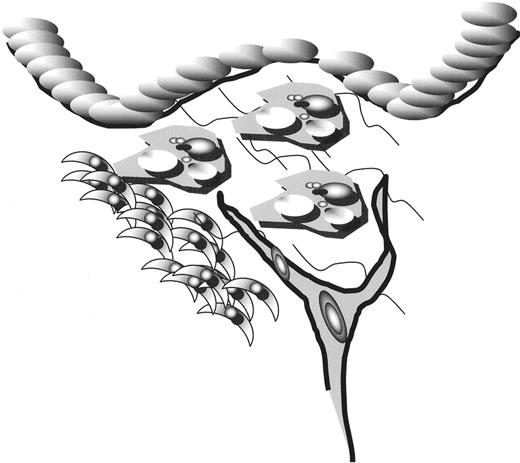
Clinically apparent disease if first noted as a result of the accumulation of foam cells.
Clinically apparent disease if first noted as a result of the accumulation of foam cells.
The clinically important lesion is characterized by intimal narrowing, many foam cells, neovascularization and flow-limiting narrowing. However, this stage of the disease is sufficiently advanced that treatments aimed at it do not impact the pathogenesis of the underlying disorder.
The clinically important lesion is characterized by intimal narrowing, many foam cells, neovascularization and flow-limiting narrowing. However, this stage of the disease is sufficiently advanced that treatments aimed at it do not impact the pathogenesis of the underlying disorder.
Acknowledgments: Dr. Crowther is a Career Investigator of the Heart and Stroke Foundation of Ontario