Abstract
Venous thrombosis occurs as a consequence of genetic and environmental risk factors. Since the discovery of factor V Leiden, the most common genetic risk factor, there has been intense interest in clarifying the roles of genes and the environment with thrombosis risk. The translation of this risk information to clinical practice is a challenging one in the setting of a rapidly expanding knowledge base that includes application of genetic medicine. There are benefits, but also potential harms, of testing for inherited disorders associated with thrombosis. This paper reviews inherited risk factors for thrombosis and discuss clinical applications of testing.
Patients with venous thrombosis (VT) commonly have an underlying genetic predisposition. The occurrence of VT is a culmination of environmental and genetic risk factors.1 Important environmental risk factors are advancing age, male sex and obesity, with “risk periods” of surgery, trauma, cancer, immobilization (including hospitalization), pregnancy and use of exogenous hormones. Genetic risk factors enhance the risk of VT during risk periods and of VT occurring in the absence of environmental triggers (e.g., idiopathic VT). About one-half of VT occur on an idiopathic basis.
Over the past 20 years several hematologic disorders have been identified that are related to the risk of VT (Table 1 ). These conditions are often referred to as “thrombophilic disorders.” Many thrombophilias are genetic disorders that are evaluated in the clinical laboratory by DNA analysis or coagulation factor activity levels. Other hemostatic factors related to thrombosis risk, such as higher D-dimer concentration,2,3 represent hemostatic activation, probably in relation to unknown genetic prothrombotic factors or combinations of known prothrombotic disorders that enhance fibrin formation.4
Use of testing for genetic or acquired thrombophilic disorders has become widespread in hematology and general practice, on the basis of the associations of these disorders with risk of a first venous thrombosis. However, few data are available on thrombophilia and prediction of recurrent events. Whether this testing provides information that is useful for clinical management in decision-making about use of long-term anticoagulation is not yet clear.5,6
Thrombophilic Disorders
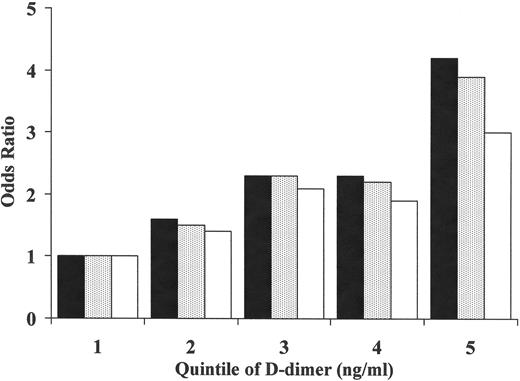
Among patients presenting with a first idiopathic VT, currently about one-half have an identifiable inherited thrombophilic disorder. These can be divided into “loss-of-coagulation function” disorders and “gain-of-coagulation function” disorders.7 Loss-of-function disorders are less common but may be more potent risk factors for thrombosis. These disorders include deficiencies of the endogenous anticoagulants, antithrombin, protein C and protein S. The gain-of-function disorders may be weaker risk factors for VT and include factor V Leiden, the prothrombin 20210A variant, and possibly elevation of procoagulant factors such as factor VIII, von Willebrand factor, and factors V, VII, IX and XI. A review of these disorders can be found elsewhere.7 Higher D-dimer, in healthy individuals, is also a risk factor for future VT, independent of other risk factors (Figure 1 ).3 Higher D-dimer may represent the sum of genetic variations reflecting gain or loss of function, but this remains to be proven. In one study D-dimer concentrations clustered with concentrations of factors V, VIII, IX and fibrinogen, suggesting common regulation or co-regulation of these factors.8
Table 1 shows the relative risk of VT associated with thrombophilic disorders, utilizing data from two population-based studies. The landmark Leiden Thrombophilia Study (LETS) is a case-control study of unselected Dutch patients aged < 70 years without cancer who experienced a first deep vein thrombosis (DVT).6 The Longitudinal Investigation of Thromboembolism Etiology (LITE) is a prospective cohort study of 21,680 US men and women aged 65–100 years, in which baseline risk factors were assessed in relation to future DVT or pulmonary embolus (PE), both idiopathic and secondary to triggers, including cancer.9 In general, the findings of the two studies were similar, although factor V Leiden was a weaker risk factor in LITE than in LETS. This may be due to the younger age in LETS, or the large number of women on oral contraceptives in LETS, as VT risk with oral contraceptives is greatly enhanced among women with factor V Leiden. In these general population studies, the risk associated with loss-of-function disorders was not higher than that associated with gain-of-function disorders, as has been suggested.7 This may be due to unstable risk estimates for loss of function disorders in these studies, because of the rarity of these disorders in the general population (< 1%). However, relative risks reported from family studies may be overestimated by studying thrombophilic families, in which the risk is higher than in the general population due to co-inherited genetic differences.
Clinical differences exist in the presentation of patients with loss- compared to gain-of-function thrombophilias. Patients with loss-of-function disorders tend to present at a younger age with idiopathic or secondary VT, may be more likely to have a family history of VT, and have a higher likelihood of recurrent thrombosis. Whether these differences are due to a higher thrombosis risk among patients with loss-of-function disorders or selection factors of research studies is uncertain.10
In our experience, among patients undergoing thrombophilia testing after VT, about 5%–10% will have an anticoagulant protein deficiency, 15%–20% factor V Leiden, 5% prothrombin 20210A, and 20% elevated factor VIII (> 200 IU/dL). Rates of positive tests are higher among patients with a family history of thrombosis.
Interaction of Risk Factors in Thrombosis
VT events often occur when multiple risk factors, including genetic and environmental, are present at the same time.1 A classic illustration of this point is the interaction of oral contraceptive use and factor V Leiden. It is estimated that women heterozygous for factor V Leiden have a 4- to 7-fold increased risk of VT. Oral contraceptives confer a 3-fold increase in risk. In the presence of both risk factors, the relative risk is 34-fold increased.11 This is likely due to the fact that oral contraceptives induce activated protein C resistance, making the biochemical defect associated with factor V Leiden worse.12
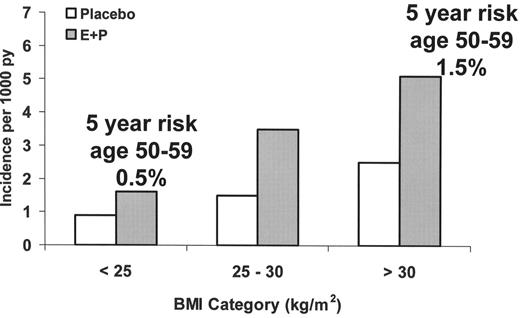
To further illustrate the additivity of VT risk factors, as a woman with factor V Leiden ages her VT risk increases. If she were to begin postmenopausal estrogen plus progestin at the age of 55, her estimated annual risk of VT would approach 1% (compared to the healthy baseline risk of 0.1%).13 If the same woman were obese her absolute annual risk would be 1.5%.13 Oral contraceptives are associated with a similar relative risk increase of VT as postmenopausal estrogen, but if this woman had used contraceptives in her twenties, her absolute annual VT risk would have been only 0.3% (0.4% if she were obese).11,14 Therefore, it would not be surprising if this patient presented in middle age with a hormone-related thrombosis, even if she had previously used oral contraceptives without complications. Figure 2 illustrates the additive effect of obesity and estrogen plus progestin on VT risk in postmenopausal women.13
Similarly, in the presence of more than one inherited risk factor for thrombosis, such as factor V Leiden and protein C deficiency, the relative risk of thrombosis is higher than in the presence of a single disorder. Some inherited traits, such as the factor V HR2 haplotype, only appear to increase the VT risk in the presence of other traits.15
Benefits of Thrombophilia Testing
Prediction of VT recurrence risk
Recurrent VT is a major clinical issue, particularly for those with idiopathic VT, in whom the recurrence rate may be 7%–10% yearly. Clinical factors associated with risk of recurrent VT include cancer-associated VT, idiopathic (versus secondary) VT, PE as the first event, male sex, and perhaps residual venous ultrasound abnormalities.16,17 After a first episode of idiopathic VT, extending anticoagulation beyond an initial 6-month course (termed “longer-term anticoagulation” here), whether administered with a target international normalized ratio (INR) of 1.5–2.018 or 2.0–3.0,19 substantially reduces the risk of recurrent VT. The higher-intensity treatment is more efficacious, and one clinical trial found no difference in rates of major hemorrhage among patients receiving higher-intensity compared to lower-intensity warfarin. Since the risk of life-threatening bleeding with anticoagulation is ~1% yearly, and anticoagulation monitoring is inconvenient, it would be desirable to identify patients at lower or higher risk of recurrence, so that therapy might be better tailored to the individual.20 In this regard, use of thrombophilia testing is controversial5 but may hold some promise.
Since the discovery of factor V Leiden and its association with VT, thrombophilia testing has increased dramatically, presumably for the purpose of identifying those with a high recurrence risk. Among patients with a first VT, earlier studies suggested that disorders such as factor V Leiden and elevated factor VIII were associated with an increased risk of recurrent VT.21–23 These findings have not been consistent in subsequent studies. The LETS group reported on a broad array of thrombophilic risk factors with 7.3-years of follow-up of 474 patients younger than age 70 with a first DVT.24 Considering levels of factors VIII, IX, XI, fibrinogen, homocysteine, anticoagulant factor deficiencies (proteins C, S and antithrombin), factor V Leiden and prothrombin 20210A, only elevated fibrinogen (> 4.1 g/L) and anticoagulant deficiencies were associated with an elevated recurrence risk (relative risks 1.7 and 1.8, respectively). It is debatable whether elevated fibrinogen is a risk factor for first VT.25 In a recent 5.6 year study of families with thrombosis attributed to factor V Leiden or deficiencies of anticoagulant proteins, antithrombin deficiency was associated with the highest incidence of recurrent VT (10.5% yearly), and factor V Leiden the lowest incidence (3.5% yearly).26 In general it is likely that patients with thrombophilic defects who belong to families with a thrombosis history are more likely to have recurrence than unselected patients with thrombophilic defects. This is probably because of unmeasured co-inherited conditions. In fact, it is believed that patients with more than one identifiable thrombophilic defect are at increased recurrence risk compared to those with no or one defect.24 Other data suggest that higher D-dimer concentration after discontinuing anticoagulation is associated with about a 2-fold increased risk of recurrent VT,27,28 although confirmation of this is required and the definition of high D-dimer needs clarification.
Given the benefits of longer-term oral anticoagulation in all patients with idiopathic VT,18,19 it is reasonable to continue anticoagulation longer-term after an initial 6-month course among patients with an anticoagulant deficiency, those with more than one thrombophilic defect, and perhaps those with a strong family history of VT. Information on the efficacy of longer-term oral anticoagulation in those with or without other coagulation factor abnormalities is needed in order to truly determine their role in anticoagulation decision-making. The findings of two trials did not demonstrate that patients with factor V Leiden or the prothrombin 20210A variant had a better or worse outcome when treated with longer-term oral anticoagulation, but the studies had limited power to address this question confidently,18,19
It is counterintuitive that common genetic conditions, such as factor V Leiden, do not increase the risk of recurrent VT. One explanation for conflicting findings among studies is that the duration of follow-up is insufficient to detect an association of these factors with recurrence. If studies could be carried out with much longer follow-up, it is possible that we would find an association of these traits with recurrence risk. Patients from families with a history of thrombosis and thrombophilic defects, even weaker ones, likely have a higher recurrence risk than patients from families without such history.
Improving patient understanding of thrombosis
Occurrence of VT is now recognized as being associated with impaired quality of life in most domains.29,30 Whether thrombophilia testing can improve patient understanding, and thus quality of life, is not known. In fact, in one study understanding of patients after factor V Leiden testing was poor, with 79% overestimating the associated risk, 64% stating they had not been given much information, and 53% believing their health care providers did not have sufficient knowledge.31 Knowledge was better among patients seen in a thrombosis clinic.31 Practitioners can use published tools for patient education32,33 and should be encouraged to refer patients to specialized centers experienced in management of thrombophilic patients. Much work is needed to understand the impact of genetic testing on quality of life in VT patients.
Family testing
Given current knowledge, thrombophilia testing yields a diagnosis in approximately one-half of patients with idiopathic VT. When results are positive, testing of asymptomatic relatives often occurs. The utility of family testing is a topic of debate. Identification of thrombophilic disorders in asymptomatic individuals would not lead to long-term treatment with anticoagulation because the risk of bleeding with this is higher than the risk of VT. However, this knowledge might improve use of VT prophylaxis in risk periods. Thus, the utility of family testing is likely to depend on how well prophylaxis against VT is applied. In regions where prophylaxis is not administered according to accepted standards, family testing that includes accurate patient education and counseling may increase the appropriate use of prophylaxis in risk settings, thereby reducing the incidence of VT. If family testing is done in a region where prophylaxis is aggressively administered to all patients, it is unlikely that this testing will reduce the incidence of secondary VT.34
Since one-half of VT cases are due to triggering factors, and genetic factors are key risk factors, it is possible that family testing could reduce the burden of VT. The practice of family testing has likely most influenced the use of prophylactic anticoagulation in pregnancy in affected female relatives, who for some disorders have a VT incidence estimated as high as 4% per pregnancy in the absence of prophylaxis. Family testing among women may also reduce VT risk through avoidance of oral contraceptives or postmenopausal hormone therapy among women testing positive. However, advantages of testing are more likely among women considering postmenopausal hormone therapy than oral contraceptives due to the much higher absolute risk of VT in middle-aged than younger women. In the latter, the risks of unwanted pregnancy versus the benefit of VT prevention are unknown.
Much research is needed in on many aspects of family testing before definitive recommendations may be made. However, for families desiring testing, careful pre- and post-test counseling must be undertaken by an experienced clinician. Issues for consideration in this testing are outlined in Table 2 .
Harms/Drawbacks of Thrombophilia Testing
Thrombophilia testing has potential harms. Several scenarios can lead to false information being conveyed to patients:
Laboratory analytical error
Laboratory or physician error in data reporting
Inherent variability in assays leading to false diagnosis (or missed diagnosis)
Diagnosing protein C or S deficiency when the test was performed in a patient taking a vitamin K antagonist or with vitamin K deficiency
Ruling out antithrombin deficiency when the test was performed in a patient taking a vitamin K antagonist (which can raise antithrombin concentration)
Diagnosing protein C, protein S or antithrombin deficiency based on a single laboratory determination
Diagnosing or ruling out any disorder using a plasma sample drawn at the time of acute VT or other illness
Erroneous diagnosis of antiphospholipid syndrome based on testing done at a single time point (this diagnosis requires abnormal testing over time)
Testing for the wrong indication
Several other areas related to thrombophilia testing require further study. The psychological effects of thrombophilia testing are not well studied. These effects are likely complicated, involving issues present any time genetic testing is considered. VT is associated with impaired quality of life.29 Whether or not thrombophilia testing would improve patient perceptions of his or her disease and quality of life, through an improved understanding, is unknown. Little information is available on the cost effectiveness of thrombophilia testing for VT patients or family members. Discrimination against patients with genetic traits in obtaining medical or life insurance is possible,35 and patients, especially asymptomatic ones, must be made aware of this prior to testing. Finally, “direct-to-consumer testing” is being promoted via internet sites, where patients can obtain genetic testing for thrombophilia without the supervision of their physician. The risks, ethics and efficacy of such testing require study.
A Practical Approach to Thrombophilia Testing
VT patients with the following characteristics may be considered for thrombophilia testing:
Idiopathic first event
Secondary, non-cancer-related first event and age < 50, including thrombosis on contraceptives or postmenopausal hormones
Recurrent idiopathic or secondary, non-cancer, events
Thrombosis at an unusual site
Thrombophilia testing should not be done in the acute setting of thrombosis, as the information does not change initial management (except in the case of a prolonged aPTT, which might indicate a lupus anticoagulant, and complicate the management of unfractionated heparin). Acute illness or thrombosis can cause transiently reduced or increased levels of several coagulation factors, including proteins C and S, antithrombin, and procoagulant factors, due to mild consumptive coagulopathy or acute inflammation. A reasonable testing policy is to continue warfarin for 6 months, and then interrupt treatment for thrombophilia testing and decision-making about whether prolonged anticoagulation will be recommended. Goals of testing are to identify patients with strongly thrombophilic disorders or multiple defects (including homozygous or double heterozygous defects) and to improve patients’ understanding of their disease. Two to three weeks after stopping warfarin, after informed consent, the following panel of laboratory tests may be used to identify thrombophilic disorders: Factor V Leiden; Factor VIII coagulant activity; Prothrombin 20210A; Fasting homocysteine; Protein C clotting activity; Screen for antiphospholipid syndrome; Protein S clotting activity; PT, aPTT; Antithrombin activity
The composition of thrombophilia testing panels used in practice varies widely. Many centers include testing for protein C, protein S and antithrombin mass determination (“antigen assays”). This testing is not necessary in the absence of a low activity level of these factors. Some employ a thrombin time to screen for dysfibrinogenemias, but these disorders are exceedingly rare. Some also include multiple coagulation factor assays (e.g., fibrinogen, factors V, VII, IX and XI). It is not clear that elevations of these factors are inherited, whether they are predictive of recurrence, what the correct cutpoint defining elevation is, and what the pre-analytical and analytical variability is at high levels. Addition of testing for activated protein C (APC) resistance might add information to the above panel of tests, by identifying APC resistance in the absence of factor V Leiden (about 10% of all APC resistance occurs in the absence of factor V Leiden). However, assay standardization, precision and variability are uncertain, and the role of this defect in VT risk is not clear.36,37
Among patients with a first idiopathic VT, there is little clinical trial evidence supporting a specific recommendation for longer-term treatment based on the results of thrombophilia testing. However, patients with the following findings, and no contraindications, should probably be treated with longer-term anticoagulation, with an intensity determined by physician and patient preference:
More than one thrombophilic defect (not including elevated homocysteine)
Antiphospholipid syndrome
Deficiency of anticoagulant proteins (proteins C, S, antithrombin)
Previous PE or thrombosis at unusual site
Strong family history of thrombosis (empiric author recommendation; requires study for confirmation)
Longer-term anticoagulation is probably not indicated for women with contraceptive-related thrombosis, but education on prophylaxis in risk periods and avoidance of retreatment with hormones is needed.38 Whether women with postmenopausal hormone-related VT should be treated longer-term is uncertain and may depend on the presence of characteristics above.
For patients without the above characteristics, with single weak thrombophilic defects or no identifiable defects, low-intensity warfarin (target INR 1.5–2.0) or observation may be considered, depending on patient characteristics and preference. Among these patients, a higher INR of 2.0–3.0 could be considered for men (higher recurrence risk than women) or for reliable older patients with multiple medical conditions, who might have a high risk of death from recurrent thrombosis.
Future Directions
Many questions remain in relation to the long-term management of patients with VT, especially those with a first idiopathic VT. The clinical role of thrombophilia testing requires further clarification in order to determine whether results should dictate management. If the goal of testing is to define recurrence risk, it is possible in the future that tests of hemostatic activation status, such as D-dimer, might be used initially, with further thrombophilia testing among those with high levels. Any role of common environmental VT risk factors, especially obesity, in decision-making is unknown since it is not clear whether obese patients have a higher recurrence risk. The broad implications of thrombophilia testing, both for VT patients and their asymptomatic family members, require rigorous study. When testing is undertaken, it must be performed by a physician experienced in the rapidly changing field of thrombosis genetics, and who is aware of the analytical problems associated with specialized coagulation tests.
Comparison of relative risks of venous thrombosis in a retrospective (LETS) and prospective (LITE) study.
| Risk Factor . | LETS . | LITE . |
|---|---|---|
| Abbreviations: LETS, Leiden Thrombophilia Study; LITE, Longitudinal Investigation of Thromboembolism Etiology; TAFI, thrombin-activatable fibrinolysis inhibitor | ||
| Factor V Leiden | 8.1 | 3.7 |
| Factor V Leiden homozygote | 80 | 24 |
| Prothrombin 20210A | 2.8 | 1.9 |
| Protein C deficiency | 3.1 | 3.4 |
| Elevated factor V | 1.3 | 1.2 |
| Antithrombin deficiency | 5.0 | Not done |
| Elevated factor VIII | 4.8 | 2.6 |
| Elevated factor VII | 0.8 | 2.4 |
| Elevated factor IX | 2.8 | Not done |
| Elevated factor XI | 2.2 | Not done |
| Elevated fibrinogen | 4.0 | 0.9 |
| Elevated TAFI | 1.7 | 1.6 |
| Elevated D-dimer | 2.5 | 3.1 |
| Elevated homocysteine | 2.5 | 1.5 |
| Risk Factor . | LETS . | LITE . |
|---|---|---|
| Abbreviations: LETS, Leiden Thrombophilia Study; LITE, Longitudinal Investigation of Thromboembolism Etiology; TAFI, thrombin-activatable fibrinolysis inhibitor | ||
| Factor V Leiden | 8.1 | 3.7 |
| Factor V Leiden homozygote | 80 | 24 |
| Prothrombin 20210A | 2.8 | 1.9 |
| Protein C deficiency | 3.1 | 3.4 |
| Elevated factor V | 1.3 | 1.2 |
| Antithrombin deficiency | 5.0 | Not done |
| Elevated factor VIII | 4.8 | 2.6 |
| Elevated factor VII | 0.8 | 2.4 |
| Elevated factor IX | 2.8 | Not done |
| Elevated factor XI | 2.2 | Not done |
| Elevated fibrinogen | 4.0 | 0.9 |
| Elevated TAFI | 1.7 | 1.6 |
| Elevated D-dimer | 2.5 | 3.1 |
| Elevated homocysteine | 2.5 | 1.5 |
Issues to consider in family testing of asymptomatic patients.
Identification of a genetic condition may:
|
Identification of a genetic condition may:
|
Relative risk of future venous thrombosis based on quintiles of D-dimer concentration: the LITE study. Dark bars represent age-adjusted relative risk, shaded bars represented models adjusted for age, sex, race and body-mass index. Open bars are models further adjusted for factor V Leiden, prothrombin 20210A and elevated factor VIII.3 This research was originally published in Blood.
Relative risk of future venous thrombosis based on quintiles of D-dimer concentration: the LITE study. Dark bars represent age-adjusted relative risk, shaded bars represented models adjusted for age, sex, race and body-mass index. Open bars are models further adjusted for factor V Leiden, prothrombin 20210A and elevated factor VIII.3 This research was originally published in Blood.
Obesity, postmenopausal hormone therapy as estrogen plus progestin, and the risk of venous thrombosis in the Women’s Health Initiative trial of estrogen plus progestin (E+P). Absolute 5-year risks are also shown.13
Obesity, postmenopausal hormone therapy as estrogen plus progestin, and the risk of venous thrombosis in the Women’s Health Initiative trial of estrogen plus progestin (E+P). Absolute 5-year risks are also shown.13