Abstract
Over 3 million people in the United Staes aged 65 years and older are anemic. This condition is associated with significant functional impairment and, perhaps, increased mortality. In March 2004, the American Society of Hematology (in conjunction with the National Institute of Aging) convened a “blue ribbon” panel of twenty physicians who are experts on various aspects of this topic. This paper highlights important consensus concepts resulting from that meeting. In particular, four areas of thought are shared. First, the epidemiology of anemia in the elderly is reviewed, including its definition, its expression in different racial groups, and its wide-ranging manifestations. Second, the pathophysiology of anemia in the elderly is reviewed as pertains to three general etiological categories (nutritional, chronic diseases, and so-called “unexplained” anemias). Particular emphasis is given to pathophysiologic mechanisms of anemia that are potentially unique to this age group. Third, a practical approach to the diagnosis and management of anemia for this patient population for the practicing hematologist is provided. Finally, the public health implications of anemia in the elderly for key stakeholder constituencies will be discussed in the oral presentation.
Hematology researchers have had a long-standing interest in the pathophysiology and clinical implications of anemia as associated with aging. Findings from the recent Third National Health and Nutrition Examination Study (NHANES III)—the only national survey that samples clinical specimens and the first iteration of this study to have no age limit—highlighted the importance of this issue with respect to the nation’s public health. The NHANES III study indicated that as many as 3,000,000 people in the United States aged 65 or over may be anemic, and that even when “mild” anemia is present, it either causes and/or is associated with both significant functional impairment and, perhaps, increased patient mortality. Given the significance of this issue, the American Society of Hematology (ASH), in conjunction with the National Institute of Aging (NIA), convened a panel of 20 experts in hematology and geriatrics in March, 2004, to address the question of anemia in the elderly in the United States. This review relates major concepts that emerged from this expert panel.
Anemia in the Elderly—Epidemiology
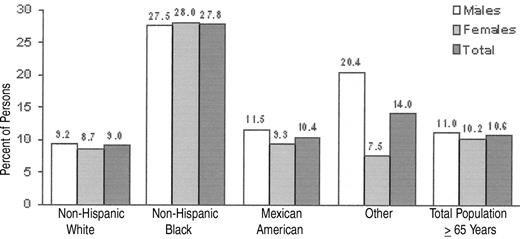
Using the World Health Organization (WHO) definition of anemia (< 13g Hb/dL for men and < 12 g Hb/dL for women), 11.0% of men and 10.2% of women were anemic according to the NHANES III data set1 (Figure 1 ). Although the prevalence of anemia is greater in women than men aged < 75, by age 75 male prevalence surpasses female prevalence by about 5 percentage points, a disparity that continues into upper age brackets.
NHANES III also shows a significant difference in the prevalence of anemia among ethnic groups. For example, the difference between the prevalence of anemia in elderly, non-Hispanic blacks versus elderly, non-Hispanic whites was 27.8% versus 9.0%. Among those with idiopathic or unexplained anemia, the difference was 6.7% versus 2.6%. Data such as these raise important issues as to what defines anemia. Are the WHO definitions of anemia appropriate for the American population? Should the definition of anemia change with age? Should the definition be physiologic and not statistical? Should there be a distinction made between anemia that is “mild” and anemia that is “severe”? If so, what is the “boundary”? With respect to the question of the relationship of race to the definition of anemia, data from several authors have suggested that black men have hemoglobin set point that is 1–2 g/dL below that of white men.2 Similar differences have been observed between black and white women and black and Asian women. These findings have been extended by Beutler et al, who used 6 years of data on ambulatory patients in the Kaiser Permanente health care system in the San Diego, California, area who are Medicare beneficiaries.3 Beutler has suggested that the observed difference in hemoglobin levels between different racial groups is biologically based and not a result of socioeconomic differences. The question thus arises as to whether or not an overall lower hemoglobin level adversely influences the black population. As such, should the definition of anemia be different for different ethnic or racial groups?
Anemia in the Elderly—Consequences
Several studies have shown decreased physical performance and strength in elderly anemic patients. Penninx and colleagues4 found that decrements in performance of three different timed functional tests (standing balance, five repetitions of sitting and rising from a chair, and an 8-foot walk) roughly correlated with declining hemoglobin concentrations in community-dwelling elderly men and women. The In CHIANTI study5 found reduced knee extensor and hand grip strength among anemic residents of the Chianti area of Italy compared with their non-anemic peers aged 65 to 102.
Perhaps of greater significance, anemia has been shown to impact mortality in elderly patients with other co-morbid conditions. For example, Esekowitz and colleagues have shown an increase in mortality in elderly patients with congestive heart failure as compared with their non-anemic cohorts.6 Improvement in hemoglobin levels can also lead to improvements in end-organ function. Hayashi and colleagues7 have shown left ventricular function improves in chronic renal failure patients treated with erythropoietin. These data begin to address the important question of the relative roles of the anemia and the co-morbid condition in the excess morbidity and mortality experienced by the anemic elderly.
Anemia in the Elderly—Causes
Three general causes for anemia in the elderly, nearly equal in frequency, emerged from the NHANES III study.1 These are 1) anemias due to blood loss/nutritional deficiencies (34%), 2) anemias associated with chronic illness/inflammation or chronic renal failure (32%), and 3) unexplained anemias (34%) (Table 1 ). A more exhaustive analysis might have better characterized those patients characterized as having unexplained anemias. Nonetheless, the question is raised: Are there unique “anemias of aging”?
Nutritional deficiencies
The prevalence of blood loss/iron deficiency as a cause of anemia in the elderly emphasizes both the importance of recognition of this diagnosis and the inadequacy of present diagnostic methods for iron deficiency anemia, especially for this patient group. This is particularly so given the frequent concomitant presence of chronic disease states in this patient population. Quantitative assessment of marrow iron stores is the “gold standard” for diagnostic accuracy, but clearly impractical for screening purposes. The measurement of both soluble transferrin receptor and serum ferritin might provide the requisite level of diagnostic accuracy, but the former test is not uniformly available and is not standardized across a wide range of laboratories. It may be that newer measurements of iron-restricted hematopoiesis, such as the identification of hypochromic reticulocytes and low reticulocyte hemoglobin levels, may ultimately provide the potential for requisite sensitivity, reproducibility, and cost necessary as a screening tool. Successful reticulocyte and hemoglobin response to therapeutic iron currently provides a high level of specificity to the diagnosis of iron deficiency anemia. It should be remembered that in elderly patients, however, oral iron might be inadequately absorbed for several reasons, including Helicobacter pylori infection.
Vitamin B12 deficiency as defined by biochemical criteria (homocysteine and methylmalonic acid deficiency) is common in the elderly, but anemia due to B12 deficiency is not. Low levels of vitamin B12 occur in 10% to 15% of the elderly, but it is estimated that only 1%–2% of the elderly are anemic due to vitamin B12 deficiency.8 Low vitamin B12 levels not associated with anemia pathogenesis may exist either as a “false” abnormality (e.g., individuals who take large quantities of ascorbic acid) or as a subclinical deficiency. The absence of macrocytosis should alert the hematologist to this possibility since macrocytosis virtually always precedes anemia when caused by vitamin B12 deficiency (except when a thalassemic trait or concomitant iron deficiency also exist). Even if a patient has both vitamin B12 deficiency and macrocytic anemia, other possibilities (e.g., alcohol abuse) must be considered. Opinion is divided on whether examination of the peripheral blood smear for hypersegmented neutrophils is the most cost-effective screening tool for the diagnosis of vitamin B12 deficiency.
Do the elderly with atrophic gastritis develop pernicious anemia? A therapeutic trial of vitamin B12, which can be given orally, could be used to identify those with clinically important vitamin B12 deficiency. Such an approach requires the use of large doses (i.e., 1000 μ g/day) of vitamin B12. The criteria for response need to be carefully specified, such as by a rise in reticulocyte count and hemoglobin coupled with a decline in mean cell volume (MCV) and normalization of either methylmalonic acid or homocysteine.
The elderly appear to be less prone to low folic acid levels than younger individuals.9 Elderly individuals are frequently prodigious vitamin takers. Folate levels are often higher in community dwelling adults due to food fortification. Elderly residents in nursing homes also receive nutritional supplements. Thus, megaloblastic anemias due to folate deficiency in the elderly appear to be rare; when they do occur, they often relate to alcohol abuse.
Chronic disease(s)
Understanding of the significance of anemias in elderly patients associated with chronic illness and/or inflammation is hampered by the absence of an unambiguous serum marker for inflammation. Candidate tests (erythrocyte sedimentation rate, fibrinogen, C-reactive protein, transferrin saturation and ferritin) are either incompletely sensitive to the inflammatory state and/or also associated with other diseases frequently seen in the elderly (e.g., cancer, diabetes, liver disease, and congestive heart failure). Hepcidin appears to play a central role in the pathogenesis of the anemia of chronic disease, but is extremely difficult to measure in the serum. Thus the “anemia of chronic disease” may include patients with a variety of pathophysiologic mechanisms.
The inflammatory response is the elderly is often aberrant. In particular, the inflammatory response to a stimulus in elderly patients can sometimes be prolonged, even after the initial inflammatory stimuli have resolved.10 The exact basis for this dysregulation is uncertain; altered inflammatory regulation by sex hormones and reduced catabolism of inflammatory cytokines have been implicated as potential causes.11 As a result, protracted elevation of interleukin (IL)-6 and tumor necrosis factor (TNF)-α in the plasma of elderly patients after exposure to inflammatory stimuli can be seen. This may be a common mechanism for the production of anemia in chronic illness unique to elderly patients.
A suggestion of the importance of an altered inflammatory response as an important cause of anemia in the elderly is suggested by the data from Artz et al, who showed that 27/60 cases (45%) anemias in a nursing home population were idiopathic in nature and characterized by average erythropoietin levels of 14.6 mU/mL, and average IL-6 levels were 8.5 pg/mL.12 Are such patients reflective of a unique “anemia of aging”? The above data also suggest that agents that help control inflammatory dysregulation might be of therapeutic benefit in this situation. However, would there be unintended consequences (e.g., increased infection rate or cancer incidence)?
Unexplained anemias (Table 2)
The above study also emphasizes the importance of the hypoxia/erythropoietin sensing mechanism to the pathophysiology of anemia in the elderly. Findings from the Baltimore Longitudinal Study on 150 individuals (W. Erschler, unpublished data) indicate that erythropoietin production increases with age among those who maintain hemoglobin levels at 14 g/dL or higher and remains constant over time, even in patients who develop diabetes or hypertension. This suggests deficiencies may occur, at least in some individuals, in the hypoxia/erythropoietin sensing mechanism with age that require increased erythropoietin production to maintain normal erythrocyte production. What produces this alteration (e.g., decreased red blood cell [RBC] progenitor mass, blunted effect of erythropoietin on RBC precursors, altered hemoglobin-oxygen affinity, decreased intracellular oxygen utilization, or alteration in sex steroid milieu) is not clear. However, what represents a “normal” or physiologically appropriate erythropoietin level may be different in the elderly than in younger individuals.
A related concept is anemia as it occurs in sarcopenic elderly (i.e., those with significant decreases in body mass). One hypothesis raised (without specific scientific corroboration as of yet) is that the decrease in muscle mass may bring about changes in red blood cell mass, oxygen utilization, and perhaps erythropoietin production. In such patients, their anemia may represent a physiological response to their sarcopenia.
The use of erythropoietin in the elderly may have both positive (e.g., lessened end-organ damage due to ischemic events) and negative (e.g., increased blood pressure) consequences. The use of synthetic erythropoietin and investigational agents that stimulate endogenous erythropoietin (e.g., hematide, FG 22156) are of clear interest in this patient group.
Also potentially related to unexplained anemias in elderly patients are changes in stem cell physiology with age. Bone marrow cellularity from marrow aspirates usually declines with age.13 In humans, the number of clones contributing to hematopoiesis (e.g., colony-forming units erythroid [CFUE]) declines with age.14 Whether this is due predominantly to an absolute decrease in stem cells (as reflected by decreased bone marrow cellularity with age) and/or altered stem cell functional characteristics remains to be defined.15
A well-known etiology of anemia that increases with age is myelodysplasia (MDS). Occult MDS may be an important cause of “unexplained” anemias in the elderly. At this point in time, MDS requires bone marrow aspiration and biopsy for clinical confirmation.
Three additional points deserve emphasis when considering unexplained anemias in the elderly. The first is the unexamined potential impact of changes in estrogen or testosterone levels with age (as alluded to above with respect to erythropoietin levels). Second, elderly patients show a propensity for polypharmaceutical usage (including alcohol). Many such drugs have the capacity to reduce erythropoiesis. Finally, careful enumeration of all significant medical conditions in an individual’s medical history is important in defining unexplained anemia. A variety of medical conditions not classically associated with inflammation (e.g., hypothyroidism) may be associated with anemia. Exactly how to account for the impact of associated disease states on the production of anemia in the elderly is a complex question that may require use of tools such as the Charlson index (a tool for assessment of geriatric co-morbidity designed to be used with Medicare databases).16
Anemia in the Elderly—Clinical Evaluation
Of particular importance in the evaluation of elderly patients with anemia are the identification of co-morbid conditions and a detailed drug history (including alcohol). Like all patients with anemia, a complete blood count with differential white count, notation of Wintrobe indices and red cell relative distribution width (RDW), reticulocyte count and peripheral smear evaluation form the basis of further evaluation. Assessment of iron stores is important for most patients, as is assessment for evidence of inflammation. Other tests may be useful as well based on the above data. A practical clinical approach to the evaluation of anemia in elderly patients is outlined in Table 3 .
Anemia in the Elderly—Conclusions
Anemia in elderly Americans is a frequent, underappreciated and potentially morbid condition. However, public health improvement for the anemic elderly involves a complex set of scientific, clinical and societal issues. Key questions posed by this issue include the following:
What is the best definition of anemia for different elderly populations?
What is the magnitude of morbidity produced by anemia in American elderly, and how readily can it be corrected using present therapies?
Are there unique “anemias of aging”? If so, how can they be diagnosed/treated?
Should elderly Americans routinely be screened for anemia?
What provider group is best able to diagnose/treat elderly patients with anemia?
Should there be a clinical guideline for the management of anemia in the elderly?
What are the economic implications to our health care system of a more aggressive approach to anemia in the elderly?
How can a coordinated research approach to the question of anemia in elderly Americans (including large-scale randomized clinical trials) best be executed?
What should the role of the hematology community (including ASH) be in pursuit of these questions?
US distribution of types of anemia in persons ≥ 65 years in 2002.Source: NHANES III: mobile examination (MEC) + home exam population.
| Type of Anemia . | Percent . | Est. Pop. . |
|---|---|---|
| Abbreviations: CKD, chronic kidney disease; ACD, anemia of chronic disease | ||
| Blood Loss/Nutrition Related | 34 | 965,544 |
| Iron deficiency and iron with folate and/or B12 deficiency (blood loss/nutrition) | 20 | 561,936 |
| Folate and/or B12 deficiencies (nutrition) | 15 | 403,608 |
| Chronic Disease (EPO Deficiency) | 32 | 904,136 |
| CKD | 8 | 229,686 |
| ACD | 20 | 554,281 |
| CKD and ACD | 4 | 120,169 |
| Unexplained Anemia | 34 | 945,195 |
| Type of Anemia . | Percent . | Est. Pop. . |
|---|---|---|
| Abbreviations: CKD, chronic kidney disease; ACD, anemia of chronic disease | ||
| Blood Loss/Nutrition Related | 34 | 965,544 |
| Iron deficiency and iron with folate and/or B12 deficiency (blood loss/nutrition) | 20 | 561,936 |
| Folate and/or B12 deficiencies (nutrition) | 15 | 403,608 |
| Chronic Disease (EPO Deficiency) | 32 | 904,136 |
| CKD | 8 | 229,686 |
| ACD | 20 | 554,281 |
| CKD and ACD | 4 | 120,169 |
| Unexplained Anemia | 34 | 945,195 |
Potential unique mechanism of anemia in the elderly.
| Dysregulation of inflammatory response |
| Blunting of hypoxia/erythropoietin sensing mechanism |
| Sarcopenia |
| Quantitative/qualitative alterations in stem cell physiology |
| Decrease in sex steroids |
| Frequent co-morbid medical conditions |
| Polypharmacy |
| Dysregulation of inflammatory response |
| Blunting of hypoxia/erythropoietin sensing mechanism |
| Sarcopenia |
| Quantitative/qualitative alterations in stem cell physiology |
| Decrease in sex steroids |
| Frequent co-morbid medical conditions |
| Polypharmacy |
Evaluation of anemia in the elderly for the clinical hematologist: a practical approach.
Always useful
|
Sometimes useful
|
Always useful
|
Sometimes useful
|
Percentage of persons age 65 and older who are anemic, by race/ethnicity and sex. Source: NHANES III, Phases I and II, 1988–1994: mobile examination (MEC) + home exam; sample excludes null and blank Hb values.
Percentage of persons age 65 and older who are anemic, by race/ethnicity and sex. Source: NHANES III, Phases I and II, 1988–1994: mobile examination (MEC) + home exam; sample excludes null and blank Hb values.