Abstract
HLA-identical sibling hematopoietic cell transplantation (HCT) for sickle cell disease (SCD) has a strong track record of efficacy and there is growing appreciation that its benefits exceed its risks in selected individuals. In contrast, the clinical utility of replacement gene therapy for sickle cell disease remains unproven. Its challenge is to ensure viral transduction into hematopoietic stem cells (HSCs) and to generate safe, stable, erythroid-specific replacement gene expression at a level that is sufficient to have a clinical effect. The clinical necessity for fulfilling all these criteria may make this genetic disorder among the most complex to treat successfully by gene therapy. But the experience of HCT for SCD has proven that eliminating the βS-globin gene is curative when the transfer is stable. Thus replacement gene therapy for sickle cell disease remains a subject of intense interest and investigation.
The objective of hematopoietic cell transplantation (HCT), and of replacement gene therapy for sickle cell disease (SCD), is to replace sickle erythropoiesis or to reduce its clinical impact by the expression of ‘anti-sickling’ β-globin chains. The clinical benefit of this cellular or molecular replacement is the elimination, or significant amelioration, of the protean clinical complications caused by the precipitation of polymerized sickle hemoglobin in erythrocytes. HLA-identical sibling HCT for SCD has a strong track record, having effected a cure in the majority of individuals treated, and there is growing appreciation that its benefits exceed its risks in selected individuals. Gene therapy, however, has no track record of success in the cure of SCD, and a complete understanding of its long-term toxicity is also lacking; this presents problems for anyone attempting to design a clinical trial with a favorable balance of risk and benefit. The challenge of replacement gene therapy for SCD is to ensure viral transduction into hematopoietic stem cells (HSCs) and to generate safe, stable, erythroid-specific replacement gene expression at a level that is sufficient to have a clinical effect. The necessity for fulfilling all these criteria may make this genetic disorder among the most complex to treat successfully by gene therapy. But the experience of HCT for SCD has proven that eliminating the βS-globin gene, and so replacing the sickle cell with healthy donor cells, is curative when the transfer is stable. Thus replacement gene therapy for sickle cell disease is a worthy objective, particularly if it obviates some of the restrictions and morbidity that apply to current transplantation regimens.
Results of Conventional Myeloablative Transplantation for Sickle Cell Disease
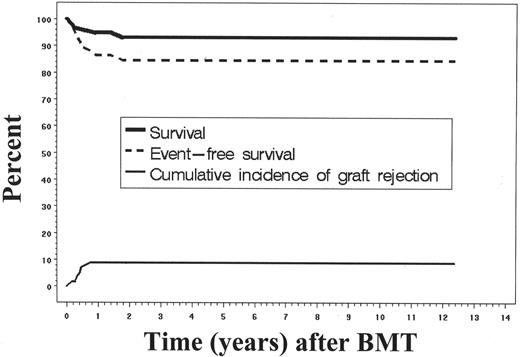
As the worldwide experience of transplantation for SCD has expanded, there has been a transition from using this modality as an experimental intervention reserved for the most severely affected patients, to one in which increasingly younger children with early signs of sickle-related morbidity are targeted for treatment. Several series in Europe and North America have reported similar results after HLA-identical sibling transplantation.1–4 The principal aim of these multicenter clinical studies was to define more completely the risks and benefits of the therapy, and to characterize the natural history of those surviving free of SCD. Myeloablative conditioning regimens have utilized a backbone of busulfan (BU) 14–16 mg/kg and cyclophosphamide (CY) 200 mg/kg, with or without antithymocyte globulin (ATG) or total lymphoid radiation (TLI). Most studies have used cyclosporine (CSP) and methotrexate (MTX) for post-transplantation immunosuppression. The results of transplantation were best when performed in children with SCD who had HLA-identical sibling donors (Figure 1; Table 1 ). Although many of the children who received allografts had significant sickle-related complications such as stroke and recurrent episodes of acute chest syndrome, encouraging results were reported showing disease-free survival of approximately 80% to 85%. However, 5% to 10% of patients died of complications related to transplantation, with GVHD and its treatment noted as the leading cause of death. In addition, very few adults are considered for transplantation.5 This experience illustrates the curative potential of HCT, but also its chief limitations, which are the severe constraints on donor availability, a rather narrow application to the youngest patients who are in good clinical condition, and rates of transplant-related morbidity and mortality that are somewhat fixed. The development of replacement gene therapy might address some of these limitations by expanding the availability of a curative therapy and avoiding the consequences of graft-versus-host disease (GVHD).
Stable Donor-Host Chimerism after Myeloablative HCT for Sickle Cell Disease
The observation of donor-host hematopoietic chimerism after conventional myeloablative HCT has lent substantial support to the notion that persistence of even a fraction of normal erythropoiesis might elicit a curative clinical effect.6 The basis of this effect appears to be 2-fold: improved survival of healthy donor erythrocytes in the blood compared to sickle erythrocytes, and ineffective erythropoiesis in the sickle cell disease marrow that lends a competitive advantage to donor erythroid progenitors. Approximately 25% of children with SCD developed stable mixed chimerism after HLA-identical sibling HCT.7 In the multicenter investigation of bone marrow transplant (BMT) for sickle cell anemia, 13 of 50 patients with clinically successful allografts developed stable mixed chimerism. The level of donor chimerism, measured in peripheral blood ≥ 6 months after transplantation, varied between 90% and 99% in 8 patients who had normal hemoglobin levels. Five additional patients had a lower proportion of donor cells (range, 11%–74%). Among these 5, hemoglobin levels varied between 11.2 and 14.2 g/dL (median, 11.3; mean, 12.0). In the patients whose donors had a normal hemoglobin genotype, the sickle hemoglobin (HbS) fractions were 0%, 0%, and 7%, corresponding to donor chimerism levels of 67%, 74%, and 11%, respectively. In the patients whose donors had sickle trait, the HbS fractions were 36% and 37%, corresponding to donor chimerism levels of 25% and 60%, respectively. Thus, allograft recipients with stable mixed chimerism had HbS levels similar to donor levels; only one patient required RBC transfusion beyond 90 days after transplantation. None of the patients experienced painful events or other clinical complications related to SCD after transplantation. One patient who had a stroke before transplantation had no further strokes after transplantation and had stable brain condition by cerebral MRI, despite having only 10% donor cells detectable in peripheral blood. These observations are consistent with the idea that chimerism even with a minority of donor cells can have a significant ameliorative effect, and that full engraftment of donor cells is not a requirement for successful HCT.
Non-myeloablative HCT for Sickle Cell Disease
Due in part to the observations discussed above, it was reasoned that pre-transplantation therapy might be adjusted to promote stable engraftment of a threshold fraction of donor cells that would be sufficient to prevent vaso-occlusion, and that this regimen could be carried out more safely than conventional myeloablative conditioning. Toward this end, several groups have attempted to apply non-myeloablative stem cell transplantation to SCD, utilizing conditioning regimens of varying intensity.8–11,12 Although these investigations are continuing, it has been difficult to identify a regimen that is sufficiently immunosuppressive to ensure stable engraftment of donor cells from HLA-identical siblings, yet also meets the objective of ‘reduced toxicity’ with a risk that is distinguishable from conventional allografting. A minimally toxic regimen was first developed in a large animal model and translated successfully into human trials for older adult patients with hematological malignancies. When applied to SCD, this approach was safe, generated little or no acute GVHD, and in most cases was associated with an initial period of donor engraftment. Unfortunately, in nearly all cases withdrawal of post-grafting immunosuppression was followed by graft rejection with disease recurrence (Table 1 ). However, serial studies of blast forming uniterythroid (BFU-E) and colony forming unit-granulocyte macrophage (CFU-GM) obtained from marrow during the course of graft rejection showed over-representation of donor erythroid progenitors compared with myeloid counterparts.13 This suggested that enrichment of donor RBC was the product of a selective advantage for donor erythroid progenitors in the marrow together with extended RBC lifespan in the blood. In another patient series, there was a 2-fold higher expression of donor β-globin RNA compared with total genomic DNA in the blood after non-myeloablative HCT. Direct bone marrow analysis revealed ineffective erythropoiesis of native HbSS erythroblasts, with a progressive increase in representation of donor RBC during erythrocyte maturation.14,15 These findings were associated with clinical benefit after transplantation, and with improvements in hemolysis, endothelial function, and nitric oxide bioavailability; however, they did not persist after graft rejection.
A smaller number of patients have received regimens that are less intense than a myeloablative regimen, but retain a moderate degree of the myelosuppressive effect in order to suppress the host-versus-graft (HVG) reaction and promote engraftment. These regimens require hospitalization, but their risk of regimen-related toxicity is reduced in comparison to conventional bone marrow transplantation. A cohort of older sickle cell patients who received such “reduced-intensity” conditioning regimens also received augmented pregrafting immunosuppression to facilitate donor cell engraftment. Nevertheless, 3 of 12 recipients experienced graft rejection (Table 1 ). Acute and chronic GVHD were also more frequent (4 of 12 patients) in this group, and GVHD was fatal in 2 cases. Thus, in older recipients the problem of transplant-related mortality was not eliminated by the reduced-intensity conditioning regimen. In contrast, donor chimerism was successfully established in children who received a reduced-intensity regimen consisting of busulfan, fludarabine, antithymocyte globulin, and total lymphoid irradiation (500 cGy).16 Five patients who were between 6 and 18 years of age received HLA-identical sibling bone marrow transplantation with cyclosporine and mycophenolate mofetil for post-grafting immunosuppression. Treatment-related toxicity was minimal, and 1 patient developed mild GVHD. All the patients had evidence of stable donor engraftment; none suffered graft rejection or recurrent SCD symptoms. Of interest, chimerism studies showed that patients with durable engraftment of donor cells tended to have full donor engraftment or a majority of donor cells, suggesting that stable mixed chimerism might not be the outcome of reduced-intensity preparation for SCD in all cases. Nonetheless, these encouraging results in younger patients suggest that future application of non-myeloablative conditioning regimens should focus on children who already have or are at risk for symptomatic disease.
To summarize these results, in the setting of HLA-identical HCT the principal barriers to success are the problems of transplant-related toxicity, the associated risk of mortality caused by the treatment itself, and immunological graft rejection accompanied by disease recurrence. These problems have been best defined in the setting of HLA-identical sibling bone marrow transplantation, but preliminary evidence (in SCD, thalassemia, and other non-malignant conditions) suggests that they will be magnified if and when transplantation is expanded to include HLA-mismatched or unrelated donors. While it might be possible to reduce the risk of transplantation by modulating the intensity of its preparation, this approach has been accompanied by a higher rate of graft rejection and, overall, a lower probability of cure. Thus the safer and broader application of transplantation is limited chiefly by an immunological barrier to tolerance, which results in graft rejection if host immunity is not sufficiently suppressed, or GVHD if donor immune reactions caused by HLA or minor histocompatibility mismatches are not adequately contained after HCT.
Mechanism of Chimerism
The immunological basis for development of stable donor-host chimerism after HCT for hemoglobinopathies remains incompletely understood, but insight has been gained from observations in patients with thalassemia who have undergone HCT. As in SCD, stable donor-host chimerism develops in approximately 10% of patients after HCT for thalassemia major; observation of its occurrence was largely serendipitous.6,17,18 It is associated with a phenotype similar to thalassemia trait, and even patients with a minority of donor cells do not require RBC transfusions. When investigated more systematically, selected individuals with stable chimerism after HCT had oligoclonal representation of Vβ family T-cell populations in unfractionated donor and host cells, as determined by spectrotype analysis of CDR3 T-cell receptor fragment length.19 This was, however, not associated with immunodeficiency, as a normal distribution of the Vβ family repertoire was restored on stimulation by T-cell mitogen or challenge to a third-party alloantigen. This emergence of selected T-cell clones after transplantation was perhaps responsible for establishing and maintaining bi-directional donor-host tolerance, but it is not clear how it can be accomplished reliably after HLA-identical sibling transplantation.
Chimerism Threshold for Clinical Effect
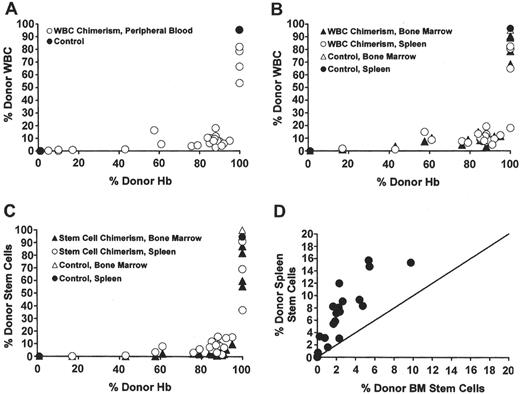
In both allogeneic HCT and replacement gene therapy, the level of chimerism must be sufficient to effect a significant clinical improvement, whether the source of corrected cells is donor or virally transduced HSCs. Murine models of sickle cell anemia have been used to assess the fraction of donor chimerism sufficient to effect a correction of sickle pathophysiology.20–22 Using nonmyeloablative conditioning by T-cell costimulation blockade, Kean et al created a panel of SCD mice with a spectrum of post-transplantation chimerism (white blood cell [WBC] and red blood cell [RBC]) that correlated with the size of the donor marrow inoculum.21 At all levels of chimerism evaluated, the donor RBC fraction in the blood was greater than donor leukocyte and progenitor cell fractions in the blood and bone marrow (Figure 2 ). This enrichment of donor RBC compared to donor WBC in the sickle mice after transplantation presumably reflected an improved survival of donor compared to native sickle erythrocytes in the blood. Unlike the clinical examples cited above, there was no apparent selective advantage of donor RBC precursors in the bone marrow. Most evidence of sickle pathophysiology was eliminated after a threshold of 70% donor hemoglobin was achieved, although histologic changes in the spleen and marrow were not eliminated until 100% donor chimerism was established.21 In another murine model, sickle mice were treated in utero by donor hematopoietic cell injection to achieve donor hemoglobin chimerism that ranged less than 30%.20 In a subsequent experiment, mice that had been grafted in utero later received a postnatal, nonmyeloablative hematopoietic cell ‘boost,’ which increased donor chimerism from 30% to 80%–90%. The extent of RBC sickling observed in vitro during a challenge of hypoxia was proportional to the level of donor chimerism, and spleen size varied inversely with the level of donor chimerism.
The murine data on the effect of donor chimerism and the clinical experience of mixed donor-host chimerism after HCT strongly suggest that replacement gene therapy for sickling disorders would not require full replacement of sickle erythropoiesis. Whether the approach is to repair ineffective erythropoiesis or to express a β-globin chain with anti-sickling properties, there might be significant clinical benefit if even a minority of erythrocyte precursors exhibit restored, native features. While a detailed description of the investigations leading up to the current status of gene therapy for SCD is beyond the scope of this review, a brief description of recent progress in this area and how it relates to transplantation issues will follow.
Optimizing the Cellular Target
The introduction of a replacement gene into the HSC requires extraction of cells from their native residence, co-cultivation with the gene delivery vector, efficient binding by the vector particle, and delivery of an intact replacement gene to the cellular nucleus (reviewed in 23). This process occurs most efficiently when performed in HSCs from umbilical cord blood or after HSC mobilization by growth factors in the blood, and when the retroviral vector and HSC target are co-cultivated in the presence of fibronectin, a substance that appears to mimic the marrow micro-environment and thereby improve the efficiency of transduction.24 Administration of hematopoietic growth factors (such as G-CSF) that mobilize HSC from the marrow cannot be carried out safely in SCD patients; marrow is thus the most likely source of HSCs for a clinical trial of gene therapy, but collection of a sufficient quantity of unmobilized HSC circulating in the blood of sickle cell patients may be feasible.25 Further improvements have been accomplished by utilizing novel pseudotype envelope proteins, such as RD114 from the feline leukemia virus, which target receptors on the HSC cell surface that are more abundant than the receptors targeted by MMLV amphotropic pseudotype envelope; however, these can adversely affect viral titers.26 Finally, the replicative state of the HSC after gene transfer also appears to affect the likelihood of marrow homing and expansion on re-introduction into the circulation: a period of induced cellular quiescence,27 or the activation of the canonical Wnt pathway,28 appears to improve the efficiency of transplantation of the ‘corrected’ HSC.
Optimizing Transduction of HSCs by Vector Selection and Design
Initial preclinical investigations of gene transfer into HSCs relied on onco-retroviral vectors to carry the β-globin gene and its regulatory elements. These suffered from problems of vector instability, low titers, and variable and unstable expression of the human β-globin gene. In addition, the requirement for active cell division, due to their inability to cross the intact nuclear membrane, limited their use in quiescent HSC targets. Finally, the potential for insertional genotoxicity has been clearly illustrated by the frequent occurrence of lymphoid leukemia after gene therapy to replace the common γ-chain in X-linked severe combined immunodeficiency. Three of 14 participants in two European clinical trials have developed T-cell acute lymphoblastic leukemia, probably caused by onco-retroviral insertion in or near the promoter of the growth-control gene LMO2.29 These difficulties are being addressed systematically on several fronts. First, the replacement of globally expressing promoters (such as the cytomegalovirus promoter) by tissue-specific erythroid promoters and linked globin cis-regulatory elements should mitigate the potential for genotoxicity in non-erythroid tissues, where erythroid regulatory elements and promoters should be less active or even inactive. Second, safety has been further enhanced by development of ‘self-inactivating’ (SIN) retro-viruses, which in their integrated proviral forms carry deletions in the flanking long terminal repeat (LTR) segments; LTRs carry the retroviral transcriptional control elements, and in their native form have the potential for significant activating effects on neighboring genes. A SIN vector was used to stably transmit the human β-globin gene coupled to regulatory elements from the Locus Control Region (LCR), resulting in correction of β-thalassemia in mice.30 While the potential for disruption of native housekeeping or critical genes remains, due to the propensity of lentiviral vectors to integrate in genes or their promoters, this toxicity appears to have its greatest impact when it causes a gain-of-function alteration in a cell growth control gene. Currently there is no evidence in patients that malignant transformation might also follow a loss-of-function mutation caused by insertional mutagenesis. Finally, by utilizing lentiviral instead of conventional onco-retroviral vectors, the requirement of cell division for transduction has largely been circumvented. A self-inactivating lentiviral vector carrying anti-sickling globin (βAS3) has been used to correct SCD in several mouse models.31–34 Lentiviral vectors have been reported to give transduction efficiencies ranging from 5% to 20%. If corrected long-term repopulating cells with high-level native globin expression were to establish durable engraftment in SCD recipients, observations after allogeneic transplantation predict that a level of chimerism that approaches 20% would have a clinical effect.
Optimizing Repopulating Activity of Target Cells after Successful Transduction
Gene therapy requires that there must be not only sufficient expression of the native or anti-sickling globin protein to exert an effect within individual cells, but also that there must be an adequate number of corrected cells in circulation to inhibit vaso-occlusion. Thus strategies to expand the pool of transduced cells in vivo are being pursued. These rely on drug resistance to enrich for transduced cells. The most promising of these applications is the use of the DNA repair enzyme O6-methyguanine-DNA-methyltransferase (MGMT).35 MGMT confers resistance to alkylating nitrosourea agents, including BCNU and temozolomide. This property has been exploited to improve the transduction efficiency in a xenotransplantation model in NOD/SCID mice, using gene-corrected human HSCs that express a mutant form of MGMT. The mutant MGMT confers resistance to the effects of exposure to O6-benzylguanine, which was administered with a nitrosourea to inhibit endogenous MGMT activity and select nitrosourea-resistant cells (Figure 3; see Color Figures, page 546). Of course, such a selection scheme would deplete HSCs that do not express the resistance gene, and thus significantly reduce the repopulating hematopoietic activity of the stem cell inoculum after the gene transfer step, and this would be particularly problematic if myeloablative therapy were administered before infusion of the transduced HSCs. To overcome this pitfall, various groups have used an inducible growth signal to promote rapid post-infusion expansion of transduced HSCs. An example is the fusion of the FK506 receptor domains to the intracellular portion of the thrombopoietin receptor.36 Strategies like this one could result in uncontrolled proliferation of an early hematopoietic progenitor cell or HSC, leading to deleterious effects, even leukemogenesis. Thus they have not been applied clinically. Ultimately, the most practical and immediately available method to promote engraftment of transduced HSCs is administration of ablative or partially ablative chemotherapy before HSC infusion, thereby providing a competitive advantage to transduced repopulating cells. This procedure would be akin to autologous transplantation and would thus extend a small but fixed risk of chemotherapy-induced morbidity and mortality. As indicated by experience with myeloablative allogeneic transplantation, ablative therapy would undoubtedly contribute to the toxicity of gene replacement therapy, particularly in older patients with end-organ damage caused by vaso-occlusion, who are most likely to be considered for enrollment in initial gene therapy trials.
Optimizing Stable Expression of the Replacement Gene
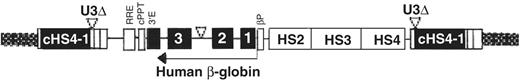
Ideally, the insertion of a native or anti-sickling globin gene should target integration to the location where the molecular controls for appropriate tissue-specific and developmentally regulated expression reside. RNA based ‘repair’ of the βS mRNA, such as use of hammerhead and trans-splicing ribozymes37 or short fragment homologous recombination38 to convert βS to β-globin, might epitomize this optimal strategy for ensuring long-term stable expression of a repaired or replacement gene. These methods, however, require improved gene delivery methods before they can move to a clinical trial. While expression from integrated retroviral cassettes increases dramatically when regulatory elements from the human β-globin LCR are juxtaposed, these elements have not been sufficient to overcome the repressive effects of neighboring chromatin in all integration locations, and so the long-term expression of the replacement gene is not universally assured after chromosomal integration.39,40 Currently there are ongoing efforts to bracket the globin gene expression cassette with ‘boundary’ or ‘insulator’ elements, which ideally might ensure the tissue-specific expression of globin RNA as influenced by its regulatory elements, and suppress the influence of neighboring chromatin or genes. Such an element might also improve safety by preventing the activation or inappropriate regulation of flanking genes by the integrated provirus. Utility of the chicken β-globin 5′HS4 insulator is being actively investigated. Current data suggest that it has insulating, activating and silencing activities, according to the context established by its non-native chromosomal location.41 As the cHS4 insulator has a potential for enhancer-blocking and chromatin insulating activities, it is possible that it (and similar elements) might also interfere with regulation of neighboring genes, particularly if retroviral integration places the insulator between a promoter and its distant regulatory elements. However, very promising preclinical results were observed after correction of β-thalassemia major CD 34+ bone marrow cells by a lentiviral vector in which the expression cassette was flanked by the cHS4 boundary element (Figure 4F3 ).33 It is likely that the utility of boundary elements and their safety will be investigated more fully in the near future.
Summary
HCT and replacement gene therapy have curative potential for SCD, and these continue to be actively investigated. HCT has a track record of success and, if applied under optimal conditions, results in clinical cure of the majority of patients. However, it is associated with short-term and long-term toxicities that limit its widespread application. Gene therapy has the potential for a lower toxicity profile compared to transplantation, but very little is known about its long-term toxicity, in particular the effects of ex-vivo manipulation of hematopoietic cells. There are well-based concerns that the genetic and cellular manipulations that are required to ensure high-level expression will carry a significant, but as yet ill-defined, risk of malignancy. In addition, there remain technical difficulties in ensuring the long-term, high-level, tissue-specific expression of replacement or anti-sickling genes, and these difficulties continue to hinder the initiation of clinical trials for gene therapy for hemoglobinopathies. In light of the high-profile nature of gene therapy and transplantation therapies, it is of utmost importance that any clinical trials be conducted in the absence of conflicting interests and with careful attention to ensuring informed consent that deals explicitly with the issues discussed above.
Hematopoietic cell transplantation for sickle cell disease.
| . | Minimal-Toxicity Conditioning Regimen . | Reduced-Intensity Conditioning Regimen . | Conventional Myeloablative Conditioning Regimen . |
|---|---|---|---|
| †Includes 2 patients with thalassemia major. | |||
| ‡Includes 1 patient with thalassemia major. | |||
| Abbreviations: ATG, antithymocyte globulin; BU, busulfan; CY, cyclophosphamide; Flu, fludarabine; Gr, grade; GVHD, graft-versus-host disease; Mel, melphalan; No., number; PBHC, peripheral blood hematopoietic cells; TBI, total body irradiation; TLI, total lymphoid irradiation; CB, umbilical cord blood | |||
| No. of patients | 11† | 12‡ | 201 |
| Patient age (median, yrs) | 11 (range, 3–30) | 22 (range, 5–56) | – (range, 0.9–22) |
| Conditioning regimen (dose) [no. of patients] | Flu (90–150 mg/m2)/TBI (200 cGy) [5]; Flu (125–150 mg/m2)/ATG/TBI (200 cGy)[6] | Flu (175 mg/m2)/BU (8 mgkg)/ATG/TLI (500 cGy) [5]; Flu (120 mg/m2)/Mel(140 mg/m2)/ATG[2]; Flu (120 mg/m2)/CY (120 mg/kg) [1]; Flu (120 mg/m2)/Mel (140 mg/m2)/Campath [2]; Flu (120 mg/m2)/BU (3.2 mg/kg) [2] | BU/CY/ATG [133] BU/CY [59] BU/CY/TLI [6] CY/TBI [3] |
| Source of stem cells (no. of patients) | Marrow (9); PBHC (2) | Marrow (9); PBHC (6); CB (2) | Marrow |
| Induction of mixed chimerism | Yes (transient in 10) | Yes | Yes, in 11% |
| No. with graft rejection/disease recurrence | 10 | 3 | 16 (8%) |
| No. with GVHD | Acute 1 (Gr I), chronic, none | Acute 4 (Gr II–IV), chronic, 3 (2 fatal) | acute 25%, chronic 12% |
| No. of deaths | None | 2 | 20 (10%) |
| No. with event-free survival | 1 (9%) | 7 (58%) | 166 (83%) |
| . | Minimal-Toxicity Conditioning Regimen . | Reduced-Intensity Conditioning Regimen . | Conventional Myeloablative Conditioning Regimen . |
|---|---|---|---|
| †Includes 2 patients with thalassemia major. | |||
| ‡Includes 1 patient with thalassemia major. | |||
| Abbreviations: ATG, antithymocyte globulin; BU, busulfan; CY, cyclophosphamide; Flu, fludarabine; Gr, grade; GVHD, graft-versus-host disease; Mel, melphalan; No., number; PBHC, peripheral blood hematopoietic cells; TBI, total body irradiation; TLI, total lymphoid irradiation; CB, umbilical cord blood | |||
| No. of patients | 11† | 12‡ | 201 |
| Patient age (median, yrs) | 11 (range, 3–30) | 22 (range, 5–56) | – (range, 0.9–22) |
| Conditioning regimen (dose) [no. of patients] | Flu (90–150 mg/m2)/TBI (200 cGy) [5]; Flu (125–150 mg/m2)/ATG/TBI (200 cGy)[6] | Flu (175 mg/m2)/BU (8 mgkg)/ATG/TLI (500 cGy) [5]; Flu (120 mg/m2)/Mel(140 mg/m2)/ATG[2]; Flu (120 mg/m2)/CY (120 mg/kg) [1]; Flu (120 mg/m2)/Mel (140 mg/m2)/Campath [2]; Flu (120 mg/m2)/BU (3.2 mg/kg) [2] | BU/CY/ATG [133] BU/CY [59] BU/CY/TLI [6] CY/TBI [3] |
| Source of stem cells (no. of patients) | Marrow (9); PBHC (2) | Marrow (9); PBHC (6); CB (2) | Marrow |
| Induction of mixed chimerism | Yes (transient in 10) | Yes | Yes, in 11% |
| No. with graft rejection/disease recurrence | 10 | 3 | 16 (8%) |
| No. with GVHD | Acute 1 (Gr I), chronic, none | Acute 4 (Gr II–IV), chronic, 3 (2 fatal) | acute 25%, chronic 12% |
| No. of deaths | None | 2 | 20 (10%) |
| No. with event-free survival | 1 (9%) | 7 (58%) | 166 (83%) |
Survival and event-free survival after bone marrow transplantation for sickle cell disease.4 The Kaplan-Meier probabilities of survival and event-free survival among 59 patients who received HLA-identical sibling bone marrow allografts are shown. In addition, the cumulative incidence of graft rejection and recurrent sickle cell disease is shown. An event was defined as death, graft rejection, or return of sickle cell disease.
Survival and event-free survival after bone marrow transplantation for sickle cell disease.4 The Kaplan-Meier probabilities of survival and event-free survival among 59 patients who received HLA-identical sibling bone marrow allografts are shown. In addition, the cumulative incidence of graft rejection and recurrent sickle cell disease is shown. An event was defined as death, graft rejection, or return of sickle cell disease.
Enrichment of peripheral blood RBC chimerism compared with WBC chimerism in the blood, hematopoietic organs, and stem cells.21 (A) In the individual mice, a striking enrichment of peripheral blood RBC versus WBC chimerism occurred. (B) Enrichment of peripheral RBC chimerism (x-axis) compared with WBC chimerism (y-axis) in the bone marrow and spleen occurred in mice that received transplants. (C) Enrichment of peripheral RBC chimerism (x-axis) compared with stem cell chimerism (y-axis) in the bone marrow and spleen also occurred in the mice that received transplants. (D) A higher percentage of splenic stem cells than bone marrow stem cells were donor derived in the chimeric mice. The line depicts the theoretical 1:1 ratio of bone marrow to spleen stem cells.
Reprinted with permission from
Enrichment of peripheral blood RBC chimerism compared with WBC chimerism in the blood, hematopoietic organs, and stem cells.21 (A) In the individual mice, a striking enrichment of peripheral blood RBC versus WBC chimerism occurred. (B) Enrichment of peripheral RBC chimerism (x-axis) compared with WBC chimerism (y-axis) in the bone marrow and spleen occurred in mice that received transplants. (C) Enrichment of peripheral RBC chimerism (x-axis) compared with stem cell chimerism (y-axis) in the bone marrow and spleen also occurred in the mice that received transplants. (D) A higher percentage of splenic stem cells than bone marrow stem cells were donor derived in the chimeric mice. The line depicts the theoretical 1:1 ratio of bone marrow to spleen stem cells.
Reprinted with permission from
Lentiviral vector design with the cHS4 insulator.33 The SIN lentiviral backbone is flanked by a 1.2-kb cHS4 insulator (cHS4-I) element to replace the 398-bp U3 promoter and/or enhancer deletion. Upon proviral integration into host genome, the U3 region containing the cHS4 is copied over to the 5′LTR. The ‘insulated’ replacement gene contains the β-globin gene and β-globin LCR elements.
Reprinted with permission from
Lentiviral vector design with the cHS4 insulator.33 The SIN lentiviral backbone is flanked by a 1.2-kb cHS4 insulator (cHS4-I) element to replace the 398-bp U3 promoter and/or enhancer deletion. Upon proviral integration into host genome, the U3 region containing the cHS4 is copied over to the 5′LTR. The ‘insulated’ replacement gene contains the β-globin gene and β-globin LCR elements.
Reprinted with permission from